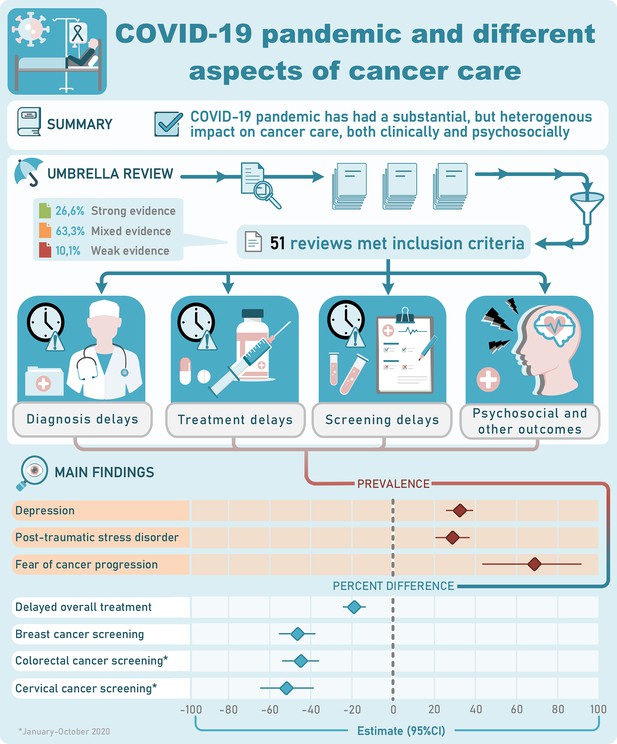
An umbrella review of systematic reviews on the impact of the COVID-19 pandemic on cancer prevention and management, and patient needs
Figures
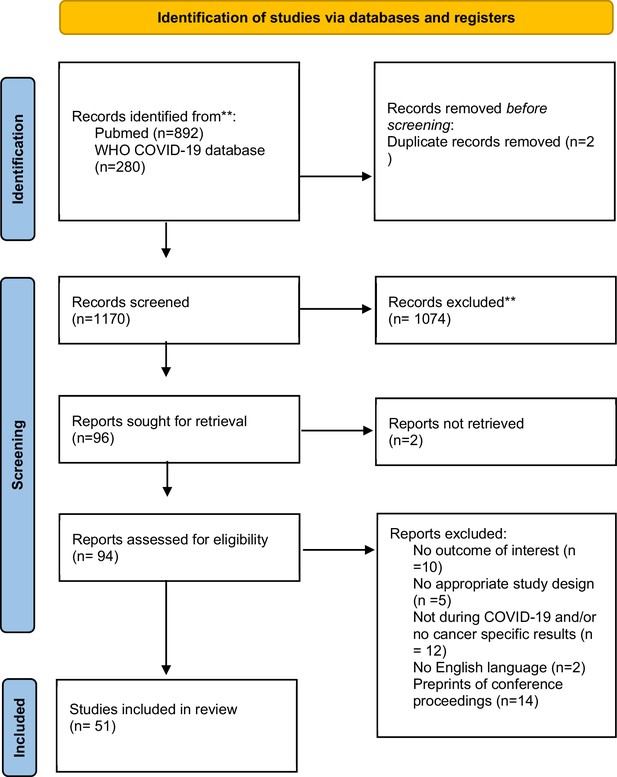
Flowchart of identification, screening, eligibility, inclusion, and exclusion of retrieved studies*.
*In the search, we did not include any language restriction filter. However, during full-text screening we included only studies that were in English. **WHO COVID-19 database does not allow to specify the search by both date and month, and the search for this specific database is up to end-December 2022. Any full text (n = 0) that was eligible and published after November 29, 2022, was excluded.
Tables
Characteristics of included systematic reviews.
