Exploring factors shaping antibiotic resistance patterns in Streptococcus pneumoniae during the 2020 COVID-19 pandemic
Figures
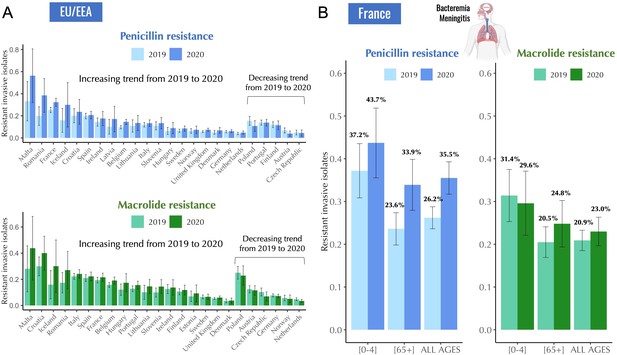
Antibiotic resistance trends in invasive Streptococcus pneumoniae isolates for the years 2019 and 2020.
(A) The proportion of invasive S. pneumoniae isolates resistant to penicillin and macrolides (azithromycin/ clarithromycin/ erythromycin) reported to EARS-Net (European Antimicrobial Resistance Surveillance Network) for 24 European countries. Error bars show 95% confidence intervals. (B) The proportion of invasive S. pneumoniae isolates resistant to penicillin (MIC >0.064 mg/L) and macrolides (erythromycin) according to age. Error bars show 95% confidence intervals. The total number of invasive pneumococcal isolates reported in France decreased by 45.1% from 2019 to 2020 (from 1119–614). Data are provided by the French National Reference Center for Pneumococci.
-
Figure 1—source data 1
European trends in antibiotic resistance in Streptococcus pneumoniae invasive isolates (2019-2020).
- https://cdn.elifesciences.org/articles/85701/elife-85701-fig1-data1-v2.xlsx
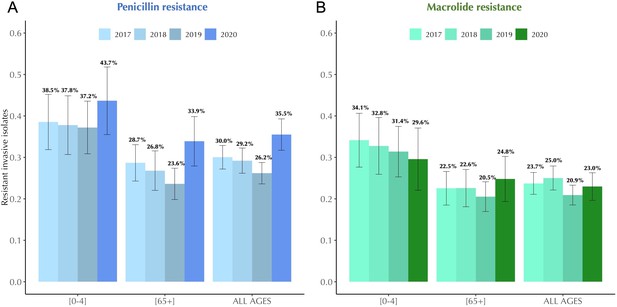
Antibiotic resistance trends in invasive Streptococcus pneumoniae isolates in France, 2017–2020.
The proportion of invasive S. pneumoniae isolates resistant to penicillin (A) and macrolides (B) according to age. Error bars show 95% confidence intervals. Across the period 2017–2020, a consistent decline in antibiotic resistance is observed for both penicillin and macrolides. Notably, this general trend experienced an anomaly in 2020, coinciding with the onset of the COVID-19 pandemic. Data are provided by the French National Reference Center for Pneumococci.
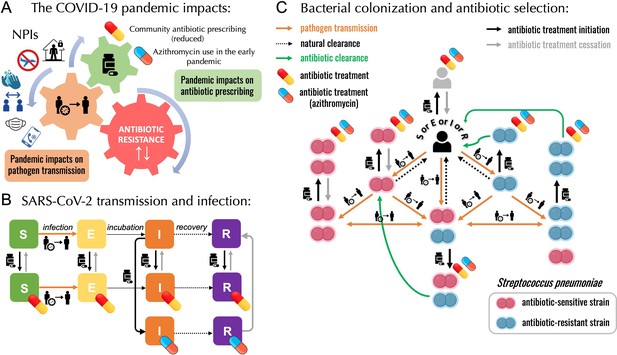
A modelling framework describing the transmission of SARS-CoV-2 and Streptococcus pneumoniae in the community setting, in the context of both general antibiotic prescribing and azithromycin prescribing for COVID-19 infected individuals.
