TLR7 activation at epithelial barriers promotes emergency myelopoiesis and lung antiviral immunity
Figures
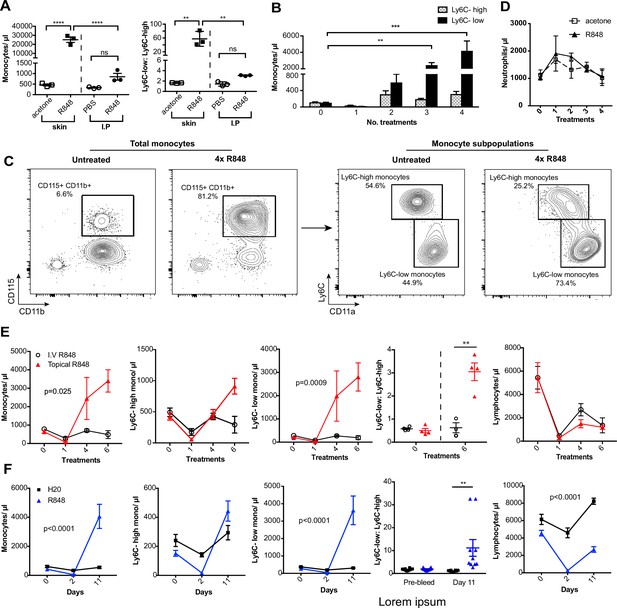
Repetitive R848 administration at barrier sites drives a profound monocytosis.
(A) BALB/c mice (n = 3 per group) received 100 µg of R848 topically or intraperitoneally (IP), 3× per week for 4 wk. Control BALB/c mice were given topical acetone or 200 µl of PBS IP. Left panel shows the number of total monocytes (CD11b+CD115+), the right panel the ratio between Ly6C-high and Ly6C-low monocytes. (B) BALB/c mice (n = 3 per group) received four treatments of topical R848 (100 µg). Numbers of Ly6C-high (grey bars) and Ly6C-low (black bars) monocyte 24 hr after each treatment. (C) Representative plots of total monocytes (left panel) and of subpopulations (right panel) in mice treated topically with acetone or R848 four times. (D) Mice treated as in (B). Neutrophil counts 24 hr after each treatment. (E) BALB/c mice (n = 4 per group) received six treatments with 100 µg topical (red line) or IV R848 (black line). Blood counts are shown for total monocytes, Ly6C-high monocytes, Ly6C-low monocytes, monocyte subpopulation ratio and lymphocytes at 24 hr after the indicated treatment. (F) C57BL/6 mice were given drinking water containing 8.3 µg/ml R848 (blue line, n = 10) or vehicle control (black line, n = 12) for 11 d. Blood counts are shown for total monocytes, Ly6C-high monocytes, Ly6C-low monocytes, monocyte subpopulation ratio and lymphocytes at the indicated time point. Data representative of at least two independent experiments (except A). One-way ANOVA with Bonferroni’s multiple-comparison test (A); two-way ANOVA with Tukey’s multiple comparison for analysis of time-course experiments (B, D–F). Data are the mean ± SEM; only significant p-values are indicated; **p<0.01; ***p<0.001; ****p<0.0001.
-
Figure 1—source data 1
FACS raw data.
- https://cdn.elifesciences.org/articles/85647/elife-85647-fig1-data1-v2.xlsx
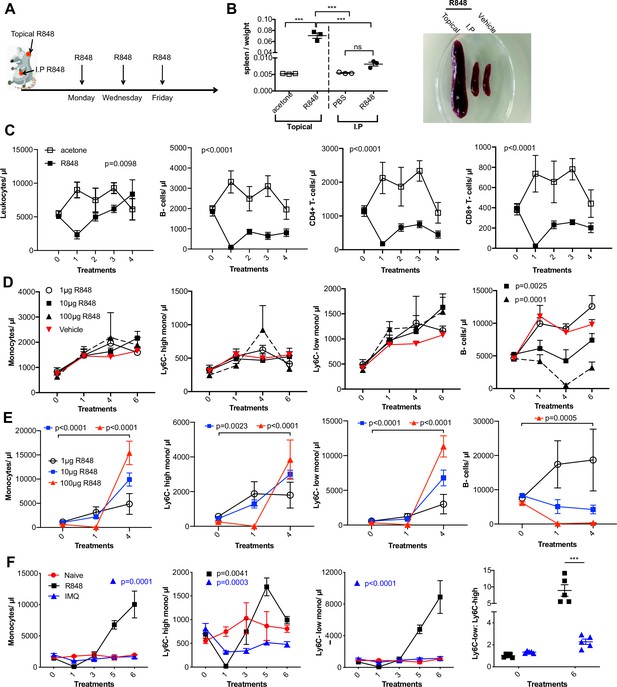
The effects of TLR7 stimulation depend on dose and route of administration.
(A) Graphical illustration of R848 treatment regimen. (B) BALB/c mice (n = 3 per group) received 100 μg of R848 topically or intraperitoneally (IP), 3× per week for 4 wk. Control mice given topical acetone or 200 μl of PBS IP. Splenomegaly quantified as spleen: body weight ratio, with representative example shown. (C) BALB/c mice (n = 3 per group) received four treatments with 100 μg topical R848 (black squares) or acetone (open squares). Blood counts are shown (left to right) for total leukocytes, B-cells, CD4+ T cells and CD8+ T cells 24 hr after each treatment. (D, E) Blood counts for total monocytes, Ly6C-high monocytes, Ly6C-low monocytes and B cells 24 hr after each treatment. (D) C57BL/6 mice (n = 4 per group) received 6× IP treatments with 1 μg (open circles), 10 μg (black squares), or 100 μg R848 (black triangles) or PBS vehicle (red triangles). Statistics versus vehicle group. (E) C57BL/6 mice (n = 4 per group) received 4× topical treatments with 1 μg (open circles), 10 μg (blue squares), or 100 μg R848 (red triangles). Statistics show pre-bleed vs. four treatments. (F) C57BL/6 mice were either naive (red circles, n = 3) or received six daily topical treatments with imiquimod (IMQ, blue triangles, n = 5) or R848 (black squares, n = 5). Blood counts 24 hr after the indicated treatments. Statistics versus naive group. Data representative of two experiments (except B and E). One-way ANOVA with Bonferroni’s multiple-comparison test for a single time point (B); two-way ANOVA with Tukey’s multiple-comparison test for analysis of time-course experiments (C–F). Data are the mean ± SEM; only significant p-values are indicated; ***p<0.001.