| Author, year of publication | Meta-analysis | Number of included studies | Countries* | Pre-pandemic controls | Cancer types | Aspects assessed | Last search |
|---|---|---|---|---|---|---|---|
| Adham et al., 2022 | No | 5 | Globally | No | H&N | MT, O | 15-Jul-20 |
| Alkatout et al., 2021 | No | 16 | Multiple countries, including US, TW, BE, NL, JP, IT, UK, AS, CA | Yes | ALL | DCS, RD | 28-Dec-20 |
| Alom et al., 2021 | No | 72 | Multiple countries | No | All | MT, TL, O | 1-Sep-20 |
| Ayubi et al., 2021 | Yes | 34 | Multiple countries | No | All | PSND, O | 3-Jan-21 |
| Azab and Azzam, 2021 | No | 51 | Multiple countries | No | Glioma | MT | End of 2020 |
| Bezerra et al., 2022 | No | 8 | NP | No | ALL | TL | 01-Apr-2021 |
| Crosby and Sharma, 2020 | No | 45 | NP | No/NS | H&N | MT | 08-Apr-2020 |
| de Bock et al., 2022 | Yes | 24 | Multiple countries | Yes | ALL, BC | Delayed and/or canceled treatment Other aspects | 21-Mar-2021 |
| Dhada et al., 2021 | No | 19 | Multiple countries, including IT, US, UK, NL | No | ALL | DCT, DCS, PSND, TL, FBD, SIA | 1-Dec-20 |
| Di Cosimo et al., 2022 | Yes | 56 | Multiple countries | Yes | ALL | MT, DCT, TL, O | 11-Dec-20 |
| Donkor et al., 2021 | No | 11 | Multiple countries, including CN, IR, BR, ZA | No | ALL | O | 3-Aug-20 |
| Fancellu et al., 2022 | No | 7 | IT | Yes | CRC | DCS, RD | 31-Jan-22 |
| Ferrara et al., 2022 | No | 33 | Multiple countries | Yes | CV | DCT, DCS, RD, RHPV | 8-Feb-22 |
| Gadsden et al., 2022 | No | 17 | Multiple countries, including IN, SL, BA | Yes | ALL | DCT, O | 15-Dec-21 |
| Garg et al., 2020 | No | 212 | Multiple countries | No | ALL | MT | 2-May-20 |
| Gascon et al., 2022 | No | 23 | Multiple countries | No | H&N | MT, O | 1-May-20 |
| Hesary and Salehiniya, 2022 | No | 22 | Multiple countries, including IT, UK, PG, NL, CN, IN, JP, TU, IR, SN | Yes | GA | MT, DCS, RD, PSND | 31-Dec-21 |
| Hojaij et al., 2020 | No | 35 | Multiple countries | No | H&N, OTO | MT, TL, O | 31-Dec-20 |
| Jammu et al., 2021 | No | 19 | Multiple countries | No | ALL | DCT, PSND, FBD | 27-Aug-20 |
| Kirby et al., 2022 | No | 56 | Multiple countries | No | ALL | PSND, FBD, SIA | 31-Mar-21 |
| Legge et al., 2022 | No | 18 | Multiple countries | No | ALL | PSND, FBD, SIA | 25-May-22 |
| Lignou et al., 2022 | No | 32 | Multiple countries | Yes | PC | DCT, RD, TL | 1-Aug-21 |
| Lu et al., 2021 | No | 41† | NP | No | ALL | TL | 1-May-20 |
| Majeed et al., 2022 | No | 60 | Multiple countries | Yes, but NS | PC | DCT, RD, TL | 3-Nov-21 |
| Mayo et al., 2021 | Yes | 13 | Multiple countries, including IT, AU, TW, US, FR, NL | Yes | ALL | DCT, DCS | 10-Feb-21 |
| Mazidimoradi et al., 2021 | No | 43 | Multiple countries | Yes | CRC | MT, DCT, RD | 1-Jun-21 |
| Mazidimoradi et al., 2022 | No | 25 | Multiple countries | Yes | CRC | DCS | 1-Jun-21 |
| Momenimovahed et al., 2021 | No | 55 | Multiple countries | No | ALL | PSND | 30-Jun-21 |
| Mostafaei et al., 2022 | No | 22 | Multiple countries | No | ALL | TL | 1-Jun-21 |
| Moujaess et al., 2020 | No | 88 | Multiple countries | No | ALL | DCT, O | 15-Apr-20 |
| Muls et al., 2022 | No | 51 | Multiple countries | No | ALL | PSND | 1-Oct-21 |
| Murphy et al., 2022 | No | 37 | Multiple countries | No | ALL | TL | 31-Mar-21 |
| Ng and Hamilton, 2022 | Yes | 31 | Multiple countries | Yes | BC | DCS, RD | 1-Oct-20 |
| Nikolopoulos et al., 2022 | No | 15 | Multiple countries | Yes, but NS | GC | MT, DCT, RD, PSND | 10-Feb-21 |
| Pacheco et al., 2021 | No | 9 | Multiple countries, including US, IT, CN, SP, UK, IR | No | ALL | DCT, O | NP |
| Pararas et al., 2022 | Yes | 10 | Multiple countries | Yes | CRC | O | NP |
| Pascual et al., 2022 | No | 12 | Multiple countries from low- and middle-income countries | Yes, but NS | Surgical Neuro-Oncology | MD, RD, TL, O | 01-Sep- 2021 |
| Piras et al., 2022 | No | 281 | Multiple countries | No | ALL | MT, DCT, SIA, PSND | 31-Dec-2021 |
| Riera et al., 2021 | No | 62 | Multiple countries | Yes | ALL | DCT | NP |
| Rohilla et al., 2021 | No | 6 | IN | No | ALL | PSND, O | 3-Feb-21 |
| Salehi et al., 2022 | No | 16 | Multiple countries | No | ALL | TL | 1-Apr-21 |
| Sarich et al., 2022 | Yes | 44 | Multiple countries | Yes | NA | RF | 5-Nov-20 |
| Sasidharanpillai and Ravishankar, 2022 | Yes | 7 | Multiple countries, including SL, IT, CA, SC, BE, US | Yes | CV | DCT, RD | 1-Sep-21 |
| Sun et al., 2021 | No | 6 | IT, AM, UK | No | BC | MT | 1-Feb-21 |
| Tang et al., 2022 | Yes | 14 | TU, CN, UK, IT, DN, AS, AU | Yes | CRC | O | 12-Jan-22 |
| Teglia et al., 2022a | Yes | 39 | Multiple countries | Yes | BC, CRC, CV | DCT, RD | 12-Dec-21 |
| Teglia et al., 2022b | Yes | 47 | Multiple countries | Yes | ALL | DCT | 12-Dec-21 |
| Thomson et al., 2020 | Yes | 54 | NP | Yes | ALL | O | 1-Jun-21 |
| Vigliar et al., 2020 | Yes | 41‡ | Multiple countries | Yes | ALL | DCS, RD | 30-Apr-20 |
| Zapała et al., 2022 | No | 160 | NP | No | ALL | DCT, PSND, TL | NP |
| Zhang et al., 2022 | Yes | 40 | Multiple countries | No | ALL | PSND | 31-Jan-22 |
-
*
Multiple countries refer to inclusion of studies for final analysis that used data from more than one country. If complete information on location from all primary studies were provided, then specific countries were listed.