(A) Non-pharmaceutical interventions (NPIs) implemented to control SARS-CoV-2 transmission (lockdown, face mask use, improved hygiene practices, travel restrictions, quarantine, telemedicine, and physical distancing) may also modify transmission of other pathogens, in addition to impacting antibiotic prescribing due to altered inter-individual contact and health-care seeking behavior. (B) SEIR (Susceptible-Exposed-Infected-Recovered) model with antibiotic treatment compartments depicts interaction between SARS-CoV-2 infection and antibiotic prescribing, including both general community prescribing and azithromycin prescribing among individuals infected with SARS-CoV-2. (C) Diagram depicting how pneumococcal colonization and the community antibiotic prescribing are affected by the COVID-19 pandemic impacts. Initiation of antibiotic treatment is assumed independent of bacterial carriage, reflecting widespread bystander selection for commensal bacteria like S. pneumoniae. For a complete modeling framework, see section S2 in Supporting Information.
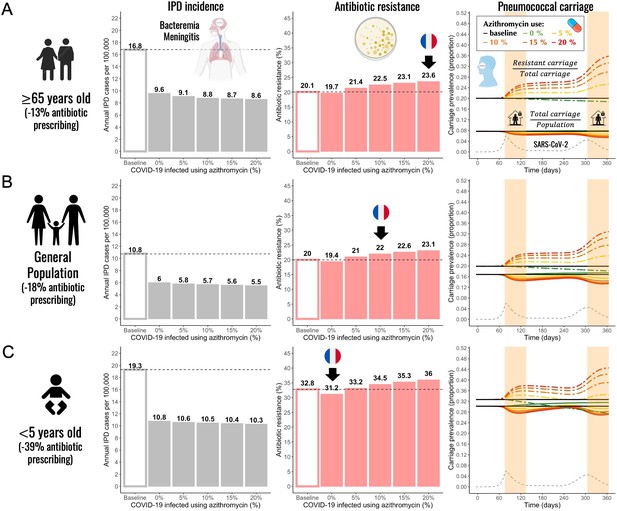
Annual incidence of invasive pneumococcal disease (IPD), antibiotic resistance (AR%), and pneumococcal carriage prevalence for three different subpopulations.
(A) The elderly (≥65 years-old) (B) general population (all ages), and (C) children (<5 years-old). Using pandemic scenario S19, which includes a combination of three different mechanisms: reduced community antibiotic prescribing, a reduced risk of developing an IPD, and community azithromycin use in COVID-19 infected individuals, we ran model simulations for three different subpopulations. For a full list of parameter values see Appendix 2—table 2. Annual IPD incidence (grey bars) decreased between 43% and 51% relative to the pre-pandemic (baseline) period with magnitude of a decrease depending on an age group and the level of azithromycin use in COVID-19 infected individuals. Antibiotic resistance (red bars) increased compared to the pre-pandemic (baseline) period in all age groups whenever azithromycin was used in COVID-19 infected. Black arrows indicate model outcomes that approximate the reported trends in antibiotic resistance in France for different age groups. Daily prevalence of total pneumococcal carriage remained relatively stable (solid-colored lines), exhibiting higher levels of decrease with increased azithromycin use. The prevalence of antibiotic-resistant pneumococcal carriage increased (dashed colored lines) over time in relation to SARS-CoV-2 outbreak (black dashed line) and higher azithromycin use. Highlighted time intervals (days 75–135 and 305–365) represent two lockdown periods.
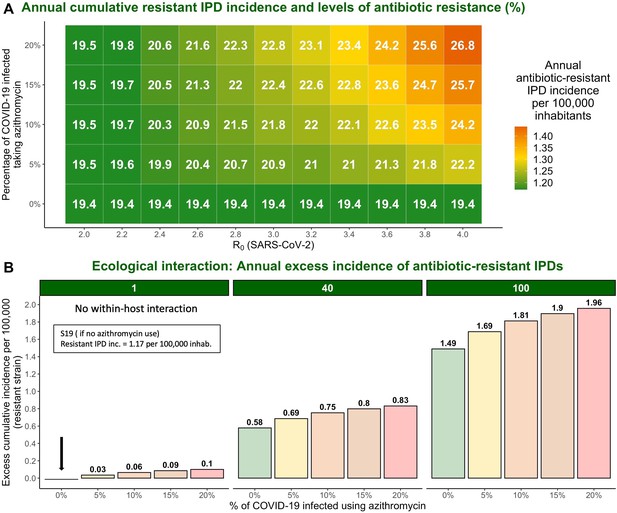
The impact of varying SARS-CoV-2 R0 and percentage of COVID-19 infected individuals taking azithromycin in scenario S19 on antibiotic resistance (%) and the annual incidence of antibiotic-resistant invasive pneumococcal disease (IPD).
