Engineering of the endogenous HBD promoter increases HbA2
Figures
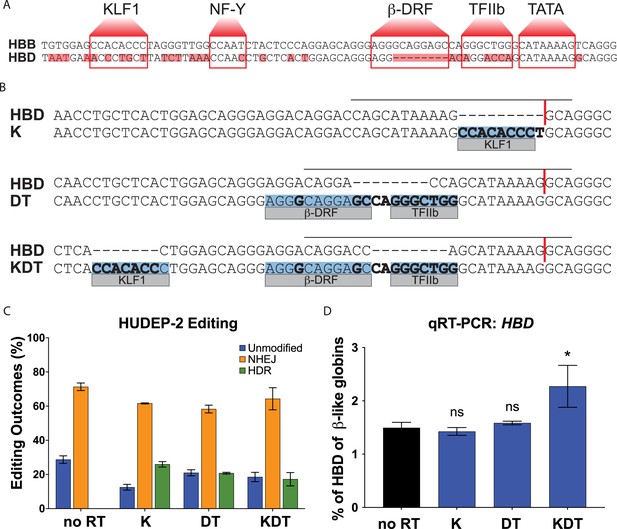
Targeting and design of the endogenous HBD promoter.
(A) Alignment of the HBB and HBD promoter sequences. Transcription factor binding sequences for KLF1, NF-Y, β-DRF, TFIIb, and TATA are shown in boxes and base pair mismatches between HBB and HBD are highlighted in red. (B) The repair template designs for insertions of KLF1 (K), β-DRF and TFIIb (DT), and KLF1, β-DRF, and TFIIb (KDT) directly compared to the HBD promoter. The inserted transcription factor binding sequences are highlighted in blue. Any base pair changes are in bold. The gRNA is indicated by a black horizontal line and the cut site is indicated by a red vertical line. (C) HUDEP-2 editing efficiencies showing percentages of unmodified, NHEJ, or HDR alleles. Conditions tested were Cas9 and sgRNA RNP with no repair template (no RT), K, DT, and KDT repair templates. This experiment was performed three times and the data is presented as mean ± SD of three biological replicates. (D) qRT-PCR of HBD after pooled editing of HUDEP-2 cells with no RT, K, DT, and KDT and 5 days of differentiation. Data is plotted as % of all β-like globins (HBB, HBG1/2, HBD). The three biological replicates from the editing experiment in (C) were each differentiated and the data is presented as mean ± SD of three biological replicates. p Value indicates paired, two-tailed student t test (ns, non-significant; *, p≤0.05; **, p≤0.01).
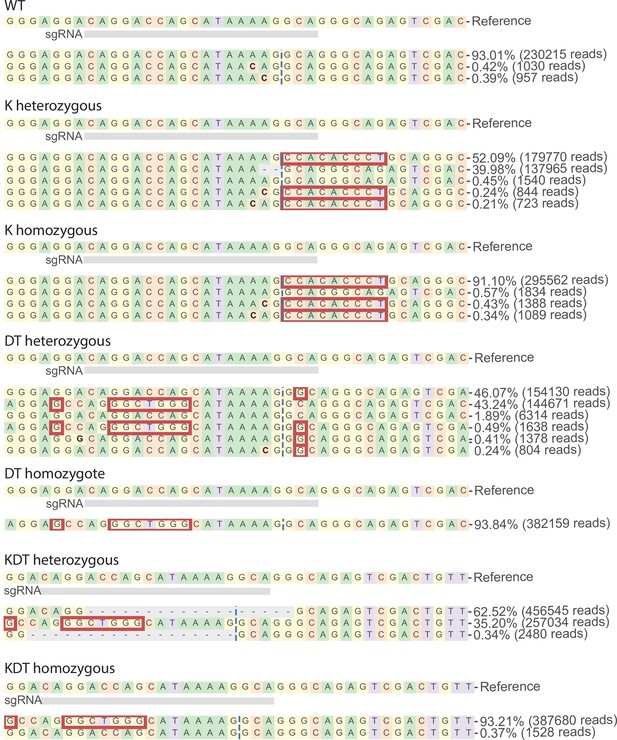
Genotypes of HUDEP-2 clones with heterozygous or homozygous knock-ins of KLF1, β-DRF, and TFIIb sequences.
Clonal HUDEP-2 cell lines of K, DT, and KDT promoter knock-ins were generated. Data shown is a representative genotype analysis of a heterozygous and homozygous clone of each knock-in using CRISPResso of NGS data. Red boxes denote insertions.