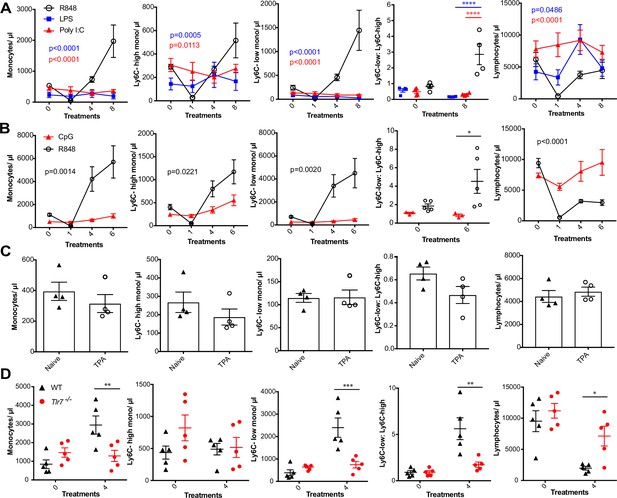
R848-induced monocytosis is specific to TLR7 activation.
Blood counts are shown for total monocytes, Ly6C-high monocytes, Ly6C-low monocytes, monocyte subpopulation ratio, and lymphocytes 24 hr after the indicated treatment. (A) BALB/c mice (n = 4 per group) received eight treatments with topical R848 (100 µg, black line), LPS (100 µg, blue line), or Poly l:C (100 µg, red line). (B) C57BL/6 mice received six treatments with topical R848 (100 µg, n = 5, black line) or CpG (100 µg, n = 3, red line). (C) BALB/c mice (n = 4 per group) received four topical treatments with 2.5 nmol TPA. (D) C57BL/6 mice (n = 5, black triangles) or Tlr7-/- mice (n = 5, red circles) received four treatments with topical R848. Data representative of two independent experiments (except A and C). Two-way ANOVA with Tukey’s multiple-comparison for time-course experiments (A, B); unpaired t-test (C, D). Data are the mean ± SEM; only significant p-values are indicated; *p<0.05; **p<0.01; ***p<0.001.
-
Figure 2—source data 1
FACS raw data.
- https://cdn.elifesciences.org/articles/85647/elife-85647-fig2-data1-v2.xlsx
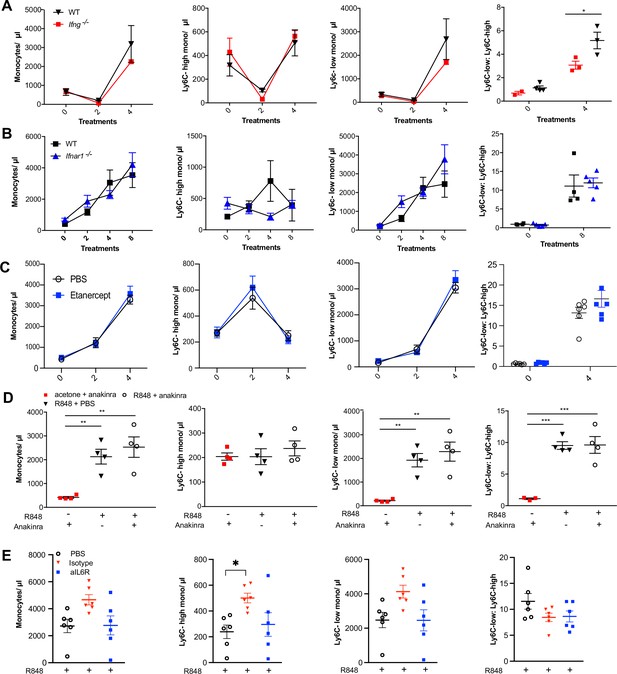
IFNs and cytokines are not involved in the R848-induced monocytosis.
(A) C57BL/6 (black triangles) or Ifng-/- mice (red squares) were either left untreated (n = 4 WT, n = 2 Ifng-/-) or given 4× topical treatments with R848 (n = 3 per group). Blood counts for total monocytes, Ly6C-high monocytes, Ly6C-low monocytes, and the monocyte subpopulation ratio at baseline and at 24 hr after the indicated treatment. Data representative of two independent experiments. (B) C57BL/6 (n = 4, black squares) or Ifnar1-/- (n = 5, blue triangles) mice received eight topical treatments with R848. Blood counts for total monocytes, Ly6C-high monocytes, Ly6C-low monocytes, and the monocyte subpopulation ratio at baseline and 24 hr after the indicated treatments. (C) BALB/c mice were treated daily intraperitoneally (IP) with etanercept (anti-TNFα) (n = 6, blue squares) or PBS (n = 6, open circles) and then received 4× topical R848 treatments. Blood counts for total monocytes, Ly6C-high monocytes, Ly6C-low monocytes, and the monocyte subpopulation ratio at baseline and at 24 hr after the indicated treatment. (D) BALB/c mice received the following combinations: topical R848 and anakinra daily IP (n = 4, open circles), topical R848 and daily PBS IP (n = 4, black triangles), and topical acetone and anakinra daily IP (n = 4, red squares). Blood counts for total monocytes, Ly6C-high monocytes, Ly6C-low monocytes, and the monocyte subpopulation ratio at baseline and at 24 hr after the fourth treatment of R848. (E) BALB/c mice were treated IP with PBS (n = 6, open circles) or anti-IL6R (n = 6, blue squares) or the isotype control (n = 6, red triangle) every 3 d and received 4× topical R848 applications. Blood counts for total monocytes, Ly6C-high monocytes, Ly6C-low monocytes, and the monocyte subpopulation ratio at baseline and at 24 hr after the fourth treatment with R848. Time-course experiments analysed using two-way ANOVA with Tukey’s multiple-comparison test to compare between time points (A–C); one-way ANOVA with Bonferroni’s multiple-comparison test for comparing >2 groups at a single time point calculated (D, E). Data are the mean ± SEM; only significant p-values are indicated; *p<0.05, **p<0.01, ***p<0.001.
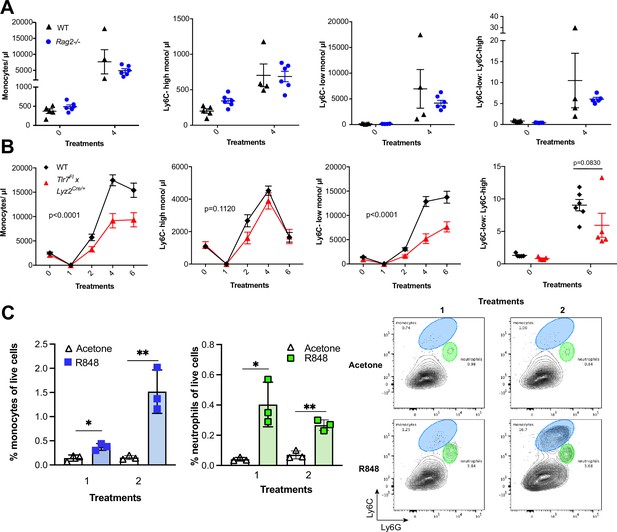
R848-induced monocytosis is driven by TLR7 activation of myeloid cells.