-
†
Apps.
-
‡
Respondents.
-
AM, America; BC; AS, Austria; AU, Australia; BA, Bangladesh; BC, breast cancer; BE, Belgium; BR, Brazil; CA, Canada; China; CRC, colorectal cancer; CV, cervical cancer; DN, Denmark; FR, France; GA, gastric cancer; GC, gynecological cancer; H&N, head and neck cancer; IN, India; IR, Iran; IT, Italy; JP, Japan; NA, not applicable; NL, Netherlands; NP, not provided; OTO, otorhinolaryngology cancer; PC, pediatric cancer; PG, Portugal; SC, Scotland; SL, Slovenia or Sri Lanka; SN, Singapore; SP, Spain; TU, Turkey; TW, Taiwan; UK, United Kingdom; United States; ZA, Zambia;MT, modification of treatment; DCT, delayed and/or canceled treatment; DCS, delayed and canceled screening; RD, reduced diagnosis: RHPV, reduced uptake of HPV vaccination; TL, telemedicine; PSND, psychological needs/distress; FBD, financial burden/distress; SIA, social isolation; O, other aspects.
Methodological rigor of included reviews.
| Author | Checklist use | Methodological rigor conclusion category | GRADE |
|---|---|---|---|
| Adham et al., 2022 | CEBM | Not provided | Not provided |
| Alkatout et al., 2021 | NOS | Strong evidence | Not provided |
| Alom et al., 2021 | NHLBI, NIH | Not provided | Not provided |
| Ayubi et al., 2021 | Not applied | Not provided | Not provided |
| Azab and Azzam, 2021 | Not applied | Not provided | Not provided |
| Bezerra et al., 2022 | Not applied | Not provided | Not provided |
| Di Cosimo et al., 2022 | CLARITY | Mixed/Intermediate | Not provided |
| Crosby and Sharma, 2020 | Not applied | Not provided | Not provided |
| de Bock et al., 2022 | ROBINS-I | Strong evidence | Not provided |
| Dhada et al., 2021 | CASP, NHLBI, NIH | Mixed/Intermediate | Not provided |
| Donkor et al., 2021 | JBI | Weak | Not provided |
| Fancellu et al., 2022 | Not applied | Not provided | Not provided |
| Ferrara et al., 2022 | NOS | Strong evidence | Not provided |
| Gadsden et al., 2022 | JBI, ROBINS-I | Mixed/Intermediate | Not provided |
| Garg et al., 2020 | Not applied | Not provided | Not provided |
| Gascon et al., 2022 | Agree II | Mixed/Intermediate | Not provided |
| Hesary and Salehiniya, 2022 | NOS | Mixed/Intermediate | Not provided |
| Hojaij et al., 2020 | Not applied | Not provided | Not provided |
| Jammu et al., 2021 | Not applied | Not provided | Not provided |
| Kirby et al., 2022 | JBI, CHEC | Mixed/Intermediate | Not provided |
| Legge et al., 2022 | MMAT | Strong evidence | Not provided |
| Lignou et al., 2022 | Not applied | Not provided | Not provided |
| Lu et al., 2021 | MARS | Mixed/Intermediate | Not provided |
| Majeed et al., 2022 | Not applied | Not provided | Low to moderate certainty |
| Mayo et al., 2021 | NOS | Mixed/Intermediate | Moderate to high |
| Mazidimoradi et al., 2021 | NOS | Mixed/Intermediate | Not provided |
| Mazidimoradi et al., 2022 | NOS | Strong evidence | Not provided |
| Momenimovahed et al., 2021 | Not applied | Not provided | Not provided |
| Mostafaei et al., 2022 | JBI | Mixed/Intermediate | Not provided |
| Moujaess et al., 2020 | Not applied | Not provided | Not provided |
| Muls et al., 2022 | MMAT | Mixed/Intermediate | Not provided |
| Murphy et al., 2022 | JBI, CHEC | Mixed/Intermediate | Not provided |
| Ng and Hamilton, 2022 | NOS | Mixed/Intermediate | Not provided |
| Nikolopoulos et al., 2022 | NOS | Mixed/Intermediate | Not provided |
| Pacheco et al., 2021 | JBI, ROBINS-I | Weak | Not provided |
| Pararas et al., 2022 | NOS | Strong evidence | Not provided |
| Pascual et al., 2022 | Not applied | Not provided | Not provided |
| Piras et al., 2022 | Not applied | Not provided | Not provided |
| Riera et al., 2021 | ROBINS-I | Mixed/Intermediate | Not provided |
| Rohilla et al., 2021 | Not applied | Not provided | Not provided |
| Salehi et al., 2022 | Not applied | Not provided | Not provided |
| Sarich et al., 2022 | ROBINS-I | Weak evidence | Not provided |