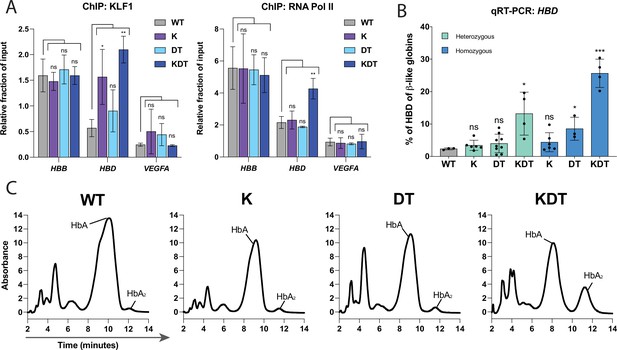
Characterization of HUDEP-2 clones with heterozygous or homozygous knock-ins of KLF1, β-DRF, and TFIIb sequences.
(A) ChIP-qPCR of KLF1 and RNA Pol II performed on WT HUDEP-2 cells and homozygous clones of K, DT, and KDT. Data is shown as relative fraction of input and normalized to SP1. The genes targeted are HBD, HBB, and VEGFA as a negative control. Cells from WT, one K homozygous clone, one DT homozygous clone, and one KDT homozygous clone were grown and harvested separately and on different days for each biological replicate. The data is presented as mean ± SD of three biological replicates. P value indicates paired, two-tailed student t test (ns, non-significant; *, p≤0.05; **, p≤0.01). (B) qRT-PCR of HBD of HUDEP-2 heterozygous and homozygous clones with K, DT, and KDT knock-in and 5 days of differentiation. Each dot represents an individual clonal population, each validated by NGS. Data is plotted as % of β-like globins (HBB, HBG1/2, HBD). Each clone was differentiated and the data is presented as one replicate for each clonal population. p Value indicates paired, two-tailed student t test (ns, non-significant; *, p≤0.05; **, p≤0.01). (C) HPLC of of HUDEP-2 homozygous clones with K, DT, and KDT knock-in and 5 days of differentiation. Hemoglobin A (HbA) and Hemoglobin A2 (HbA2) peaks are annotated. HPLC of one homozygous clone of K, DT, and KDT was performed in triplicate, with a representative dataset of one replicate shown.
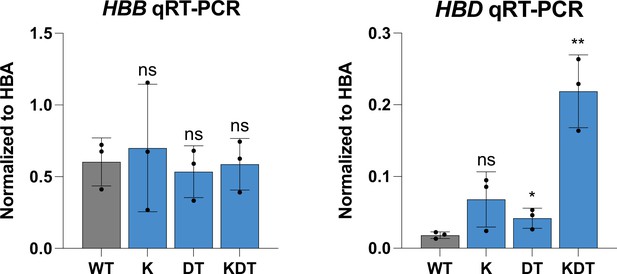
qRT-PCR of HUDEP-2 clones with homozygous knock-ins of KLF1, β-DRF, and TFIIb sequences.
qRT-PCR of HBD of HUDEP-2 homozygous clones with K, DT, and KDT knock-in and 5 days of differentiation. Data is plotted as normalized to HBA. Each clone was differentiated on 3 separate days and the data is presented as mean ± SD of three biological replicates. p Value indicates paired, two-tailed student t test (ns, non-significant; *, p≤0.05; **, p≤0.01).
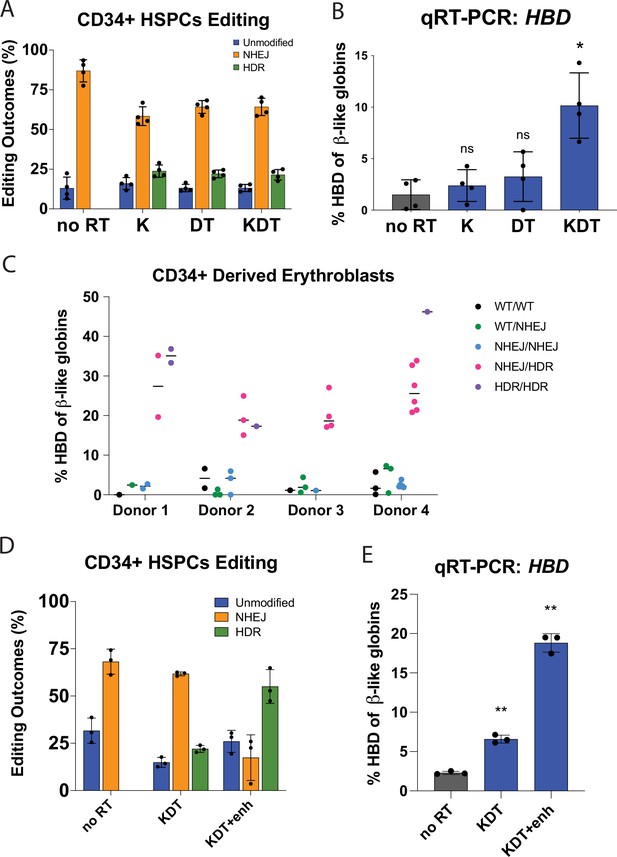
Endogenous editing of the HBD promoter in HSPCs.
