Evolution of an extreme hemoglobin phenotype contributed to the sub-Arctic specialization of extinct Steller’s sea cows
Figures
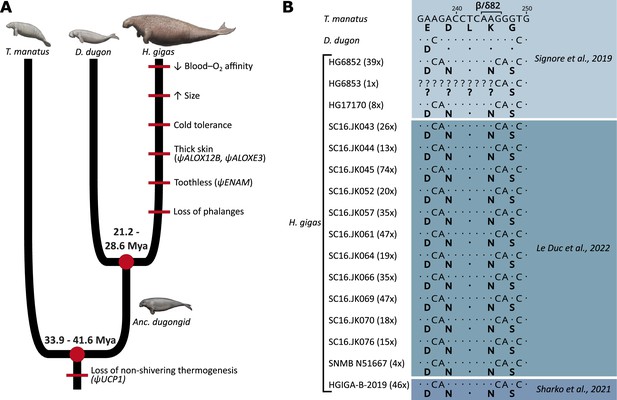
Evolution of notable morphological and genetic attributes within Sirenia.
(A) Phenotypic innovations contributing to the unique biology of Steller’s sea cows are mapped along the sirenian phylogeny (red bars) with the underlying genetic causes shown in brackets where known. Note that the red bars do not represent the dating of these traits and that their placement order is arbitrary. Divergence dates in millions of years (Mya) are based on and Heritage and Seiffert, 2022. The ancestral dugongid (Anc. dugongid) is represented by a late-Oligocene Metaxytherium spp. Sirenian paintings by Carl Buell are adapted from Figure 3 of Springer et al., 2015 and are used with permission of J. Gatesy. (B) Partial nucleotide alignment of the sirenian β/δ-globin gene encoding the central region of the 2,3-diphosphoglycerate binding pocket of hemoglobin; corresponding amino acid residues (bolded) are provided below each sequence. The G→C nucleotide mutation underlying the otherwise invariant amino acid substitution (β/δ82Lys→Asn; K→N) of Steller’s sea cow (Hydrodamalis gigas) hemoglobin is apparent in all 16 individuals for which sequence data is available. Values in brackets next to each H. gigas specimen represent the depth of sequence coverage for this nucleotide. Dots represent sequence identity with the Florida manatee (Trichechus manatus).
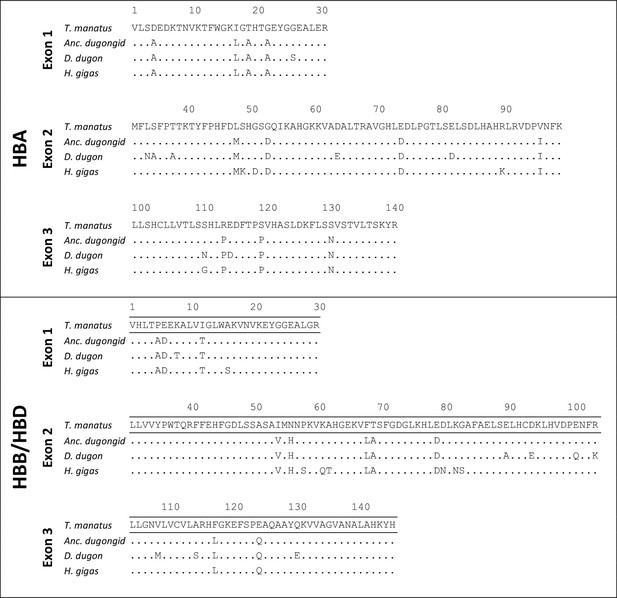
Amino acid sequences of sirenian HBA and HBB/HBD genes and the reconstructed sequences of the last common ancestor (‘Anc. dugongid’) shared by the dugong (Dugong dugon) and Steller’s sea cow (Hydrodamalis gigas).
Dots represent sequence identity with the Florida manatee (Trichechus manatus latirostris).