| Sasidharanpillai and Ravishankar, 2022 | NHLBI, NIH | Strong evidence | Not provided |
| Sun et al., 2021 | Not applied | Not provided | Not provided |
| Tang et al., 2022 | NOS | Strong evidence | Not provided |
| Teglia et al., 2022a | CASP | Mixed/Intermediate | Not provided |
| Teglia et al., 2022b | CASP | Mixed/Intermediate | Not provided |
| Thomson et al., 2020 | ASTRO | Mixed/Intermediate | Not provided |
| Vigliar et al., 2020 | Not applicable | Not provided | Not provided |
| Zapała et al., 2022 | Not applied | Not provided | Not provided |
| Zhang et al., 2022 | JBI | Mixed/Intermediate | Not provided |
-
CEBM, Critical appraisal tool of qualitative studies from Centre of Evidence-based Medicine (CEBM), University of Oxford; ASTRO, The American Society of Radiation Oncology; CASP, https://casp-uk.net/casp-tools-checklists/; CHEC, Consensus on Health Economic Criteria: CLARITY, ‘Risk of bias instrument for cross-sectional surveys of attitudes and practices’ from the CLARITY Group at McMaster University; JBI, Joanna Briggs Institute; MARS, Mobile Apps Rating Scale; MMAT, Mixed Methods Appraisal Tool; NHLBI, NHI, National Institute of Health Checklist; NOS, Newcastle-Ottawa Quality Assessment: RBC, Risk of Bias Checklist for Prevalence Studies by Hoy et al., 2012.
Methodological assessment of the included reviews – AMSTAR-2 evaluation (16 questions)*.
| Authors, year of publication | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9† | Q10 | Q11 | Q12 | Q13 | Q14 | Q15 | Q16 | Overall assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adham et al., 2022 | n | n | n | py | n | n | n | n | y | n | na | na | na | n | na | n | Critical low |
| Alkatout et al., 2021 | n | py | y | py | n | n | n | py | y | n | na | na | n | n | na | y | Critical low |
| Alom et al., 2021 | n | n | n | py | n | y | n | py | y | n | na | na | y | n | na | y | Critical low |
| Ayubi et al., 2021 | y | n | n | py | n | n | n | y | n | n | y | n | n | n | y | y | Critical low |
| Azab and Azzam, 2021 | n | n | n | py | y | y | n | y | py | n | y | n | n | n | y | y | Critical low |
| Bezerra et al., 2022 | y | n | n | n | n | n | n | y | n | n | na | na | n | n | na | y | Critical low |
| Crosby and Sharma, 2020 | n | n | n | n | n | n | n | n | n | n | na | na | na | n | na | y | Critical low |
| de Bock et al., 2022 | y | n | y | py | y | y | n | y | y | n | y | n | n | y | n | y | Critical low |
| Dhada et al., 2021 | n | py | n | py | n | n | n | y | y | n | na | na | n | n | na | y | Critical low |
| Di Cosimo et al., 2022 | n | n | n | py | y | n | n | y | y | n | y | y | y | y | y | y | Critical low |
| Donkor et al., 2021 | n | n | n | py | y | y | n | y | y | n | na | na | na | n | na | y | Critical low |
| Fancellu et al., 2022 | y | n | n | n | n | n | n | n | n | n | na | na | n | n | n | n | Critical low |
| Ferrara et al., 2022 | n | py | n | py | y | y | n | n | y | n | na | na | y | n | na | y | Low |
| Gadsden et al., 2022 | y | py | n | py | y | n | n | y | y | n | na | na | y | n | na | y | Low |
| Garg et al., 2020 | n | n | n | py | y | y | n | n | n | n | na | na | n | y | na | y | Critical low |
| Gascon et al., 2022 | y | y | n | y | y | y | n | na | y | y | na | na | na | n | na | y | Low |
| Hesary and Salehiniya, 2022 | n | py | n | py | n | n | n | n | y | n | na | na | n | n | na | y | Critical low |
| Hojaij et al., 2020 | n | n | n | n | n | n | n | n | n | n | na | na | na | n | na | y | Critical low |
| Jammu et al., 2021 | n | n | n | py | y | y | n | n | n | n | na | na | n | n | na | y | Critical low |
| Kirby et al., 2022 | y | py | n | y | n | y | n | py | y | n | na | na | n | n | na | y | Critical low |
| Legge et al., 2022 | y | py | y | py | y | y | n | y | y | n | na | na | n | n | na | y | Critical low |
| Lignou et al., 2022 | y | n | n | n | y | y | n | y | n | n | na | na | n | n | na | y | Critical low |
| Lu et al., 2021 | y | n | na | py | n | n | n | y | na | n | na | na | na | n | na | y | Critical low |