Hypothetical within-host interactions contribute to an excess incidence of antibiotic-resistant IPDs. (A) Cumulative incidence of antibiotic-resistant IPDs and antibiotic resistance increase with greater values of SARS-CoV-2 R0 and higher percentage of the COVID-19 infected individuals taking azithromycin. The reproduction number for SARS-CoV-2 (R0) in the community corresponds to the most common estimates of R0 in France and other European countries ranging from R0=2–4 (Allieta et al., 2022; D’Arienzo and Coniglio, 2020; Di Domenico et al., 2020; Flaxman et al., 2020; Liu et al., 2020; Roux et al., 2020; Salje et al., 2020). (B) Annual excess in cumulative antibiotic-resistant IPD incidence in scenario S19 due to synergistic within-host ecological interactions compared to the same scenario with no within-host interactions and no azithromycin use (1.17 resistant IPD cases/100,000 inhabitants). A rate of disease progression increased by a factor (no within-host interaction) and in scenario S19 applied to the general population assuming azithromycin use in 10% of the infected individuals resulted in approximately 0.06 and 0.75 additional cases of antibiotic-resistant disease per 100,000 inhabitants over the course of 1 year, respectively, compared to the scenario S19 assuming no within-host interaction and no azithromycin use (indicated by the black arrow). For more details, see Appendix 2—figure 1.
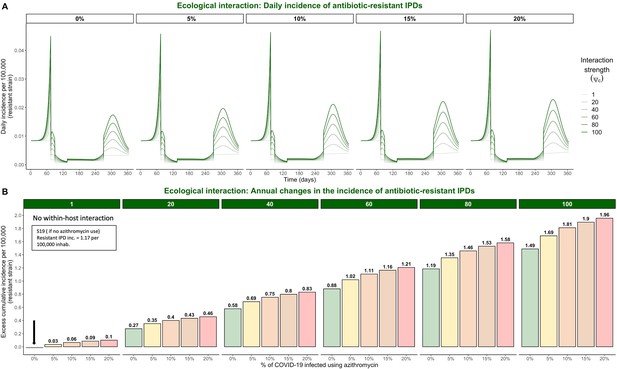
Hypothetical within-host interactions in scenario S19 promote the incidence of antibiotic-resistant invasive pneumococcal disease (IPD).
(A) Impacts of ecological interactions between pathogens. When SARS-CoV-2 infection leads to faster progression from pneumococcal colonization to disease , surges in COVID-19 cases accompanied by increasing levels of azithromycin use lead to substantial increases in the daily incidence of antibiotic-resistant IPD. (B) Annual excess in cumulative IPD incidence due to synergistic within-host ecological interactions. A rate of disease progression increased by a factor (no within-host interaction) and in scenario S19 applied to the general population resulted in approximately 0.06 and 0.75 additional cases of antibiotic-resistant disease per 100,000 inhabitants over the course of one year, respectively, compared to the scenario S19 assuming no within-host interaction and no azithromycin use indicated by the black arrow (1.17 cases/100,000 inhabitants).