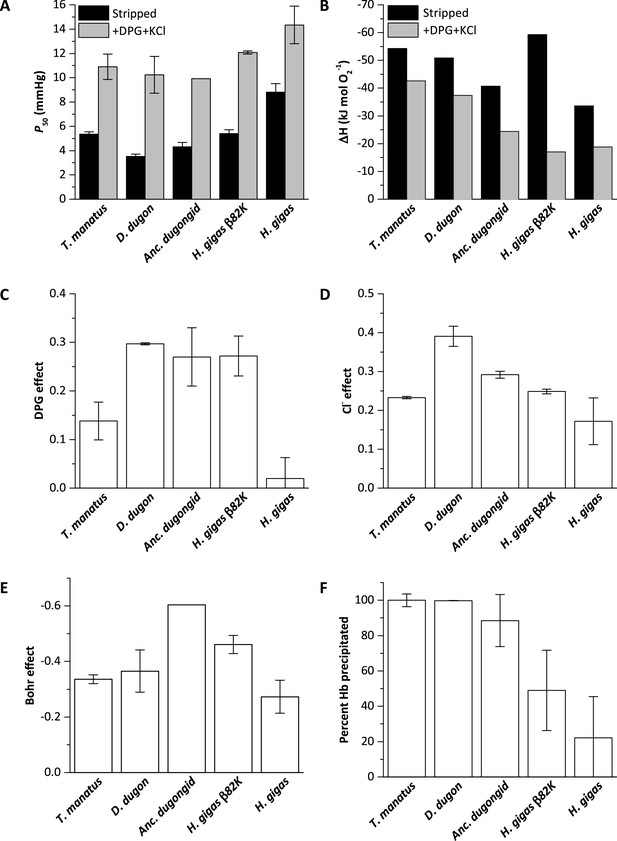
Biochemical properties of hemoglobins (Hb) from manatee (Trichechus manatus), dugong (Dugong dugon), ancestral dugongid (Anc. dugongid), Steller’s sea cow (Hydrodamalis gigas), and a Steller’s sea cow β/δ82Asn→Lys mutant (H. gigas β82K).
All values were measured at 37°C and corrected to pH 7.2, with error bars representing the standard error of the regression estimate. (A) Oxygen tensions at half O2 saturation (P50) in the absence (stripped) and presence of allosteric cofactors (twofold molar excess of 2,3-diphosphoglycerate (DPG) and 0.1 M KCl). (B) The enthalpy of oxygenation (ΔH) between 25 and 37°C in stripped Hb and in the presence of allosteric cofactors (twofold molar excess DPG and 0.1 M KCl). (C) The effect of DPG on sirenian Hbs determined from logP50(0.5 mM DPG)-logP50(stripped). (D) The effect of chloride on sirenian Hbs determined from logP50(0.1 M KCl)-logP50(stripped). (E) The Bohr effect of sirenian Hbs in the presence of allosteric cofactors (twofold molar excess 2,3-diphosphoglycerate (DPG) and 0.1 M KCl), as calculated from ΔlogP50/ΔpH over the pH range 6.9 and 7.8. (F). The relative solubility of sirenian Hbs is denoted by the percentage of Hb protein precipitated by the addition of 3 M ammonium sulfate.
-
Figure 2—source data 1
Source data for Figure 2.