| Majeed et al., 2022 | n | y | n | py | n | y | n | n | py | n | na | na | n | n | na | y | Critical low |
| Mayo et al., 2021 | n | y | n | py | y | y | n | n | py | n | n | y | y | n | n | y | Critical low |
| Mazidimoradi et al., 2022 | n | py | n | py | n | n | n | py | y | n | na | na | n | n | na | y | Critical low |
| Mazidimoradi et al., 2021 | n | py | n | py | n | n | n | y | y | n | na | na | n | n | na | y | Critical low |
| Momenimovahed et al., 2021 | n | n | n | py | n | n | n | n | n | n | na | na | n | n | na | y | Critical low |
| Mostafaei et al., 2022 | n | py | n | n | n | n | y | py | y | n | na | na | n | n | na | y | Critical low |
| Muls et al., 2022 | y | py | y | py | n | y | n | y | y | n | na | na | n | n | na | y | Critical low |
| Murphy et al., 2022 | n | n | n | y | n | n | n | y | y | n | na | na | n | n | na | y | Critical low |
| Ng and Hamilton, 2022 | n | py | n | py | n | n | n | py | y | n | y | n | y | y | y | y | Low |
| Nikolopoulos et al., 2022 | n | py | n | py | n | n | n | n | y | n | na | na | n | n | na | y | Critical low |
| Pacheco et al., 2021 | y | y | y | py | y | y | y | py | y | y | na | na | y | n | na | y | High quality |
| Pararas et al., 2022 | n | y | n | y | y | n | n | n | y | n | n | n | n | y | y | y | Critical low |
| Pascual et al., 2022 | y | n | y | py | y | y | n | y | n | n | na | na | n | y | na | n | Critical low |
| Piras et al., 2022 | n | n | n | py | n | n | n | py | n | n | na | na | n | n | na | y | Critical low |
| Riera et al., 2021 | n | py | y | py | y | y | y | y | y | y | na | na | n | y | na | y | Moderate quality |
| Rohilla et al., 2021 | n | n | n | py | n | y | n | n | n | n | na | na | n | n | na | y | Critical low |
| Salehi et al., 2022 | n | n | n | py | y | n | n | n | n | n | na | na | n | n | na | y | Critical low |
| Sarich et al., 2022 | y | y | y | py | y | y | n | y | y | n | y | y | n | y | n | y | Critical low |
| Sasidharanpillai and Ravishankar, 2022 | n | py | n | py | n | n | n | y | y | n | y | y | y | y | y | y | Low |
| Sun et al., 2021 | n | n | n | py | n | n | n | n | n | n | na | na | na | n | na | n | Critical low |
| Tang et al., 2022 | y | n | n | n | n | n | n | n | y | py | n | n | n | y | n | y | Critical low |
| Teglia et al., 2022a | y | py | y | py | y | y | n | n | y | n | n | n | n | n | y | y | Critical low |
| Teglia et al., 2022b | y | py | y | py | y | y | n | py | y | n | n | n | n | y | n | y | Critical low |
| Thomson et al., 2020 | n | n | n | n | n | n | n | n | y | n | y | n | n | n | na | y | Critical low |
| Vigliar et al., 2020† | na | na | na | na | na | na | na | na | na | na | na | na | na | na | na | na | NA |
| Zapała et al., 2022 | n | n | n | n | n | n | n | n | n | n | na | na | n | n | na | y | Critical low |
| Zhang et al., 2022 | y | y | y | py | n | y | n | py | y | n | y | y | y | y | y | y | Low |
-
AMSTAR-2 overall assessment rating: high—the review provides an accurate and comprehensive summary of the results of the available studies that addresses the question of interest; moderate—the review has more than one weakness, but no critical flaws. It may provide an accurate summary of the results of the available studies; low—the review has a critical flaw and may not provide an accurate and comprehensive summary of the available studies that address the question of interest; or critically low—the review has more than one critical flaw and should not be relied on to provide an accurate and comprehensive summary of the available studies.
-
Q1: Did the research questions and inclusion criteria for the review include the components of PICO?
-
Q2: Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review and did the report justify any significant deviations from the protocol?
-
Q3: Did the review authors explain their selection of the study designs for inclusion in the review?