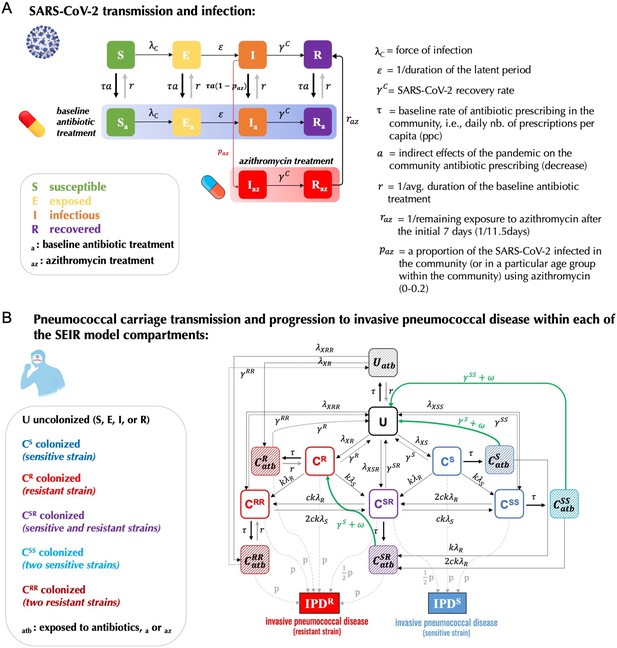
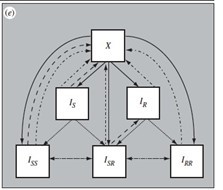
Model schematic describing co-circulation of SARS-CoV-2 infection and pneumococcal carriage transmission.
(A) Individuals in each of the SARS-CoV-2 transmission model compartments (S, E, I, R) can be either (B) uncolonized (U) or colonized by the bacteria (CS, CR, CSS, CRR, CSR) with or without concomitant antibiotic treatment (subscript atb denotes compartments exposed to antibiotics – both standard baseline antibiotic exposure and azithromycin exposure). Full list of model parameters can be found in Appendix 2—table 2.
Tables
Five mechanisms implemented in 31 pandemic scenarios proposed to explain the reported trends of IPD incidence, antibiotic resistance, and pneumococcal carriage in S. pneumoniae.
Scenarios explore all possible combinations of mechanisms proposed to test hypotheses that can explain the reported trends of annual invasive pneumococcal disease incidence (annual no. of cases per 100,000 inhabitants), antibiotic resistance (% of annual antibiotic-resistant IPD cases among total IPD cases), and % change in the pneumococcal carriage prevalence at the end of the first 60-day lockdown compared the prevalence before the lockdown. Model simulations were initiated assuming the initial 20% antibiotic resistance. Two pre-pandemic scenarios assume no SARS-CoV-2 circulation in the population and allow for the same 30-day carriage duration of both antibiotic-sensitive and -resistant strains (dS = dR) or a longer, 40-day carriage duration of -resistant strains (dR > dS) . When implemented, these five mechanisms assume 18% reduction in community antibiotic prescribing, a reduced risk of developing an IPD during (0.2) and after the first lockdown (0.4), a 25% reduction in transmission of pneumococcal carriage during the first lockdown, a 10% of azithromycin use among COVID-19 infected individuals, and a longer 40-day carriage duration of -resistant strains. For a full list of parameters see Appendix 2—table 2. Reported trends in European countries showed a decrease in annual IPD incidence by 44.3% on average, an increase in antibiotic resistance, and generally stable asymptomatic pneumococcal carriage in healthy individuals during the first lockdown period. Only scenarios S19 and S29 fulfill all three reported trends during the COVID-19 pandemic in 2020 simultaneously while accounting for the reported reduction in community antibiotic prescribing ( = carriage duration of antibiotic-sensitive pneumococcal strains; = carriage duration of antibiotic-resistant pneumococcal strains; PENI = penicillin; ERY = erythromycin).