- https://cdn.elifesciences.org/articles/85414/elife-85414-fig2-data1-v2.xlsx
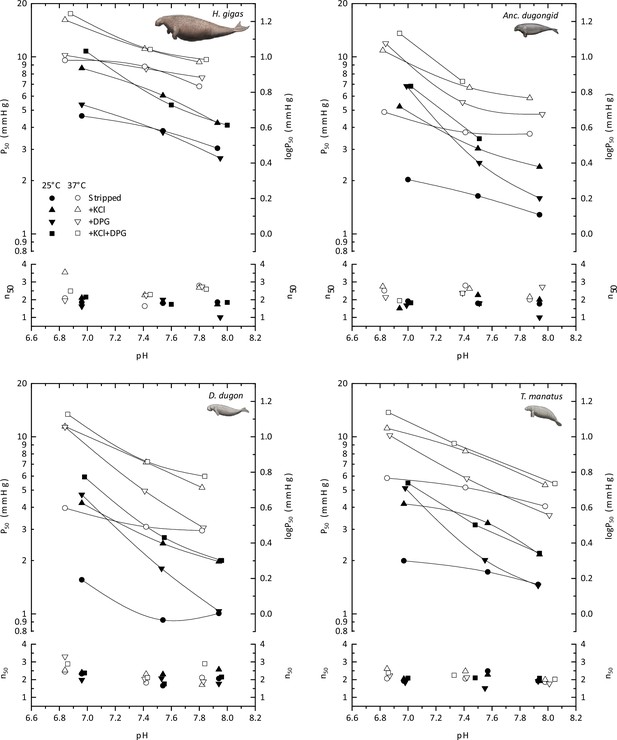
The pH dependence of oxygen tensions and the cooperativity coefficients at half O2 saturation (P50 and n50, respectively) for hemoglobins of the Florida manatee (Trichechus manatus latirostris), dugong (Dugong dugon), Steller’s sea cow (Hydrodamalis gigas), and the last common dugonid ancestor (‘Anc. dugongid’) in stripped Hb (circles), and in the presence of 0.1 M KCl (triangles), of a twofold molar excess of 2,3-diphosphoglycerate (DPG; inverted triangles), and of both KCl and DPG (squares), at 25°C (solid symbols) and 37°C (open symbols).
Images of sirenians are adapted from Figure 3 of Springer et al., 2015 and are used with permission of J. Gatesy.
-
Figure 2—figure supplement 1—source data 1
Source data for Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/85414/elife-85414-fig2-figsupp1-data1-v2.xlsx
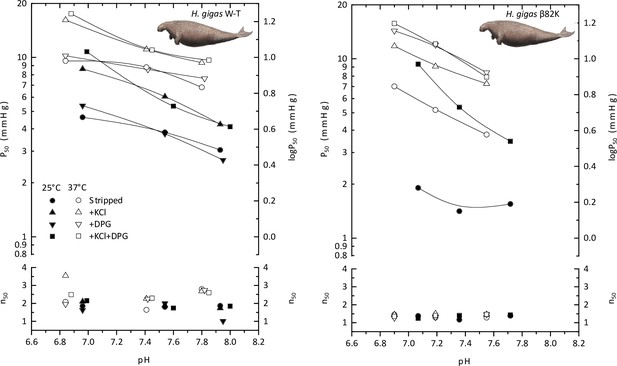
The pH dependence of oxygen tensions and the cooperativity coefficients at half O2 saturation (P50 and n50, respectively) of wild-type Steller’s sea cow hemoglobin (H.gigas W-T) and a mutated Steller’s sea cow β/δ82Lys variant (H.gigas β82K) in the absence and presence of allosteric effectors (0.1 M KCl and/or twofold molar excess of 2,3-diphosphoglycerate (DPG)) at 25°C (solid symbols) and 37°C (open symbols).
Images of sirenians are adapted from Figure 3 of Springer et al., 2015 and are used with permission of J. Gatesy.
-
Figure 2—figure supplement 2—source data 1
Source data for Figure 2—figure supplement 2.
- https://cdn.elifesciences.org/articles/85414/elife-85414-fig2-figsupp2-data1-v2.xlsx
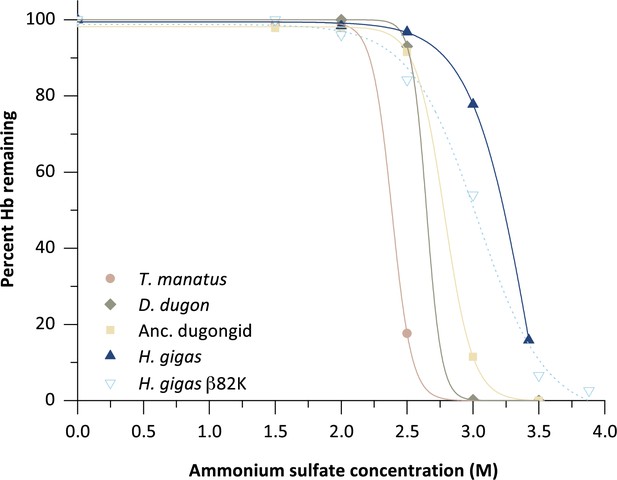
Solubility assay of five sirenian hemoglobins (Hb) illustrating the percentage of Hb protein remaining in solution after precipitation by the addition of ammonium sulfate.