-
Q4: Did the review authors use a comprehensive literature search strategy?
-
Q5: Did the review authors perform study selection in duplicate?
-
Q6: Did the review authors perform data extraction in duplicate?
-
Q7: Did the review authors provide a list of excluded studies and justify the exclusions?
-
Q8: Did the review authors describe the included studies in adequate detail?
-
Q9: Did the review authors use a satisfactory technique for assessing the risk of bias (RoB) in individual studies that were included in the review?
-
Q10: Did the review authors report on the sources of funding for the studies included in the review?
-
Q11: If meta-analysis was performed did the review authors use appropriate methods for statistical combination of results?
-
Q12: If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis?
-
Q13: Did the review authors account for RoB in individual studies when interpreting/discussing the results of the review?
-
Q14: Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review?
-
Q15: If they performed quantitative synthesis did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review?
-
Q16: Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review?
-
*
The review scored yes if study used a checklist to evaluate methodological rigor, and partial yes if only GRADE assessment was provided without applying a checklist for assessing methodological rigor.
-
†
Individual participant meta-analysis and thus not applicable the AMSTAR evaluation.
-
n, no; na, not applicable; py, partially yes; y, yes.
Summary estimates of the meta-analysis included.
| Author | No. of studies | Outcome | Estimate | LCI | UCI | I2 | p-heterogeniety | Metric |
|---|---|---|---|---|---|---|---|---|
| Ayubi et al., 2021 | 15 | Depression | 0.37 | 0.27 | 0.47 | 99 | <0.001 | Prev† |
| 17 | Anxiety | 0.38 | 0.31 | 0.46 | 99 | <0.001 | Prev† | |
| 4 | Anxiety | 0.25 | 0.08 | 0.42 | 68 | 0.02 | SMD† | |
| Zhang et al., 2022 | 28 | Depression | 0.325 | 0.263 | 0.392 | 99 | <0.001 | Prev† |
| 34 | Anxiety | 0.313 | 0.254 | 0.375 | 99 | <0.001 | Prev† | |
| 8 | PTSD | 0.288 | 0.207 | 0.368 | 99 | <0.001 | Prev† | |
| 5 | Distress | 0.539 | 0.469 | 0.609 | 67 | 0.016 | Prev† | |
| 5 | Insomia | 0.232 | 0.171 | 0.293 | 91 | <0.001 | Prev† | |
| 3 | Fear of cancer progression | 0.674 | 0.437 | 0.91 | 93 | <0.001 | Prev† | |
| Di Cosimo et al., 2022 | 28 | Cancellation/delay of treatment | 0.58 | 0.48 | 0.67 | 98 | <0.01 | Prop*† |
| 14 | Modification of treatment | 0.65 | 0.53 | 0.75 | 98 | <0.01 | Prop*† | |
| 10 | Delay of clinic visits | 0.75 | 0.49 | 0.95 | 99 | <0.01 | Prop*† | |
| 14 | Reduction in activity | 0.58 | 0.47 | 0.68 | 93 | <0.01 | Prop*† | |
| 25 | Use of remote consultation | 0.72 | 0.59 | 0.84 | 99 | <0.01 | Prop*† | |
| 7 | Routine use of PPE (patients) | 0.81 | 0.75 | 0.95 | 96 | <0.01 | Prop*† | |
| 16 | Routine use of PPE (workers) | 0.8 | 0.61 | 0.94 | 99 | <0.01 | Prop*† | |
| 18 | Routine screening SARA-CoV-2 swab | 0.41 | 0.3 | 0.53 | 96 | <0.01 | Prop*† | |
| de Bock et al., 2022 | 5 | ≥T2 stage during the COVID-19 pandemic compared to the pre-pandemic control group | 1.00 | 0.72 | 1.38 | 58 | 0.05 | OR‡ |
| 4 | ≥T3 stage during the COVID-19 pandemic compared to the pre-pandemic control group | 0.95 | 0.69 | 1.32 | 39 | 0.18 | OR‡ | |
| 5 | ≥N1 stage during the COVID-19 pandemic compared to the pre-pandemic control group | 1.55 | 0.87 | 2.74 | 3 | 0.39 | OR‡ | |
| Mayo et al., 2021 | 6 | Screening breast cancer | 0.63 | 0.53 | 0.77 | 100 | <0.001 | IRR‡ |