| Scenarios | Mechanisms | IPD inc. | AR (%) | Sp. (%) | ||||
| 1 | 2 | 3 | 4 | 5 | ||||
| Pre-pandemic 1: (dS = dR) | 10.8 | 20.0 | NA | |||||
| Pre-pandemic 2: (dR > dS) | x | 11.3 | 20.0 | NA | ||||
| Pandemic: S1 | x | 10.9 | 19.2 | +1.3 | ||||
| S2 | x | 8.9 | 20.1 | –36.1 | ||||
| S3 | x | 5.9 | 20.0 | 0 | ||||
| S4 | x | 9.9 | 23.7 | –9.1 | ||||
| S5 | x | 11.3 | 20.0 | 0 | ||||
| S6 | x | x | 9.1 | 19.4 | –35.2 | |||
| S7 | x | x | 6.0 | 19.4 | +1.3 | |||
| S8 | x | x | 10.1 | 22.9 | –8.0 | |||
| S9 | x | x | 11.5 | 19.3 | +1.3 | |||
| S10 | x | x | 5.2 | 20.0 | –36.1 | |||
| S11 | x | x | 8.9 | 20.1 | –36.1 | |||
| S12 | x | x | 9.4 | 20.9 | –34.3 | |||
| S13 | x | x | 5.6 | 22.5 | –9.1 | |||
| S14 | x | x | 6.2 | 20.0 | 0 | |||
| S15 | x | x | 10.4 | 23.4 | –9.1 | |||
| S16 | x | x | x | 5.3 | 19.6 | –35.2 | ||
| S17 | x | x | x | 8.3 | 22.4 | –41.3 | ||
| S18 | x | x | x | 9.6 | 20.3 | –33.5 | ||
| S19 | x | x | x | 5.7 | 22.0 | –8.0 | ||
| S20 | x | x | x | 6.3 | 19.5 | +1.3 | ||
| S21 | x | x | x | 10.6 | 22.7 | –7.9 | ||
| S22 | x | x | x | 5.0 | 22.0 | –42.1 | ||
| S23 | x | x | x | 5.5 | 20.6 | –34.3 | ||
| S24 | x | x | x | 8.7 | 23.9 | –40.2 | ||
| S25 | x | x | x | 5.9 | 22.3 | –9.1 | ||
| S26 | x | x | x | x | 5.0 | 21.6 | –41.3 | |
| S27 | x | x | x | x | 5.6 | 20.1 | –33.5 | |
| S28 | x | x | x | x | 8.8 | 23.2 | –39.4 | |
| S29 | x | x | x | x | 5.9 | 21.8 | –7.9 | |
| S30 | x | x | x | x | 5.2 | 22.5 | –40.2 | |
| S31 | x | x | x | x | x | 5.3 | 22.1 | –39.4 |
| REPORTED TRENDS: | IPD inc. | AR (%) | Sp. (%) | |||||
| Pre-pandemic (FR, 2019) | 10.5 [10.3–10.7] | 26.2 (PENI) and 20.9 (ERY) | NA | |||||
| Pandemic (FR, 2020) | 5.8 [5.7–5.9] | 35.5 (PENI) and 23.0 (ERY) | Stable | |||||
| Pandemic (EU/EEA, 2020) General trends | Decrease by 44.3% on avg. | Majority of EU countries report an increase | Generally stable | |||||
-
Mechanisms: (1) Reduced community antibiotic prescribing; (2) Lockdown effect on reducing transmission of S. pneumoniae; (3) Reduced risk of developing an IPD; (4) Community azithromycin use in COVID-19 infected individuals; (5) Longer carriage duration of antibiotic-resistant pneumococcal strains.
Total number of invasive S. pneumoniae isolates from blood or cerebrospinal fluid tested in 2019 and 2020 and reported to EARS-Net (European Antimicrobial Resistance Surveillance Network).
| No. of invasive Streptococcus pneumoniae isolates in the EU/EEA | |||
|---|---|---|---|
| Country | 2019 | 2020 | % decrease in the total number of reported invasive isolates from 2019–2020 |
| Austria | 550 | 301 | 45.3% |
| Belgium | 1548 | 858 | 44.6% |
| Bulgaria | 46 | 28 | 39.1% |
| Croatia | 156 | 55 | 64.7% |
| Czechia | 387 | 204 | 47.3% |
| Denmark | 601 | 351 | 41.6% |
| Estonia | 161 | 80 | 50.3% |
| Finland | 678 | 293 | 56.8% |
| France | 1264 | 668 | 47.2% |
| Germany | 2035 | 1314 | 35.4% |
| Hungary | 222 | 124 | 44.1% |
| Iceland | 44 | 20 | 54.5% |
| Ireland | 348 | 136 | 60.9% |
| Italy | 1351 | 685 | 49.3% |
| Latvia | 79 | 42 | 46.8% |
| Lithuania | 120 | 96 | 20.0% |
| Luxembourg | 38 | 24 | 36.8% |
| Malta | 27 | 16 | 40.7% |
| Netherlands | 1552 | 997 | 35.8% |
| Norway | 507 | 243 | 52.1% |
| Poland | 364 | 165 | 54.7% |
| Portugal | 983 | 588 | 40.2% |
| Romania | 107 | 42 | 60.7% |
| Slovakia | 40 | 15 | 62.5% |
| Slovenia | 283 | 172 | 39.2% |
| Spain | 1038 | 611 | 41.1% |
| Sweden | 1071 | 551 | 48.6% |
| United Kingdom | 3468 | 1412 | 59.3% |
Summary of the model parameters.