-
Figure 2—figure supplement 3—source data 1
Source data for Figure 2—figure supplement 3.
- https://cdn.elifesciences.org/articles/85414/elife-85414-fig2-figsupp3-data1-v2.xlsx
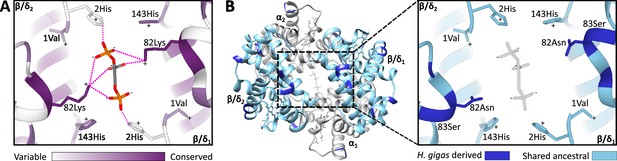
Homology models of the 2,3-diphosphoglycerate (DPG) binding site in ancestral dugongid and Steller’s sea cow (Hydrodamalis gigas) hemoglobin.
(A) Model of the ancestral dugongid DPG binding site. Amino acids are colored according to the degree of sequence conservation. Notably, β/δ82Lys shows the highest level of sequence conservation, as it is able to bind to multiple sites on the DPG molecule (indicated by dashed pink lines), whereas β/δ2His is only able to directly interact with DPG in the protonated state. (B) Model of Steller’s sea cow hemoglobin (left) and a close-up of the DPG binding pocket (right). Dark blue colored residues represent the 11 H. gigas specific substitutions, while those in light blue denote the ancestral state. Homology modeling illustrates how the replacement of β/δ82Lys with neutral Asn inhibits DPG binding to the hemoglobin molecule.
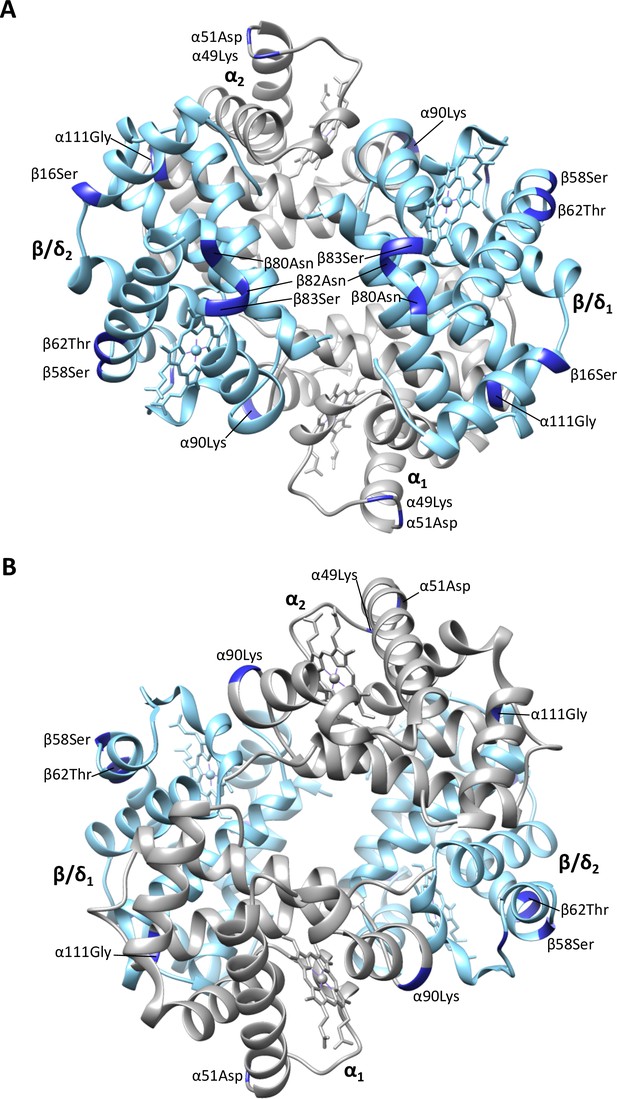
Homology model of Steller’s sea cow (Hydrodamalis gigas) adult-expressed (α2β/δ2) hemoglobin with (A) the β/δ-subunits (blue) in the foreground and (B) the α-subunits (gray) in the foreground.
