Multiplexed microfluidic screening of bacterial chemotaxis
Figures
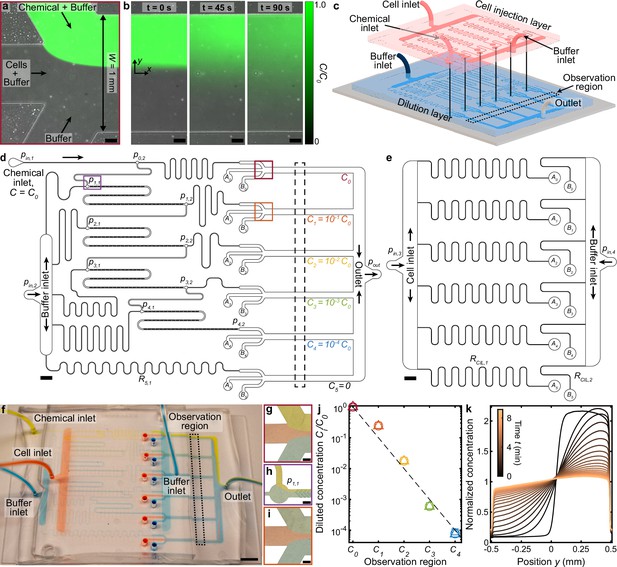
Multiplexed microfluidic device for simultaneous chemotaxis assays.
(a,b) Continuous flow through a microfluidic junction (a) stratifies chemostimulus, cell, and buffer solutions, demonstrated here with fluorescein, DI water, and DI water, respectively. Upon halting the flow (b) diffusion establishes a chemical gradient across the channel, which is repeated at each observation channel in the MCD (d, red and orange boxes). Scale bars, 0.1 mm. (c) Assembly of the MCD showing the PDMS dilution layer (blue) and cell injection layer (red) microchannels mounted on a glass slide (grey; Materials and methods). (d) Scaled drawing of the dilution layer, which receives chemical (pressure, ) and buffer () solutions. Initial chemical concentration (C0) is sequentially diluted 10-fold to each of four additional concentrations (), plus a control solution (). These six chemostimulus solutions are merged separately with additional cell () and buffer () solutions from the cell injection layer (e) for chemotaxis assays in respective observation channels (dashed black box, corresponding to c and f). (e) Scaled drawing of the cell injection layer which injects a cell suspension () and buffer solution () into the dilution layer (; Materials and methods). Scale bars d,e, 2 mm. (f) Photograph of the completed MCD with dyed water to visualize the chemical (yellow), cell (red), and buffer (blue) fluid streams in the channel network. Scale bar, 5 mm. (g) Stratified chemical (C0), cell, and buffer solutions in the first observation region (d, red box). (h) Dilution of the chemical (C0) by the buffer prior to mixing in the first micromixer (Stroock et al., 2002) to produce concentration C1 (d, purple box). (i), Stratified chemical solution after initial dilution (C1, green) in the second observation region (d, orange box). Scale bars g-i, 0.2 mm. (j) Measured chemical concentrations (see Materials and methods) generated from the dilution microchannels (d) for various driving pressures (square, circle, and triangle, respectively). (k) Measured evolution of the chemical gradient (b) produced in the C0 observation region (Figure 1—figure supplement 3; Materials and methods) by the MCD shows the chemical diffusion across the channel with increasing time t.

Micromixer geometry and mixing performance.
(a,b) The herringbone micromixer (Stroock et al., 2002) - used to incorporate chemical and buffer during serial dilution - consists of a main rectangular channel (, ) with herringbone ridges (, ), which enhance mixing by generating transverse flow. Each herringbone half cycle ( long) consists of six ridges (pitch, θ = 45°; a). The distance, q, from the sidewall alternates every half cycle between 2 W/3 and W/3. (c) The degree of mixing (DOM; Materials and methods) (Stroock et al., 2002) was quantified for a long test channel having 29 herringbone cycles for two different flow rates: (blue triangles) and (red circles) using fluorescein dye (Petrášek and Schwille, 2008) (). Distance indicates the downstream position from the point where the dye and water solutions meet. The solution is considered mixed when (90% complete mixing, dashed line), which is achieved after 9 cycles for both flow rates. The final micromixer design comprised 26 herringbone cycles (see Figure 1—figure supplements 2 and 4) to ensure complete mixing across a range of potential chemostimulants.