| 5 | Screening conlonc cancer | 0.11 | 0.05 | 0.24 | 100 | <0.001 | IRR‡ | |
| 3 | Screening cervical cancer | 0.1 | 0.04 | 0.24 | 100 | <0.001 | IRR‡ | |
| Ng and Hamilton, 2022 | 3 | Screening breast cancer registry-based study | 0.59 | 0.46 | 0.7 | 100 | <0.001 | RR‡ |
| 10 | Screening breast cancer non-registry-based study | 0.47 | 0.38 | 0.58 | 100 | <0.001 | RR‡ | |
| 4 | Diagnosis breast cancer registry-based study | 0.82 | 0.63 | 1.06 | 99 | <0.001 | RR‡ | |
| 18 | Diagnosis breast cancer non-registry-based study | 0.71 | 0.63 | 0.8 | 92 | <0.001 | RR‡ | |
| Pararas et al., 2022 | 5 | Tis-T1 stage | 1.14 | 0.87 | 1.48 | 41 | 0.15 | OR‡ |
| 5 | T2 stage | 0.91 | 0.78 | 1.06 | 0 | 0.6 | OR‡ | |
| 5 | T3 stage | 1.18 | 0.82 | 1.7 | 88 | <0.001 | OR‡ | |
| 6 | T4 stage | 1.19 | 0.79 | 1.8 | 80 | <0.001 | OR‡ | |
| 6 | N+ stage | 1 | 0.89 | 1.11 | 0 | 0.54 | OR‡ | |
| 6 | M+ stage | 1.65 | 1.02 | 2.67 | 91 | <0.001 | OR‡ | |
| 7 | Right-sided tumors | 0.88 | 0.51 | 1.52 | 99 | <0.001 | OR‡ | |
| 7 | Left-sided tumors | 0.91 | 0.56 | 1.5 | 96 | <0.001 | OR‡ | |
| 8 | Rectal tumors | 0.93 | 0.63 | 1.37 | 95 | <0.001 | OR‡ | |
| 3 | Emergency presantations | 1.74 | 1.07 | 2.84 | 95 | <0.001 | OR‡ | |
| 3 | Complicated tumor | 1.72 | 0.78 | 3.78 | 82 | 0.004 | OR‡ | |
| 3 | Neoadjuvant therapy | 1.22 | 1.09 | 1.37 | 0 | 0.4 | OR‡ | |
| 4 | Palliative internt surgery | 1.95 | 1.13 | 3.36 | 54 | 0.09 | OR‡ | |
| 6 | Minimally invasive surgery | 0.68 | 0.37 | 1.24 | 98 | <0.001 | OR‡ | |
| 5 | Stoma formation | 0.91 | 0.51 | 1.62 | 94 | <0.001 | OR‡ | |
| 2 | Morbidity | 0.92 | 0.55 | 1.55 | 25 | 0.25 | OR‡ | |
| 3 | Leng of hospital stay | 0.51 | −0.93 | 1.94 | 79 | 0.008 | WMD‡ | |
| 3 | Lymph node harvest | 1.57 | −1.99 | 5.13 | 64 | 0.06 | WMD‡ | |
| Sarich et al., 2022 | 12 | Smoking prevalence | 0.87 | 0.79 | 0.97 | 99 | <0.001 | PR‡ |
| 17 | Among smokers, smoking less prevalence | 0.21 | 0.14 | 0.3 | 99 | <0.001 | Prev† | |
| 22 | Among smokers, smoking more | 0.27 | 0.22 | 0.32 | 98 | <0.001 | Prev† | |
| 17 | Among smokers, smoking unchanged | 0.5 | 0.41 | 0.58 | 99 | <0.001 | Prev† | |
| 6 | Among smokers, quit smoking | 0.04 | 0.01 | 0.09 | 95 | <0.001 | Prev† | |
| 4 | Among non-smokers, started smoking | 0.02 | 0.01 | 0.03 | 92 | <0.001 | Prev† | |
| Sasidharanpillai and Ravishankar, 2022 | 7 | Women screened before the COVID-19 pandemic | 0.0979 | 0.06 | 0.1359 | 100 | <0.001 | Prop |
| 7 | Women screened during the COVID-19 pandemic | 0.0424 | 0.0277 | 0.0571 | 100 | <0.001 | Prop | |
| Tang et al., 2022 | 10 | Postoperative morbidity | 0.9 | 0.8 | 1.01 | 26 | 0.22 | OR‡ |
| 8 | Postoperative mortality | 1.27 | 0.92 | 1.75 | 0 | 0.57 | OR‡ | |
| 4 | Converion rate | 1.07 | 0.75 | 1.52 | 31 | 0.23 | OR‡ | |
| 5 | Incidence of anastomotic leakage | 0.71 | 0.07 | 19.22 | 0 | 0.74 | OR‡ | |
| 2 | Intensive care unit demand rate | 0.73 | 0.29 | 1.85 | 0 | 0.5 | OR‡ | |
| 4 | R1 resections rate | 0.46 | 0.11 | 1.9 | 0 | 0.48 | OR‡ | |
| 5 | Mean lymph node yield | 0.16 | −2.26 | 2.59 | 54 | 0.07 | MD‡ | |
| 7 | Length of hospital stay | −0.05 | −2.28 | 2.19 | 98 | <0.001 | MD‡ | |
| Teglia et al., 2022a | 21 | Breast cancer screening January–October 2020 | 0.467 | 0.378 | 0.378 | NP | NP | PRED‡ |
| 21 | Breast cancer screening April 2020 | 0.74 | 0.567 | 0.918 | NP | NP | PRED‡ | |
| 21 | Breast cancer screening June–October 2020 | 0.13 | −0.07 | 0.33 | NP | NP | PRED‡ | |
| 22 | Colorectal cancer screening January–October 2020 | 0.449 | 0.361 | 0.538 | NP | NP | PRED‡ | |