(A, B) Blood counts for total monocytes, Ly6C-high monocytes, Ly6C-low monocytes, monocyte subpopulation ratio, and lymphocytes at baseline and 24 hr after the indicated treatment. (A) C57BL/6 mice (n = 5, black triangles) and Rag2-/- mice (n = 6, blue circles) received four topical treatments with R848. (B) C57BL/6 mice (n = 6, black rhombi) and Tlr7fl × Lyz2Cre/+ mice (n = 5, red triangles) received six topical treatments with R848. (C) C57BL/6 mice (n = 3 per group) were treated once or twice with topical R848 or acetone. Treated ear skin was harvested at 24 hr post-treatment and anal- ysed by flow cytometry. Proportion of monocytes (CD11b+Ly6C+Ly6G-low) and neutrophils (CD11b+Ly6G-high- Ly6C-low) among total live cells (left panels). Representative flow cytometry plots gated on CD11b+ cells (right panels). Data represent a single experiment (A, C) or two experiments (B). Two-way ANOVA, with Tukey’s multiple-comparison for time-course experiments (A, B); unpaired t-test (C). Data are the mean ± SEM; only significant p-values are indicated; *p<0.05; **p<0.01.
-
Figure 3—source data 1
FACS raw data.
- https://cdn.elifesciences.org/articles/85647/elife-85647-fig3-data1-v2.xlsx
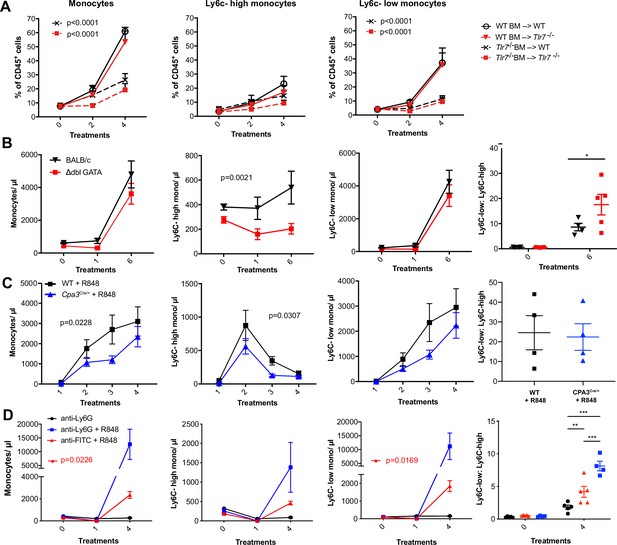
The role of myeloid cell populations in R848-driven monocytosis.
(A) Lethally irradiated WT or Tlr7-/- host mice were reconstituted with bone marrow (BM) from WT or Tlr7-/- donor mice as follows: WT BM into WT host (n = 5, open circles), WT BM into Tlr7-/- host (n = 5, red triangles), Tlr7-/- BM into WT host (n = 5, crosses), and Tlr7-/- BM into Tlr7-/- host (n = 5, red squares). Each group received 4× topical treatments with R848. Total blood monocytes, Ly6C-high monocytes, and Ly6C-low monocytes are shown as a proportion of CD45+ cells, at baseline and 24 hr after the indicated treatments. (B–D) Blood counts for total monocytes, Ly6C-high monocytes, Ly6C-low monocytes, and the monocyte subpopulation ratio at baseline and 24 hr after the indicated R848 treatments. (B) BALB/c (n = 4, black triangles) or ΔdblGATA (n = 6, red squares) mice received six topical treatments with R848. (C) BALB/c mice were either treated with topical R848 (n = 4, black squares) alongside a group of Cpa3-Cre-/+ mice (n = 4, blue triangles). (D) BALB/c mice were treated with combinations of topical R848 and intraperitoneally (IP) anti-Ly6G or anti-FITC isotype control, with the antibody injected 4 hr prior to the first R848 treatment. Treatments were in the following groups: isotype control and R848 (n = 5, red triangles), anti-Ly6G and R848 (n = 4, blue squares), and anti-Ly6G alone (n = 5, black circles). Statistics compare anti-Ly6G+R848 to anti-FITC+R848. Time-course experiments analysed using two-way ANOVA with Tukey’s multiple-comparisons test to compare between groups at a given time point (A–D); one-way ANOVA with Bonferroni’s multiple-comparison test for comparing >2 groups at a single time point; unpaired t-test for comparing two groups at a single time point. Data are the mean ± SEM; only significant p-values are indicated; * p<0.05, **p<0.01, ***p<0.001.
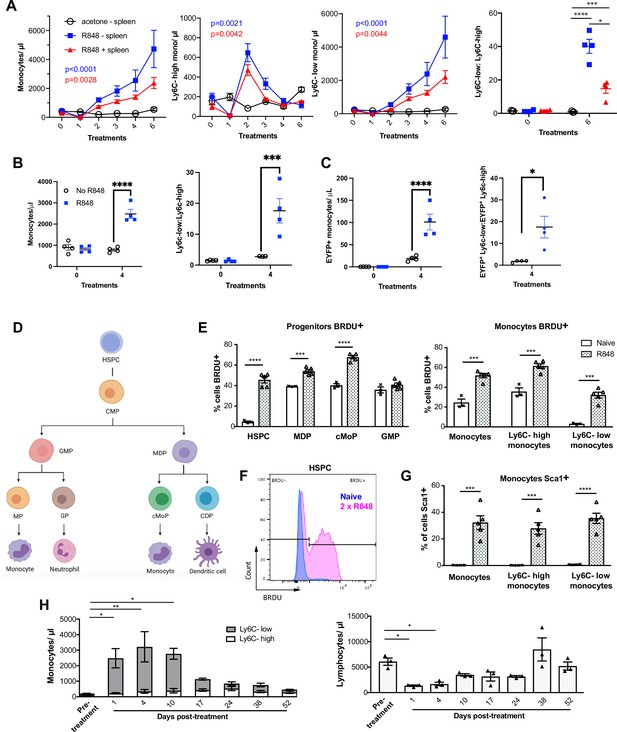
R848-induced monocytes are derived from the bone marrow (BM) and have features of emergency myelopoiesis.