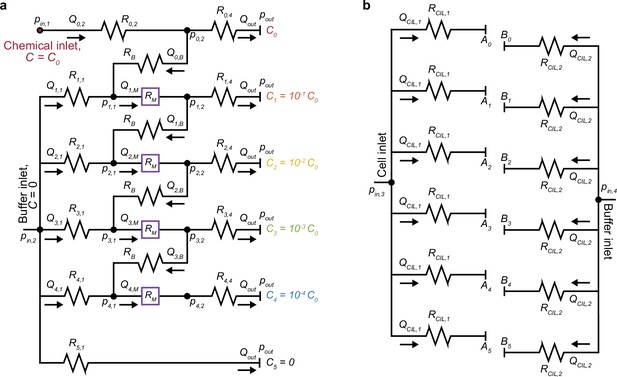
Hydraulic circuit design of MCD dilution layer and cell injection layer.
(a) Circuit representation of the dilution layer (Sugiura et al., 2010) of the MCD (Figure 1d). The two inlets (, ) receive the base chemical solution (C0) and the buffer solution (), respectively, and the outlet is open to atmospheric pressure (). The hydraulic network was designed such that a prescribed inlet pressure from a pressure controller produces outlet flow rates of . The resistances of the bridge channels (), micromixer channels (), and observation channels () were predetermined (Materials and methods). The remaining flow rates, pressures, and hydraulic resistances were determined by solving a linear set of equations for the hydraulic circuit (Kirby, 2010), and the resulting resistances determine the individual channel geometries (Appendix 1). (b) Circuit representation of the cell injection layer of the MCD (Figure 1e). The cell injection layer network was designed to flow the cell () and buffer () solutions into the dilution layer to ensure the cell solution would be centered in the observation region. To do so, the applied inlet pressures were designed to be and flow rates in each microchannel for the cell () and buffer () were and, respectively. The resulting channel resistances and dimensions were determined using the Hagen-Poiseuille law (Kirby, 2010; Oh et al., 2012; Materials and methods), and accounted for the resistance of the observation region (; Appendix 1). The labels in a and b corresponds to that in Figure 1d and e.
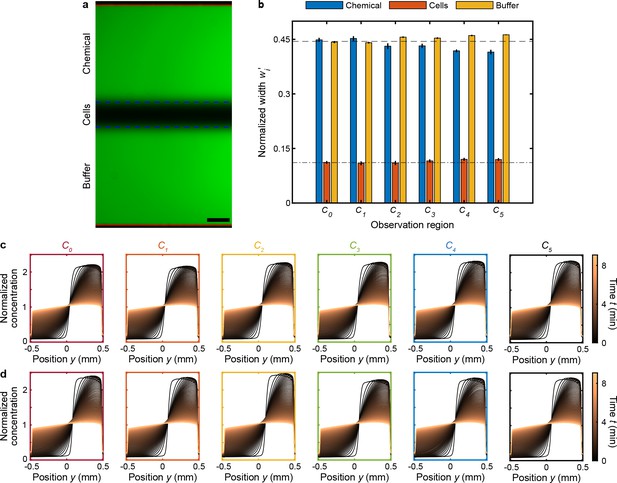
Validation of chemostimulus distribution and gradient evolutionin in MCD observation regions.
(a,b) The initial symmetry of the stratified chemical, cell, and buffer solutions flowing in the observation channel was verified by measuring the widths of the three fluid streams through fluorescent dye visualization (Materials and methods). (a) A fluorescein solution was flowed through both inlets of the dilution layer (chemical and buffer inlets; Figure 1d) and also the buffer solution inlet of the cell injection layer (Figure 1e). Solid red and dashed blue lines indicate measured channel wall and stream interface locations, respectively, for a driving pressure and (Figure 1—figure supplement 2). Scale bar, 100 μm. The normalized measured widths,, of the chemical, cell, and buffer streams illustrate their high degree of symmetry across all observationchannels (); where wi are the fluid stream widths (a; dashed blue lines) and is the total width of the intensity profile (a; solid red lines). The bar plots and error bars indicate the mean and standard deviation of the normalized stream widths, investigated for three different driving pressures of the dilution layer and cell injection layer: 100/70 mbar, 150/105mbar, and 200/140 mbar (). The applied inlet pressure of the cell injection layer was tuned (Materials and methods) until symmetric flow was achieved in the observation channels. The mean percent difference of the measured widths of the chemical, cell, and buffer solution relative to the theoretical widths (Materials and methods) was only 2.6%, 3.0%, and 1.8%, respectively. (c,d) The uniformity of the chemostimulus gradient evolution across the various observation regions () was determined through fluorescent dye (fluorescein) visualization of stop-flow experiments (Materials and methods). Each observation region started with the same initial (fixed) concentration () by injecting the same dye concentration into both the chemical and buffer inlets of the dilution layer (see also Figure 2—figure supplement 1b). The fluorescence intensity enables visualization of the chemical concentration profile evolution across each channel over the course of approximately 9 min (starting from post flow). Measurements were performed for two different driving pressures to ensure that the resulting gradients were insensitive to flow rate: 100/70 mbar (c) and 200/140 mbar (d).