(A) Editing efficiencies showing percentages of unmodified, NHEJ, or HDR alleles. Conditions tested were Cas9 and sgRNA RNP with no repair template (no RT), K, DT, and KDT repair templates. The data is presented as independent editing experiments with four different donor samples. (B) qRT-PCR of HBD after pooled editing of HSPCs with no RT, K, DT, and KDT. Cells were expanded in erythroid expansion conditions and differentiated for 5 days. Data is plotted as % of all β-like globins (HBB, HBG1/2, HBD). The data is presented as independent editing experiments with four different donor samples. (C) qRT-PCR of HBD of clonal erythroblast populations after 5 days of differentiation. Genotypes were determined by NGS. Data is plotted as % of β-like globins (HBB, HBG1/2, HBD). The data is presented as independent editing experiments with four different donor samples and each dot denotes an individual clonal population. (D) Editing efficiencies showing percentages of unmodified, NHEJ, or HDR alleles. Conditions tested were Cas9 and sgRNA RNP with no repair template (no RT), KDT repair template, and KDT repair template with AZD-7648 (KDT +enh). The data is presented as one editing experiment with three different donor samples. (E) qRT-PCR of HBD after pooled editing of HSPCs with no RT, KDT, or KDT +enh. Cells were expanded in erythroid expansion conditions and differentiated for 5 days. Data is plotted as % of all β-like globins (HBB, HBG1/2, HBD). The data is presented as one editing experiment with three different donor samples.
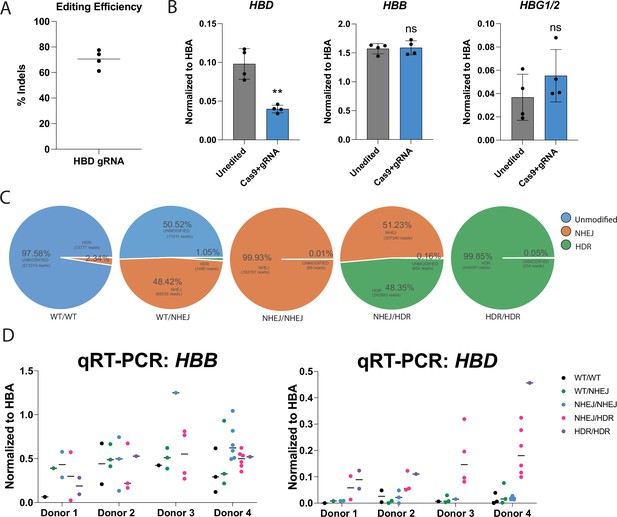
Characterization of HBD promoter edited CD34 +HSPCs.
(A) Editing efficiencies showing percentages of indels after pooled editing of HSPCs with no RT. The data is presented as one editing experiments with four different donor samples. (B) qRT-PCR of HBD after pooled editing of HSPCs with no RT. Cells were expanded in erythroid expansion conditions and differentiated for 5 days. Data is plotted as % of all β-like globins (HBB, HBG1/2, HBD). The data is presented as one editing experiment with four different donor samples. P value indicates paired, two-tailed student t test (ns, non-significant; *, p≤0.05; **, p≤0.01). (C) An example subset of genotypes of WT/WT, WT/NHEJ, NHEJ/NHEJ, NHEJ/HDR, and HDR/HDR clonal erythroblasts as analyzed by CRISPResso2 of NGS data. (D) qRT-PCR of HBD of clonal erythroblast populations after 5 days of differentiation. Genotypes were determined by NGS. Data is plotted as normalized to HBA. The data is presented as independent editing experiments with four different donor samples and a dot denotes individual clonal populations.
Additional files
-
Supplementary file 1
Oligo sequences used for the HBD IVT templates for the generation of sgRNAs, repair template sequences, NGS primers, qRT-PCR primers, and ChIP qRT-PCR primers.
- https://cdn.elifesciences.org/articles/85258/elife-85258-supp1-v2.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/85258/elife-85258-mdarchecklist1-v2.pdf