(A) BALB/c mice underwent splenectomy or sham surgery and were left to recover for 7 wk. Among the splenectomised mice, a group was treated 6× with topical R848 (n = 4, blue line) and the remaining mice with acetone (n = 3, solid black line). The sham surgery group was treated with R848 (n = 4, red line). Blood counts are shown for total monocytes, Ly6C-high monocytes, Ly6C-low monocytes, and the monocyte subpopulation ratio at baseline and 24 hr after the last treatments. (B, C) HSC-SCL-Cre-ERT;R26R-EYFP mice (n = 4 per group) received tamoxifen (4 mg/100 µl) by oral gavage for five consecutive days. Three days later, the left ears were treated topically 4× with R848 (blue squares) or left untreated (open circles). Shown are the total blood monocyte counts and the monocyte subpopulation ratio (B); the total blood EYFP+ monocyte counts and the subpopulation ratio among the EYFP-expressing monocytes at baseline and 24 hr after the last treatment (C). (D) Diagram illustrating BM myeloid progenitor differentiation: haematopoietic stem and progenitor cells (HSPC); common myeloid progenitor (CMP); monocyte-dendritic precursor (MDP); granulocyte-monocyte progenitor (GMP); common monocyte progenitor (cMoP); common dendritic progenitor (CDP); monocyte-committed progenitor (MP); granulocyte-committed progenitor (GP). (E–G) C57BL/6 mice were injected with 2 mg BRDU intraperitoneally (IP), either naïve (n = 3, white bars) or after two topical R848 treatments (n = 5, grey bars). Mice culled at 16 hr after the BRDU injection and BM harvested. (E) Percentage of BRDU positivity in HSPC, MDP, cMoP, GMP, total monocytes, Ly6C-high monocytes, and Ly6C-low monocytes. (F) Representative histogram of BRDU expression in HSPC at baseline (blue) or after 2× topical R848 (magenta). (G) Percentage of Sca1 positivity in total monocytes, Ly6C-high monocytes and Ly6C-low monocytes in the BM. (H) BALB/c mice (n = 3) received four treatments with topical R848. Blood counts for Ly6C-high monocytes (white bars), Ly6C-low monocytes (grey bars), and lymphocytes were monitored at the indicated time points after the cessation of treatment. Data representative of two independent experiments (except A and H). Two-way ANOVA with Tukey’s multiple comparison for time-course experiments (A–C, H); unpaired t-test (E, G). Data are the mean ± SEM; only significant p-values are indicated; *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
-
Figure 4—source data 1
FACS raw data.
- https://cdn.elifesciences.org/articles/85647/elife-85647-fig4-data1-v2.xlsx
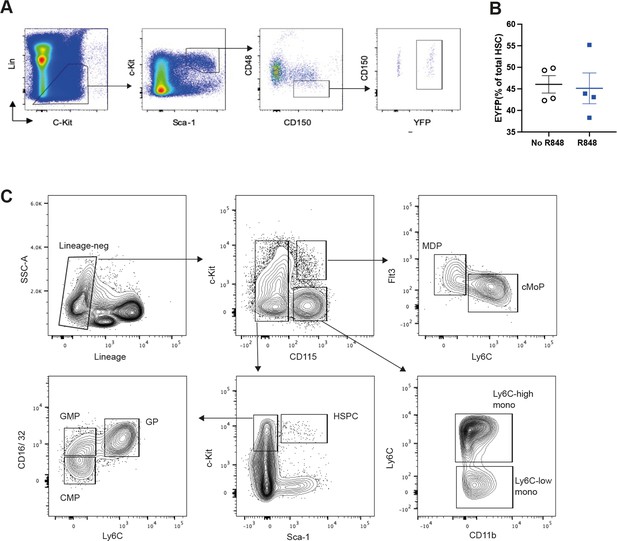
Bone marrow (BM) gating strategy.
(A, B) HSC-SCL-Cre-ERT;R26R-EYFP mice (n = 4 per group) were treated for five consecutive days with tamoxifen (4 mg/100 μl) by oral gavage. Three days after the tamoxifen administration, the left ears were treated topically 4× with R848 or left untreated. (A) Gating strategy for YFP+ HSCs in BM. HSCs are excluded for doublets using side scatter area and side scatter height prior and gated lineage negative (B220, CD5, CD4, CD8, TER119, and GR-1). HSCs were then defined as CD117+Sca-1+CD150+CD48-. EYFP was then gated to determine recombination efficiency. (B) Percentage of EYFP+ HSCs within the HSCs in the BM after tamoxifen and 4xR848 treatment (R848 group, blue squares) or tamoxifen only (no R848 group, open circles). Representative of two independent experiments. (C) Gating strategy for BM cells. All populations are gated lineage-negative (CD3, B220, Ly6G, CD49b). HSPC: CD115-neg, c-kit+, Sca-1-neg; MDP: CD115+, c-Kit+, Ly6C-low; cMoP: CD115+, c-Kit+, Ly6C-high, Flt3-neg; GMP: CD115-neg, cKit+, Sca-1-neg, CD16/32+ , Ly6C-low; CMP: CD115-neg, cKit+, Sca-1-neg, CD16/32-neg, Ly6C-low; GP: CD115-neg, cKit+, Sca-1-neg, CD16/32+ , Ly6C-high; Ly6C-high monocytes: CD115+, c-Kit-neg, Ly6C-high; Ly6C-low monocytes: CD115+, c-Kit-neg, Ly6C-low. HSPC: haematopoietic stem and progenitor cells; CMP: common myeloid progenitor; MDP: monocyte-dendritic precursor; GMP: granulocyte-monocyte progenitor; cMoP: common monocyte progenitor; CDP: common dendritic progenitor; MP: monocyte-committed progenitor; GP: granulocyte-committed progenitor.
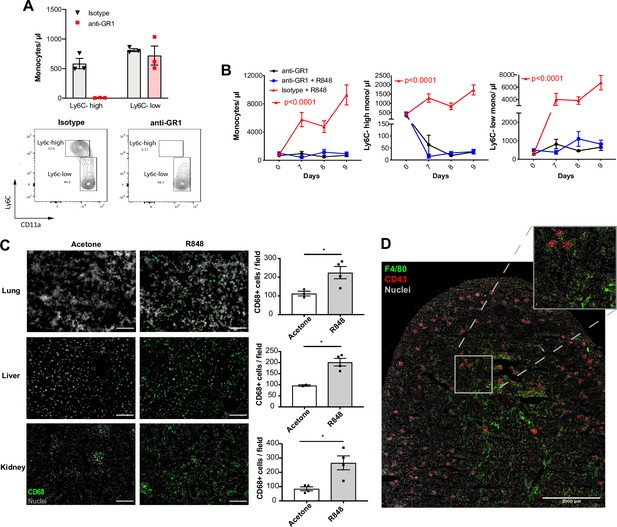
Topical R848 drives Ly6C-high monocyte differentiation to intravascular and tissue macrophages.