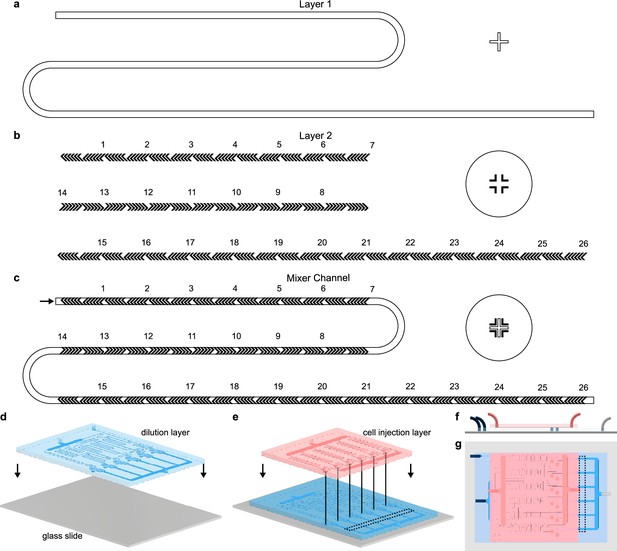
Two-layer photolithography and soft lithography microfabrication of the MCD.
(a–c) Herringbone micromixers (Stroock et al., 2002) were fabricated in the dilution layer of the MCD using multilayer photolithography (subsection of full photomasks shown; see also Figure 1). The first photolithography layer produced the rectangular cross-section main channel (a; 200 μm wide), and a second photolithography layer formed the herringbone ridges on top (Anderson et al., 2000) (b; see also Figure 1—figure supplement 1). Complementary alignment markers were used to align the herringbone ridges (b, hollow cross and circle) with the main channel (a, cross). (c) Overlay of the herringbone micromixer channel components from a and b (see also Figure 1—figure supplement 1). (d,e) The dilution layer (d, blue) and cell injection layer (e, red) PDMS microchannels for the MCD were cast individually from SU-8 molds via soft lithography (McDonald et al., 2000; Materials and methods). The dilution layer microchannel was first plasma bonded (McDonald et al., 2000) to a standard double-wide glass slide (d; grey; 75 mm × 50 mm×1 mm). Subsequently, the cell injection layer was aligned and plasma bonded (Eddings et al., 2008; McDonald et al., 2000) on top of the dilution layer (e). (f,g) Side and top-down view of the assembled MCD, respectively, showing the locations of the four inlets, single outlet, and observation regions (dashed box; see also Figure 1).
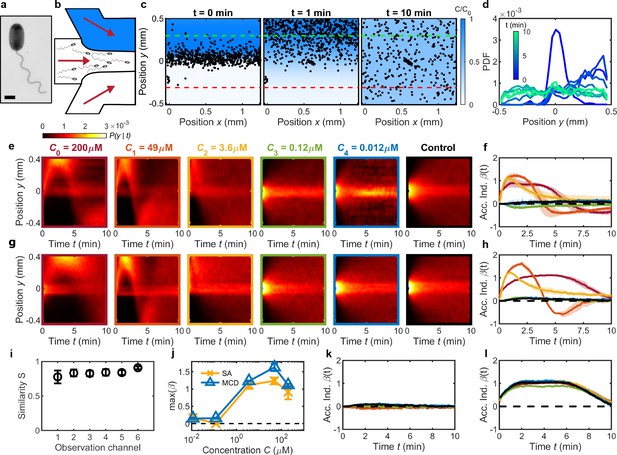
Validation of MCD and measurement of V. alginolyticus chemotactic performance toward serine.