Dark blue colored residues represent the 11 H. gigas specific substitutions.
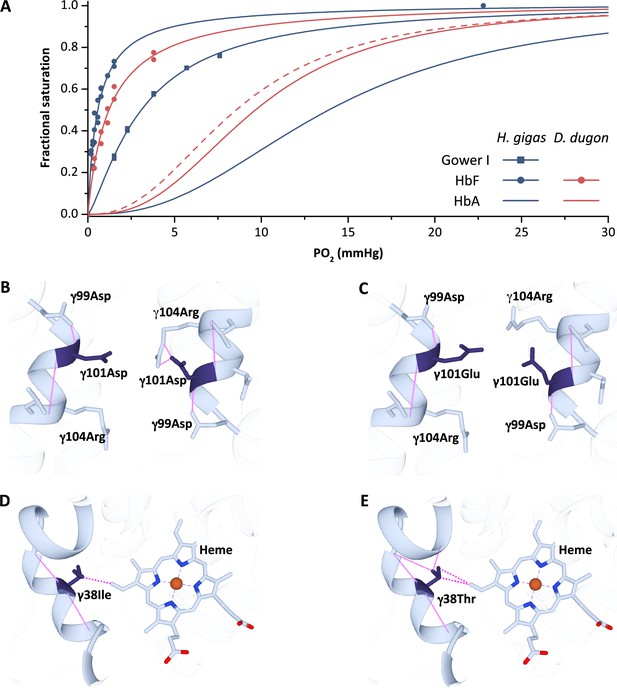
Oxygen equilibrium curves of prenatal and adult sirenian hemoglobins.
(A) Oxygen equilibrium curves for Steller’s sea cow (Hydrodamalis gigas; blue) hemoglobin (Hb) Gower I (ζ2ε2), HbF (α2γ2), and HbA (α2β/δ2) and dugong (Dugong dugon; red) HbF and HbA in the presence of allosteric cofactors 2,3-diphosphoglycerate (DPG) (twofold molar excess) and KCl (100 mM) at pH 7.1 (prenatal Hbs) or 7.2 (HbA).The dashed red line is for dugong HbA in the absence of DPG which illustrates relative differences in O2 affinity between the maternal (solid line) and fetal (dashed line) circulations. Homology models of Steller’s sea cow and dugong HbF denote structural alterations arising from the H. gigas specific 101 (B) vs. (C), respectively, and 38 (D) vs. (E), respectively, replacements. Solid pink lines denote predicted hydrogen bonds while the dashed pink lines represent predicted Van der Waals interactions.