(A) C57BL/6 mice were injected intraperitoneally (IP) with either anti-GR1 antibody (n = 3, red squares) or matched isotype control (n = 3, black triangles). Blood Ly6C-high and Ly6C-low monocyte counts (upper panels) and representative flow cytometry plots (bottom panels) are shown at 24 hr post-injection. (B) C57BL/6 mice were treated with combinations of topical R848 and IP anti-GR1 antibody or matched isotype control, with the antibody injected daily for three consecutive days starting 1 hr prior to the third R848 treatment (day 6). The groups were isotype control and R848 (n = 4, red triangle), anti-GR1 and R848 (n = 4, blue squares), and anti-GR1 alone (n = 4, black circles). Blood counts for total monocytes, Ly6C-high monocytes, and Ly6C-low monocytes at baseline and on the indicated days are shown. Statistics compare anti-GR1+R848 to isotype +R848. (C) C57BL/6 mice received four treatments with topical R848 (n = 4 per group, grey bars) or acetone (n = 4 per group, white bars). Immunofluorescent staining for CD68 (green) and nuclei (grey) was performed on tissue sections from lung, liver, and kidneys. Staining was quantified as mean CD68+ cells per field. (D) C57BL/6 mice (n = 4) received six treatments with topical R848. Kidney sections were stained for CD43 (red), F4/80 (green), and nuclei (grey). A representative tile scan is shown. Data representative of two independent experiments (except D). Time-course experiments analysed with two-way ANOVA with Tukey’s multiple-comparison used to compare between groups at a given time point (B); comparison of two groups at a single time point calculated using unpaired t-test (C). Data are the mean ± SEM; only significant p-values are indicated; *p<0.05.
-
Figure 5—source data 1
FACS raw data.
- https://cdn.elifesciences.org/articles/85647/elife-85647-fig5-data1-v2.xlsx
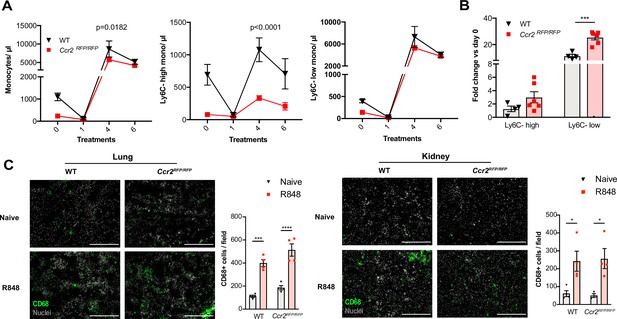
Immature monocytes egress from the bone marrow (BM) independently of CCR2.
(A, B) C57BL/6 mice (n = 4, black triangles) or Ccr2RFP/RFP mice (n = 6, red squares) were treated topically six times with R848. (A) Blood counts for total monocytes, Ly6C-high and Ly6C-low monocytes at 24 hr after each treatment are shown. (B) Fold change of monocyte subpopulations versus baseline after 6× R848 treatments. (C) C57BL/6 mice or Ccr2RFP/RFP mice were either naive (WT n = 4, Ccr2RFP/RFP n = 4, grey bars) or received six R848 treatments (WT n = 4, Ccr2RFP/RFP n = 4, red bars). Immunofluorescent staining for CD68 (green) and nuclei (grey) was performed on tissue sections from lung and kidneys. Staining was quantified as mean CD68+ cells per field. Data representative of two (C) or four (A, B) independent experiments. Time-course experiments analysed using two-way ANOVA with Tukey’s multiple-comparison test to compare between time points (A); for a single time point one-way ANOVA with Bonferroni’s multiple-comparison test for >2 groups (B, C). Data are the mean ± SEM; only significant p-values are indicated; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
-
Figure 6—source data 1
FACS raw data.
- https://cdn.elifesciences.org/articles/85647/elife-85647-fig6-data1-v2.xlsx
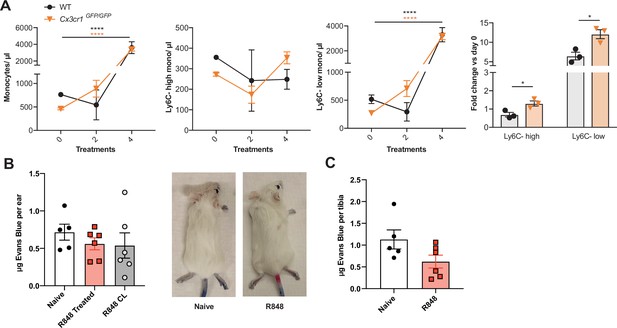
R848-induced myeloid response is independent from CX3CR1 and vascular permeability.
(A) C57BL/6 mice (n = 3, black lines) or Cx3cr1GFP/GFP (n = 3, orange lines) were treated topically four times with R848. Blood counts for total monocytes, Ly6C-high monocytes, Ly6C-low monocytes, and the fold change of monocyte subpopulations after four treatments versus baseline. (B, C) BALB/c mice were naive (n = 5) or treated topically four times with R848 (n = 6). 1 mg Evans Blue was injected intravenously (IV) at 24 hr after the final R848 treatment. (B) Quantity of Evans Blue per ear is shown for naive mice (white bars), the R848-treated ear (red bars), and the contralateral ear (grey bars), along with representative photographs. (C) Evans Blue is quantified as µg per tibia in naïve and R848-treated mice. Data representative of two independent experiments. Time-course experiments analysed using two-way ANOVA with Tukey’s multiple-comparison test to compare between time points (A); fold change data analysed using unpaired t-test (A); two groups at a single time point compared using unpaired t-test (C); one-way ANOVA with Bonferroni’s multiple-comparison test for >2 groups at a single time point (B). Data are the mean ± SEM; only significant p-values are indicated; *p<0.05, ****p<0.0001.
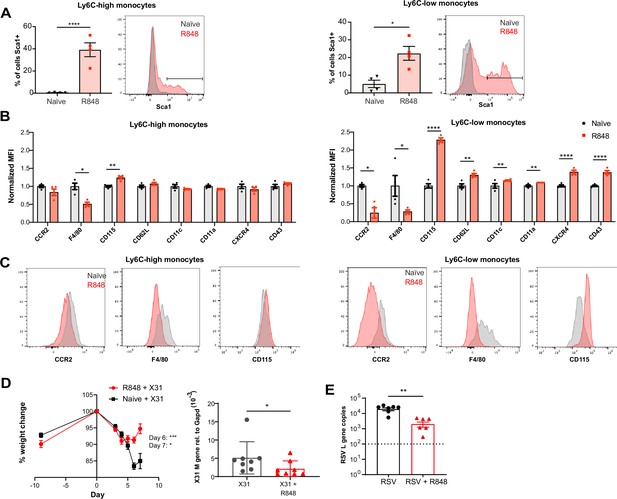
R848-induced emergency monocytes have antiviral effects in the lung.