(a) TEM image of V. alginolyticus (Materials and methods). Scale bar, 1 μm. (b) A single chemotaxis assay (SA) with a single conventional microfluidic device flows chemostimulus (top, blue), cell suspension (middle), and buffer (bottom) streams into the observation region (Materials and methods). (c) SA with chemostimulus (serine, ) showing measured cell positions (V. alginolyticus, black dots) at various times after initial flow stratification () relative to the chemostimulus distribution (blue, from measurements in Figure 1b). Cells migrate up the gradient () followed by uniform dispersal as the gradient dissipates (). Degree of cell accumulation is determined from the number of cells, , in a 200 μm wide region on the chemostimulus side (positive; green dashed line) and buffer side (negative; red dashed line), respectively (Seymour et al., 2010; Stocker et al., 2008). (d) The measured cell distribution across the microchannel evolves over time (from c) and is represented as a conditional probability density of cell position, (shown as a kymograph). (e) for V. alginolyticus chemotactic response to serine from a series of SA devices having the same geometry as the MCD observation regions (Figure 1). SA measurements illustrate the transition from positive chemotactic response at high attractant concentration () to no response at low concentration () compared to control () (Altindal et al., 2011). (f) Accumulation index, , for SA measurements from e. (g) measured by the MCD under the same conditions of the SA. (h) measured from g accurately captures the behavior of V. alginolyticus to serine compared to SA results (f). (i) Sørensen similarity metric (Cha, 2007) comparing e and g, which is calculated at each time point and averaged. (j) Comparing MCD and SA peak chemotactic response quantified by max () from f,h. (k,l) in the absence of a chemical gradient (k; Figure 2—figure supplement 1) and for fixed gradients of (l; Figure 2—figure supplement 1b) across each observation channel in the MCD indicates no significant bias. No gradient (k) conditions () were obtained by injecting buffer into the chemical inlet (setting ). Fixed gradient (l) conditions ( of serine) were obtained by injecting of serine into both the chemical and buffer inlets of the dilution layer. Shading in f,h,k,l indicates one standard deviation (N=3). Error bars in i,j are one standard deviation across biological replicates.
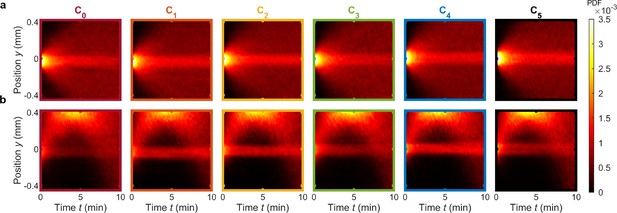
Kymographs for control chemotaxis experiments corresponding to Figure 2k and l with no chemostimulus present () and with a fixed chemostimulus concentration (), respectively.
(a) Control chemotaxis assay with V. alginolyticus in the absence of a chemical gradient. In place of a chemostimulus, ASW () was injected into the chemical inlet of the dilution layer, illustrating that bacteria exhibit no significant bias (see also Figure 2k). (b) Control experiment with V. alginolyticus in a set of fixed chemical gradients across all observation channels illustrates consistent (positive) chemotaxis. ASW with serine at a concentration was injected into both the chemical and buffer inlets of the dilution layer. This approach results in each of the observation regions exhibiting the same chemostimulus gradient, based on the serine concentration (see also Figure 2l).
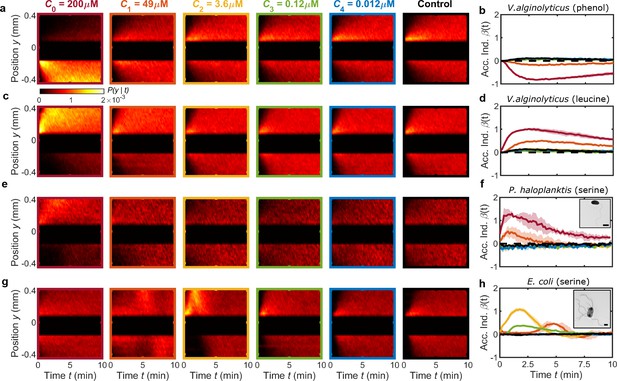
MCD enables rapid quantification of chemotactic responses across different chemostimulants and microbial species.
(a,b) The negative chemotactic response of V. alginolyticus to the repellent phenol (Homma et al., 1996) is evident in kymographs of cell position, , and the accumulation index, , respectively. Central 250 μm wide band, which often contains a significant subpopulation of non-motile cells, is omitted from for visualization purposes and has no impact on . (c,d) MCD measurements demonstrate the positive chemotactic response of V. alginolyticus to leucine observed in and , respectively. (e–h) in response to various concentrations of serine for bacteria P. haloplanktis (e,f) and E. coli (g,h). TEM images of P. haloplanktis (f, inset) and E. coli (h, inset). Scale bars, 1 μm. β(t) for P. haloplanktis (f) illustrates a monotonically increasing response to increased concentrations (extracted from e). Accumulation index, , for E. coli (h) reveals a peak response to an intermediate serine concentration () and delayed accumulation at higher concentrations (Bhattacharjee et al., 2021). Shaded regions are standard deviation (N=2 and N=3 for P. haloplanktis and E. coli, respectively) across biological replicates. Color bar corresponds to kymographs in a,c,e,g.