| 21 | Colonoscopy screening January–October 2020 | 0.525 | 0.388 | 0.663 | NP | NP | PRED‡ | |
| 21 | Fecal occult blood test or fecal immunochemical test January–October 2020 | 0.378 | 0.258 | 0.499 | NP | NP | PRED‡ | |
| 21 | Colorectal cancer screening April 2020 | 0.693 | 0.369 | 1 | NP | NP | PRED‡ | |
| 21 | Colorectal cancer screening June–October 2020 | 0.234 | 0.024 | 0.444 | NP | NP | PRED‡ | |
| 11 | Cervical cancer screening January–October 2020 | 0.518 | 0.389 | 0.647 | NP | NP | PRED‡ | |
| 21 | Cervical cancer screening March 2020 | 0.788 | 0.583 | 0.993 | NP | NP | PRED‡ | |
| PRED‡ | ||||||||
| Teglia et al., 2022b | NP | Overall treatment January–October 2020 | 0.187 | 0.133 | 0.241 | NP | NP | PRED‡ |
| NP | Overall treatment January–February 2020 | 0.027 | 0.045 | 0.1 | NP | NP | PRED‡ | |
| NP | Overall treatment March 2020 | 0.156 | 0.076 | 0.237 | NP | NP | PRED‡ | |
| NP | Overall treatment April 2020 | 0.283 | 0.194 | 0.372 | NP | NP | PRED‡ | |
| NP | Overall treatment May 2020 | 0.262 | 0.176 | 0.041 | NP | NP | PRED‡ | |
| NP | Overall treatment June–October 2020 | 0.16 | 0.041 | 0.279 | NP | NP | PRED‡ | |
| NP | Overall surgical treatment January–October 2020 | 0.339 | 0.279 | 0.399 | NP | NP | PRED‡ | |
| NP | Overall surgical treatment January–February 2020 | 0.072 | −0.093 | 0.238 | NP | NP | PRED‡ | |
| NP | Overall surgical treatment March 2020 | 0.307 | 0.219 | 0.396 | NP | NP | PRED‡ | |
| NP | Overall surgical treatment April 2020 | 0.342 | 0.239 | 0.445 | NP | NP | PRED‡ | |
| NP | Overall surgical treatment May 2020 | 0.416 | 0.318 | 0.514 | NP | NP | PRED‡ | |
| NP | Overall surgical treatment June–October 2020 | 0.351 | 0.186 | 0.516 | NP | NP | PRED‡ | |
| NP | Overall medical treatment January–October 2020 | 0.126 | 0.048 | 0.204 | NP | NP | PRED‡ | |
| NP | Overall medical treatment January–February 2020 | 0.015 | −0.055 | 0.084 | NP | NP | PRED‡ | |
| NP | Overall medical treatment March 2020 | 0.116 | −0.012 | 0.233 | NP | NP | PRED‡ | |
| NP | Overall medical treatment April 2020 | 0.248 | 0.09 | 0.407 | NP | NP | PRED‡ | |
| NP | Overall medical treatment May 2020 | 0.196 | 0.085 | 0.306 | NP | NP | PRED‡ | |
| NP | Overall medical treatment June–October 2020 | 0.079 | −0.078 | 0.236 | NP | NP | PRED‡ | |
| PRED‡ | ||||||||
| Vigliar et al., 2020 | 41 | Cytological samples over 4 weeks of the COVID-19 pandemic | 0.453 | 0.001 | 0.98 | NP | NP | PRED‡ |
| 41 | Ratio of exfoliative to fine needle aspiration samples | 0.89 | 0.74 | 1.08 | 95 | <0.01 | OR‡ | |
| 27 | Malignant diagnosis | 0.0556 | 0.0377 | 0.0735 | 81 | <0.01 | RD‡ |
-
*
Surveyed centers/operators.
-
†
Estimates are during pandemic.
-
‡
Estimates are pandemic vs. pre-pandemic.
-
LCI, lower confidence interval; IRR, incidence rate ratio; MD, mean difference; OR, odds ratio; PRED, percent reduction; PR, prevalence ratio; Prev, prevalence: Prop, proportion; RD, risk difference; RR, rate ratio; PPE, personal protective equipment; NP, not provided; UCI, upper confidence interval; SMD, standardized mean difference; WMD, weighted mean difference.
Additional files
-
Supplementary file 1
(1a-l) Table characteristics, main findings, PRISMA, and search strategy.
- https://cdn.elifesciences.org/articles/85679/elife-85679-supp1-v2.docx
-
Supplementary file 2
Bibliographic databases used from each review (see excel file).
- https://cdn.elifesciences.org/articles/85679/elife-85679-supp2-v2.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/85679/elife-85679-mdarchecklist1-v2.pdf