(A–C) C57BL/6 mice were either naive (n = 4, black circles) or treated four times with topical R848 (n = 4, red squares). (A) Percentage of cells positive for Sca-1 and representative histograms in blood Ly6C-high and Ly6C-low monocytes from naive mice (grey) or R848-treated mice (red). (B) Blood Ly6C-high and Ly6C-low monocytes were gated by flow cytometry and surface expression of the indicated proteins was quantified and expressed as MFI, normalised to the mean of the naive group. (C) Representative histograms of CCR2, F4/80, and CD115 staining in blood Ly6C-high and Ly6C-low monocytes. (D) Percentage of original weight (left panel) and lung viral load (right panel) in C57BL/6 mice (n = 8/group) pretreated five times with or without topical R848 infected intranasally with the influenza virus strains X31. Lung viral load at day 7 post inoculation was measured by quantification of matrix X31 gene copies in whole lung tissues. (E) Lung viral load after respiratory syncytial virus (RSV) infection in C57BL/6 mice (n = 6–7/group) pretreated or not with topical R848 (five times) was determined by quantification of viral L gene copies in lung tissues at day 4 post inoculation. Data representative of two independent experiments. Statistical analysis using unpaired t-test (A, B; D right panel, E); two-way ANOVA with Bonferroni’s post hoc test (D, left panel) Data are the mean ± SEM; only significant p-values are indicated; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
-
Figure 7—source data 1
FACS raw data.
- https://cdn.elifesciences.org/articles/85647/elife-85647-fig7-data1-v2.xlsx
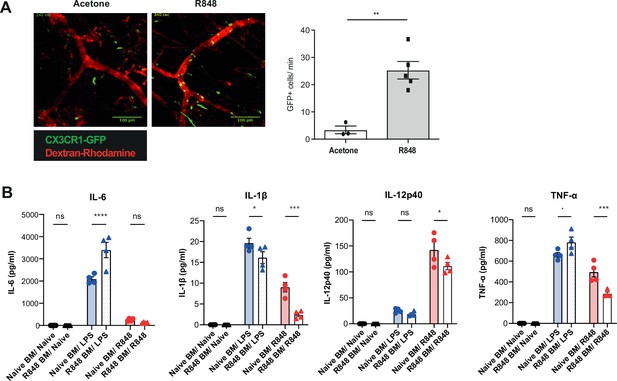
R848-induced monocytes are activated and functionally impaired.
(A) Cx3cr1GFP/+ mice were treated topically four times with R848 (n = 5, grey bars) or acetone (n = 3 white bars) on the right ear. 24 hr after the last treatment, mice were anaesthetised and the contralateral ear was visualised using intravital microscopy. Representative images are shown of the contralateral ears (red = dextran, green = CX3CR1-GFP). Non-classical monocytes are quantified as intravascular GFP+ cells per minute (left panel). (B) C57BL/6 mice were treated topically four times with 100 μg of R848. Bone marrow (BM)-sorted monocytes (four biological replicates per group) were plated in vitro at a concentration of 1 × 106 per ml and restimulated overnight with 100 μg/ml of R848 or 1 μg/ml of LPS. Unstimulated monocytes were used naïve controls. Cytokines in the supernatant were quantified using a Legendplex Mix and expressed as pg/ml. Data representative of two independent experiments. Statistical analysis using unpaired t-test to compare two groups (A) and two-way ANOVA with Bonferroni’s multiple-comparison test for >2 groups (B). Data are the mean ± SEM; only significant p-values are indicated; * p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
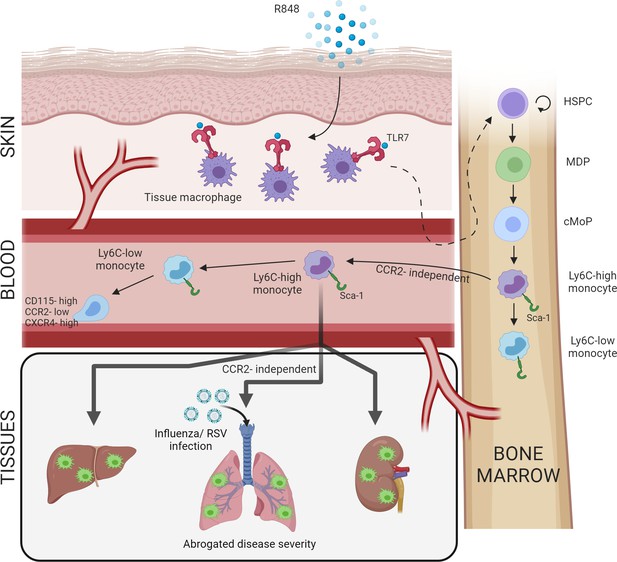
Repetitive application of a TLR7 agonist (R848) to the skin activates tissue macrophages which signal to the bone marrow to drive the expansion of haematopoietic stem and progenitor cells (HSPCs) and their differentiation into monocytes.
These Ly6C-high monocytes have an immature phenotype and egress from the bone marrow independently of CCR2. Subsequently, Ly6C-high monocytes differentiate into Ly6C-low blood monocytes and into tissue macrophages in multiple organs such as liver, lung, and kidneys. When challenged with a secondary viral stimulus such as influenza or respiratory syncytial virus (RSV) infection, the emergency myelopoiesis is associated with reduced disease severity.
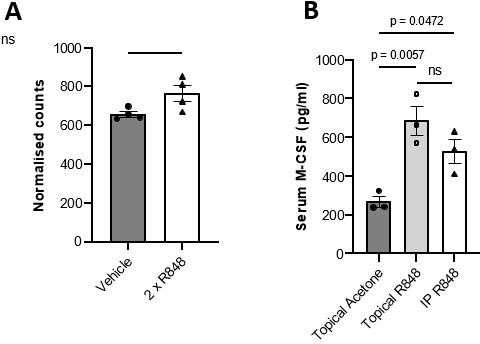
(A) BALB/c mice (n=4 per group) received 2x topical ear treatments with 100ug R848, while vehicle mice received 2x treatments with acetone. Ear skin was harvested at 24 hours after the final treatment for transcriptomic analysis. Csf1 transcript counts shown are normalised to a panel of reference genes. Data represent a single experiment. (B) BALB/c mice (n=3 per group) received 100μg of R848 topically or I.P, 3x per week for 4 weeks. Control mice were given topical acetone. Serum CSF-1 levels were quantified by ELISA. Panel (A) was analysed by Mann-Whitney test; panel (B) by oneway ANOVA with Bonferroni’s multiple comparison test versus vehicle group. Data are the mean ± SEM; ns = non significant; p values are indicated.
Videos
Cx3cr1GFP/+ mice were treated topically on the right ear for four times with R848 (panel on the right) or acetone (panel on the left).