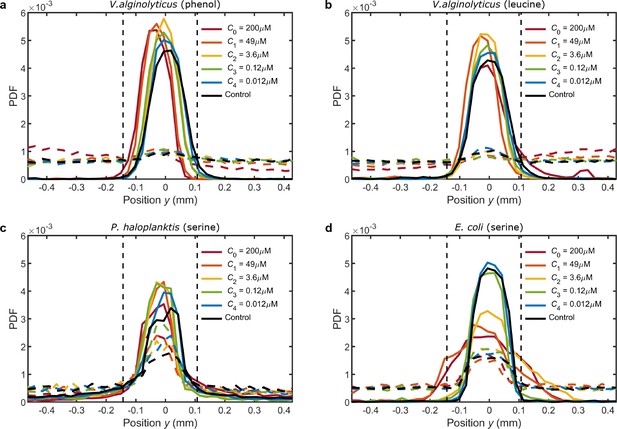
Initial and final cell probability distributions () for the organism/chemostimulant systems in Figure 3.
(a) V. alginolyticus and phenol, (b) V. alginolyticus and leucine, (c) P. haloplanktis and serine, and (d) E. coli and serine. In all experiments, cells begin in a narrow central band localised in the center of the channel (; solid lines). Minor differences in the initial distributions are a consequence of cell motility and available chemostimulus. Comparing the final cell positions (; dashed lines) to the initial peaked distributions (solid lines) reveals that each population contains varying proportions of non-motile cells. After 10 min, few V. alginolyticus cells remain in their original positions relative to the background distribution across the channel (a,b). In contrast, both P. haloplanktis (c) and E. coli (d) have a larger non-motile fraction, with a moderate portion of the population remaining in the center of the channel after 10 min. The spatial distribution of non-motile cells remained approximately constant across all experiments and was comparable to the theoretical width of the fluid injection stream (Figure 1—figure supplement 3). Hence, a fixed 250 μm wide exclusion zone (vertical dashed lines) was chosen to encapsulate the majority of non-motile cells for all experiments. This choice aids in visualisation of (Figure 3) and has no bearing on the outcome of our analyses: Cell accumulation is only measured within 200 μm of the upper/lower channel boundaries. Therefore cells that remain in the center of the channel over the full experiment duration are never considered in the calculation of .
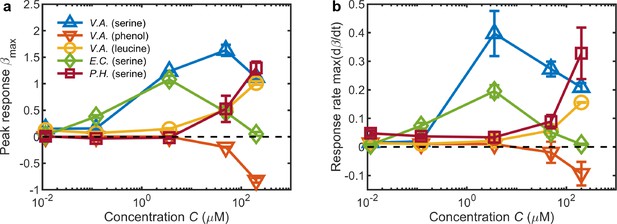
Summary of chemotactic responses across various species, chemostimulants, and concentrations measured using the MCD.
(a) The primary metric for quantifying the chemotactic response of the bacteria was the peak of the accumulation index , where the sign is determined by the positive or negative chemotactic behavior for each chemostimulus concentration (from Figures 2h, 3b, d, f and h). (b) The maximal response rate (prior to ) is indicative of the speed of cell accumulation. These metrics distinguish chemotactic behaviors, for example: The accumulation of V. alginolyticus to serine is greatest at high concentrations (a; ), but the fastest response occurs at weaker concentrations (a; ). Error bars are one standard deviation across biological replicates.
Tables
Multiplexed chemotaxis device (MCD) microchannel resistance and dimensions (corresponding to Figure 1—figure supplement 2f).
| Channel | Resistance(mPamPa.s. μm-3) | Height(μm) | Width(μm) | Length(mm) |
|---|---|---|---|---|
| 0.0082 | 90 | 90 | 19.06 | |
| 0.0095 | 90 | 90 | 22.08 | |
| 0.0147 | 90 | 90 | 34.06 | |
| 0.0201 | 90 | 90 | 46.43 | |
| 0.0277 | 90 | 90 | 64.13 | |
| 0.0300 | 90 | 90 | 69.45 | |
| 0.0201 | 90 | 90 | 46.50 | |
| 0.0149 | 90 | 90 | 34.54 | |
| 0.0098 | 90 | 90 | 22.60 | |
| 0.0046 | 90 | 90 | 10.76 | |
| 0.0035 | 90 | 150 | 19.93 | |
| 0.0043 | - | 200 | 40.7 | |
| 7.7 × 10−4 | 90 | - | - | |
| 0.0575 | 75 | 75 | 64.24 | |
| 0.0142 | 75 | 75 | 15.89 |