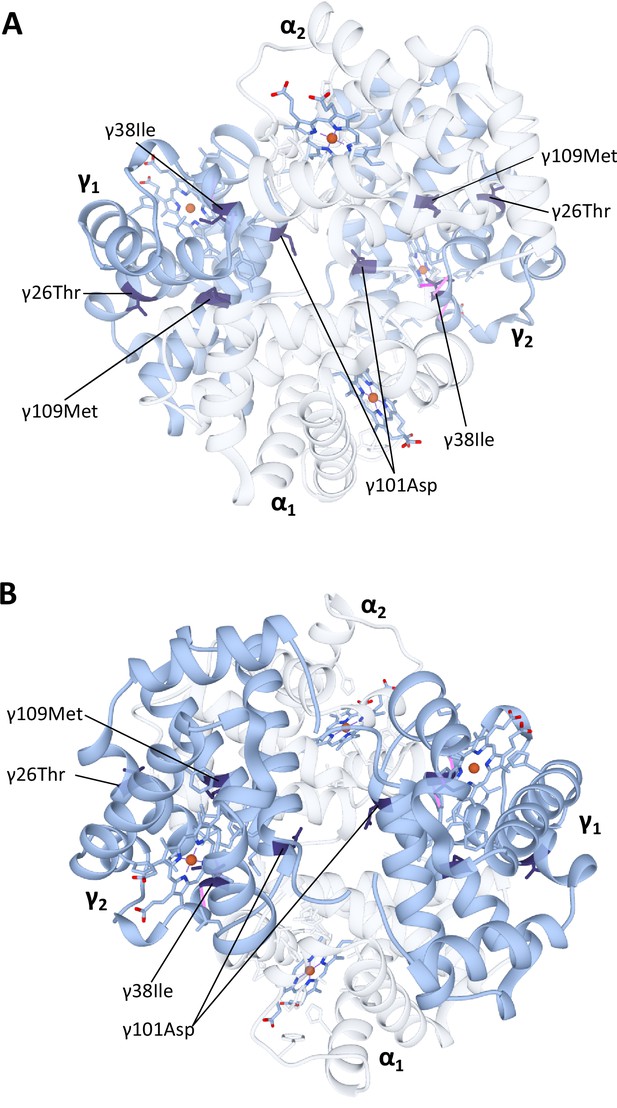
Homology model of Steller’s sea cow (Hydrodamalis gigas) HbF hemoglobin (α2γ2) with (A) the α-subunits in the foreground and (B) the γ-subunits in the foreground.
The α-subunits are identical to those in the H. gigas adult-expressed hemoglobin (see Figure 4 for details) and have been made transparent to improve the clarity of the γ-subunit substitutions. Dark blue colored residues denote the four H. gigas specific γ-chain substitutions (γ26Lys→Thr, γ38Thr→Ile, γ101Glu→Asp, and γ109Val→Met) identified by Signore et al., 2019.

DNA alignment of the 5’ untranslated region and transcriptional promoter regions for representative paenungulate and human HBG genes. Note that hyrax HBG is pseudogenized (Signore et al., 2019) and hence is not included in the alignment.
Regulatory elements and the ‘ATG’ start codons are highlighted in gray. Dots represent sequence identity with Homo sapiens, while hyphens represent alignment gaps. Note that the ‘CACCC’ promoter element required for fetal suppression of HBB is mutated in both Steller’s sea cows (Hydrodamalis gigas) and dugongs (Dugong dugon). Conversely, this box element is intact in both the African elephant (Loxodonta africana) and Florida manatee (Trichechus manatus latirostris) HbF promoter region, consistent with a fetal expression pattern in these species. Note also the A→G mutation at the putative mRNA ‘CAP’ site of H. gigas HBG that has been shown to downregulate the level of human HBB transcription by approximately twofold (Myers et al., 1986).
Additional files
-
Supplementary file 1
Raw oxygen binding data and GenBank accession numbers.
(a) Intrinsic oxygen affinities (P50, mmHg) for the adult-expressed hemoglobins (α2β/δ2) of the Florida manatee (Trichechus manatus latirostris), dugong (Dugong dugon), Steller’s sea cow (Hydrodamalis gigas), and the ancestral dugongid (‘Anc. dugongid’), and their sensitivity to allosteric effectors at 25 and 37°C in 0.1 M HEPES buffer. All values are corrected to pH 7.2. (b) Intrinsic oxygen affinities (P50, mmHg) for the prenatally-expressed hemoglobins Gower I (ζ2ε2) and HbF (α2γ2) of Steller’s sea cow (Hydrodamalis gigas) and HbF (α2γ2) of the dugong (Dugong dugon), and their sensitivity to allosteric effectors at 37°C in 0.1 M HEPES buffer. All values are corrected to pH 7.1 to account for the lower pH of prenatal blood. (c) Accession numbers for beta-globin amino acid sequences used for ConSurf analyses.
- https://cdn.elifesciences.org/articles/85414/elife-85414-supp1-v2.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/85414/elife-85414-mdarchecklist1-v2.pdf