24 hr after the last treatment, the mice were anaesthetised and the contralateral ear was visualised using intravital microscopy (red = dextran, green = CX3CR1-GFP).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | Rat anti-mouse Ly6G FITC (1A8) | BioLegend | Cat# 127606 | Flow (1:100) |
| Antibody | Rat anti-mouse MHC-II FITC (M5/114.15.2) | BioLegend | Cat# 107606 | Flow (1:400) |
| Antibody | Rat anti-mouse Ly6C PerCP-Cy5.5 (HK1.4) | BioLegend | Cat# 128012 | Flow (1:200) |
| Antibody | Rat anti-mouse Ly6C Brilliant Violet 605 (HK1.4) | BioLegend | Cat# 128036 | Flow (1:100) |
| Antibody | Rat anti-mouse CD11b PE-Cy7 (M1/70) | BioLegend | Cat# 101216 | Flow (1:400) |
| Antibody | Rat anti-mouse CD11b APC (M1/70) | BioLegend | Cat# 101212 | Flow (1:400) |
| Antibody | Rat anti-mouse CD115 PE (AFS98) | BioLegend | Cat# 135506 | Flow (1:100) |
| Antibody | Rat anti-mouse CD115 APC (AFS98) | BioLegend | Cat# 135510 | Flow (1:100) |
| Antibody | Rat anti-mouse B220 Brilliant Violet 605 (RA3-6B2) | BioLegend | Cat# 103244 | Flow (1:400) |
| Antibody | Rat anti-mouse B220 FITC (RA3-6B2) | BD Biosciences | Cat# 553087 | Flow (1:400) |
| Antibody | Hamster anti-mouse CD3e Brilliant Violet 711 (145-2C11) | BioLegend | Cat# 100349 | Flow (1:100) |
| Antibody | Hamster anti-mouse CD3e FITC (145-2C11) | BD Biosciences | Cat# 553062 | Flow (1:100) |
| Antibody | Rat anti-mouse CD11a APC (M17/4) | BioLegend | Cat# 101120 | Flow (1:100) |
| Antibody | Rat anti-mouse CD117 PE-Cy7 (2B8) | BioLegend | Cat# 105814 | Flow (1:100) |
| Antibody | Rat anti-mouse CD11b PerCP-Cy5.5 (M1/70) | BioLegend | Cat# 101228 | Flow (1:400) |
| Antibody | Rat anti-mouse CD135 Brilliant Violet 421 (A2F10.1) | BioLegend | Cat# 135315 | Flow (1:100) |
| Antibody | Rat anti-mouse Sca1 Brilliant Violet 711 (D7) | BioLegend | Cat# 108131 | Flow (1:100) |
| Antibody | Rat anti-mouse CD16/CD32 APC-Cy7 (93) | BioLegend | Cat# 101328 | Flow (1:100) |
| Antibody | Rat anti-mouse CD135 APC (A2F10.1) | BioLegend | Cat# 135310 | Flow (1:100) |
| Antibody | Rat anti-mouse CD49b FITC (DX5) | BD Biosciences | Cat# 553857 | Flow (1:100) |
| Antibody | Hamster anti-mouse CD11c APC (HL3) | BD Biosciences | Cat# 550261 | Flow (1:100) |
| Antibody | Anti-mouse CD45 APC-eFluor 780 (30-F11) | eBioscience | Cat# 47-0451-82 | Flow (1:100) |
| Antibody | Rat anti-mouse Ly6G Brilliant Violet 421 (1A8) | BioLegend | Cat# 127628 | Flow (1:100) |
| Antibody | Rat anti-mouse F4/80 Brilliant Violet 785 (BM8) | BioLegend | Cat# 123141 | Flow (1:400) |
| Antibody | Rat anti-mouse F4/80 Alexa Fluor 488 (BM8) | BioLegend | Cat# 123120 | IF (1:200) |
| Antibody | Rat anti-mouse MHC-II PerCP eFluor710 (M5/114.15.2) | eBioscience | Cat# 46-5321-82 | Flow (1:400) |
| Antibody | Rat anti-mouse Sca1 PE-Dazzle 594 (D7) | BioLegend | Cat# 108138 | Flow (1:100) |
| Antibody | Rat anti-mouse CD43 Alexa Fluor 700 (S11) | BioLegend | Cat# 143214 | Flow (1:400) |
| Antibody | Rat anti-mouse CD43 | BioLegend | Cat# 143202 | IF (1:50) |
| Antibody | Goat anti-Art IgG (H+L) Cross-Adsorbed secondary antibody, Alexa-Fluor 555 | Thermo Fisher | Cat# A-21434 | IF (1:400) |
| Antibody | Rat anti-mouse CXCR4 Alexa Fluor 647 (L276F12) | BioLegend | Cat# 146504 | Flow (1:100) |
| Antibody | Rat anti-mouse CCR2 Brilliant Violet 510 (SA203G11) | BioLegend | Cat# 150617 | Flow (1:100) |
| Antibody | Hamster anti-mouse CD11c PE-Cy7 (HL3) | BD Biosciences | Cat# 561022 | Flow (1:100) |
| Antibody | Rat anti-mouse Ly6C eFluor450 (HK1.4) | eBioscience | Cat# 48-5932-82 | Flow (1:100) |
| Antibody | Rat anti-mouse CD68 Alexa Fluor 488 (FA-11) | BioLegend | Cat# 137012 | IF (1:100) |
| Antibody | Rat anti-mouse Ly6G eFluor450 (1A8) | eBioscience | Cat# 48-9668-82 | Flow (1:100) |
| Antibody | Rat anti-mouse B220 PE-Cy5 (RA3-6B2) | BioLegend | Cat# 103210 | Flow (1:400) |
| Antibody | Rat anti-mouse CD4 PE-Cy5 (RM4-5) | BioLegend | Cat# 100513 | Flow (1:100) |
| Antibody | Rat anti-mouse CD5 PE-Cy5 (53-7.3) | BioLegend | Cat# 100604 | Flow (1:100) |
| Antibody | Rat anti-mouse CD8 PE-Cy5 (53-6.7) | BioLegend | Cat# 100710 | Flow (1:100) |
| Antibody | Rat anti-mouse TER119 PE-Cy5 (TER119) | BioLegend | Cat# 116210 | Flow (1:100) |
| Antibody | Rat anti-mouse GR1 PE-Cy5 (RB6-8C5) | BioLegend | Cat# 108410 | Flow (1:100) |
| Antibody | Rat anti-mouse CD150 PE-Cy7 (TC15-12F12.2) | BioLegend | Cat# 115914 | Flow (1:100) |
| Antibody | Rat anti-mouse CD117 APC eFluro780 (2B8) | eBioscience | Cat# 47-1171-82 | Flow (1:100) |
| Antibody | Rat anti-mouse Sca1 BV785 (D7) | BioLegend | Cat# 108139 | Flow (1:100) |
| Antibody | Hamster anti-mouse CD48 APC (HM48-1) | BioLegend | Cat# 103412 | Flow (1:100) |
| Antibody | Anti-mouse Ly6G (1A8, mouse chimeric) | Absolute Antibody | Cat# Ab00295-2.3 | Flow (1:100) |
| Antibody | Anti-mouse GR1 (RB6-8C5, mouse chimeric) | Absolute Antibody | Cat# Ab01030-2.0 | Flow (1:100) |
| Antibody | Rat anti-mouse IL-6R (15A7) | BioXcell | Cat# BE0047 | |
| Antibody | Rat IgG2b isotype control, anti-keyhole limpet hemocyanin (LTF-2) | BioXcell | Cat# BE0090 | |
| Peptide, , recombinant protein | R848 (water soluble) | Invivogen | tlrl-r848 | |
| Peptide, , recombinant protein | R848 | Enzo | ALX-420-038M025 | |
| Peptide, , recombinant protein | Poly(I:C) (LMW) | Invivogen | tlrl-picw | |
| Peptide, , recombinant protein | CpG (ODN 2395) | Invivogen | tlrl-2395 | |
| Peptide, , recombinant protein | LPS (E. coli 055:B5) | Invivogen | tlrl-b5lps | |
| Chemical compound, drug | Aldara (Imiquimod) | Meda Pharmaceuticals | 5% cream | |
| Chemical compound, drug | Anakinra | Swedish Orphan Biovitrum | 150 mg/ml | |
| Chemical compound, drug | Etanercept (Enbrel) | Pfizer Europe | 25 mg | |
| Chemical compound, drug | Baytril | Bayer Corporation | ||
| Chemical compound, drug | Tamoxifen | Sigma | Cat# T5648-1G | |
| Chemical compound, drug | Phorbol 12-myristate 13-acetate (TPA) | Sigma-Aldrich | Cat# P8139 | |
| Chemical compound, drug | Tetramethylrhodamine isothiocyanate–Dextran, 70 kDa | Sigma-Aldrich | Cat# T11-62 | |
| Chemical compound, drug | Bromodeoxyuridine (BrDU) | BioLegend | Cat# 423401 | |
| Chemical compound, drug | Liberase TM Research grade | Roche | Cat# 5401119001 | |
| Chemical compound, drug | DNase I (grade II) from bovine pancreas | Sigma-Aldrich | Cat# 10104159001 | |
| Commercial assay or kit | RNA-Later | Thermo Fisher | Cat# AM7020 | |
| Commercial assay or kit | LIVE/DEAD Fixable Aqua Dead Cell Stain Kit | Life Technologies | Cat# L34597 | |
| Commercial assay or kit | BD FACS Lysis Solution 10X Concentrate | BD Biosciences | Cat# 349202 | |
| Commercial assay or kit | RNEasy Mini Kit | QIAGEN | Cat# 74104 | |
| Commercial assay or kit | RNEasy Micro Plus Kit | QIAGEN | Cat# 74034 | |
| Commercial assay or kit | iScript cDNA Synthesis Kit | Bio-Rad | Cat# 1708891 | |
| Commercial assay or kit | QuantiTect Probe PCR Kit | QIAGEN | Cat# 204343 | |
| Commercial assay or kit | High-Capacity RNA-to-cDNA kit | Applied Biosystems | Cat# 4387406 | |
| Commercial assay or kit | Legendplex Mix and Match Kit | BioLegend | ||
| Commercial assay or kit | BrdU Staining Kit | eBioscience | Cat# 8817-6600 | |
| Strain, strain background (Mus musculus, C57BL/6) | B6.129P-Cx3cr1tm1Litt/J | Jackson Laboratory | JAX stock #005582 | Jung et al., 2000 |
| Strain, strain background (M. musculus, C57BL/6) | B6(Cg)-Ifnar1tm1.2Ees/J | Jackson Laboratory | JAX stock #028288 | Hwang et al., 1995 |
| Strain, strain background (M. musculus, C57BL/6) | B6(Cg)-Rag2tm1.1Cgn/J | Jackson Laboratory | JAX stock #08449 | Hao and Rajewsky, 2001 |
| Strain, strain background (M. musculus, C57BL/6) | B6.129P2-Lyz2tm1(cre)Ifo/J | Jackson Laboratory | JAX stock #004781 | Clausen et al., 1999 |
| Strain, strain background (M. musculus, C57BL/6) | B6.129(Cg)-Ccr2tm2.1Ifc/J | Jackson Laboratory | JAX stock #017586 | Saederup et al., 2010 |
| Strain, strain background (M. musculus, C57BL/6) | B6.129P2-Tlr7tm1Aki | Hemmi et al., 2002 | ||
| Strain, strain background (M. musculus, C57BL/6) | B6.129S7-Ifngtm1Ts/J | Jackson Laboratory | JAX stock #002287 | Dalton et al., 1993 |
| Strain, strain background (M. musculus; C57BL/6) | Tlr7flox/flox | Solmaz et al., 2019 | ||
| Strain, strain background (M. musculus; BALB/c) | Cpa3- cre4Glli | Jackson Laboratory | JAX stock #026828 | Feyerabend et al., 2011 |
| Strain, strain background (M. musculus; BALB/c) | Gata1tm6Sho/J | Jackson Laboratory | JAX stock #05653 | Yu et al., 2002 |
| Strain, strain background (M. musculus; C57BL/6) | HSC-SCL-Cre-ERT;R26R-EYFP | Göthert et al., 2005 | ||
| Strain, strain background (influenza A virus) | strain X31 | John McCauley | Davidson, S., 2014 | |
| Strain, strain background (RSV) | Strain A2 | ATCC | ATCC VR-1540 | |
| Sequence-based reagent | RSV L gene forward | Invitrogen | GAACTCAGT GTA GGT AGAATGTTTGCA | |
| Sequence-based reagent | RSV L gene reverse | Invitrogen | TTCAGCTATCATTTTCTCTGCCAAT | |
| Sequence-based reagent | RSV L FAM-TAMRA probe | Eurofins MWG Operon | TTTGAACCTGTCTGAACATTCCCGGTT | |
| Sequence-based reagent | Flu M1 gene forward | Invitrogen | AAGACCAATCCTGTCACCTCTGA | |
| Sequence-based reagent | Flu M1 gene reverse | Invitrogen | CAAAGCGTCTACGCTGCA | |
| Sequence-based reagent | Flu M1 FAM-TAMRA probe | Eurofins MWG Operon | TTTGTGTTCACGCTCACCGT | |
| Software, algorithm | GraphPad Software (Prism) | GraphPad Software, Inc, La Jolla, California, USA | Version 9 | |
| Software, algorithm | FlowJo | Tree Star Inc Ashland, OR, USA | Version 10.7.1 | |
| Software, algorithm | Imaris 8.0.1 | Bitplane AG | ||
| Software, algorithm | FIJI | ImageJ2 (open source) | ||
| Software, algorithm | 7500 Fast System SDS v1.4 21 CFR Part 11 Module | Applied Biosystems | ||
| Software, algorithm | QuantStudio Software V1.2.4 | Applied Biosystems |