| Symbol | Interpretation | Value(s) | References |
|---|---|---|---|
| SARS-CoV-2 infection parameters: | |||
| transmission rate of SARS-CoV-2 | 0.46 days–1 | Liu et al., 2020 | |
| SARS-CoV-2 recovery rate (mild cases) | 1/7 days–1 | Lauer et al., 2020; Rhee et al., 2020 | |
| SARS-CoV-2 incubation rate (latent period from exposed to infectious state) | 1/5 days–1 | Elias et al., 2021 | |
| Relative risk of SARS-CoV-2 transmission due to lockdown implementation | 0.23 | Salje et al., 2020 | |
| Pneumococcal colonization and invasion parameters: | |||
| transmission rate of antibiotic-sensitive strain | 0.056 days–1 0.046 days–1 0.034 days–1 | Davies et al., 2019; Olesen et al., 2020 | |
| fitness of antibiotic-resistant strain (assuming there is a fitness cost on transmissibility) | 0.9652 0.949 0.926 | Dagan et al., 2008; Melnyk et al., 2015 | |
| transmission rate of antibiotic-resistant strain | calculated | ||
| Relative risk of pneumococcal transmission due to lockdown implementation | 1 or 0.75 | assumed | |
| rate of natural bacterial clearance (assumed to be the same for antibiotic-sensitive and -resistant strains) | 1/20 days–1 1/30 days–1 1/45 days–1 | Abdullahi et al., 2012; Davies et al., 2019; Ekdahl et al., 1997; Högberg et al., 2021Melegaro et al., 2004 | |
| relative infectiousness with each strain for dually colonized | 0.5 | assumedColijn et al., 2010 | |
| fraction of dually colonized returning to single-colonized upon reinfection | 0.5 | assumed (Colijn et al., 2010) | |
| probability of acquiring secondary bacterial carriage | 0.5 | assumed (Colijn et al., 2010) | |
| probability of transmitting antibiotic-sensitive strain | 0.5 | assumed (Colijn et al., 2010) | |
| probability of a single infection | 0.5 | assumed (Colijn et al., 2010) | |
| pneumococcal invasion rate (summer and winter) | [3x10-6 day–1,9x10-6 day–1] in the elderly and general population, and [1x10-6 day–1, 2.5x10-6 day–1] in <5 years-old | Domenech de Cellès et al., 2019; Opatowski et al., 2013 | |
| initial states – initial prevalence of the total pneumococcal carriage (antibiotic-sensitive and -resistant) in different populations | 10% 20% 30% | Cohen et al., 2023; Rose et al., 2021; Rybak et al., 2022; Tinggaard et al., 2023; Wang et al., 2017 | |
| Antibiotic exposure parameters: | |||
| rate of antibiotic-induced pneumococcal clearance for sensitive strains(1/time before antibiotic action) | 1/3 days–1 | Kuitunen et al., 2023 | |
| rate of return to antibiotic unexposed compartment (1/duration of antibiotic treatment) | 1/7 days–1 | Grant and Saux, 2021; Kuitunen et al., 2023 | |
| rate of return to antibiotic unexposed compartment (1/the remainder of how long azithromycin stays in the body) | 1/11.5 days–1 | calculated (Foulds et al., 1990; Girard et al., 2005) | |
| baseline rate of antibiotic exposure in the community (France) | 0.0014 average daily ppc (prescriptions per capita) | Bara et al., 2022 | |
| A reduction factor for antibiotic exposure in the community resulting from changes in healthcare-seeking behavior in response to the COVID-19 pandemic | [0.51, 0.77, 0.84]to represent annual 13%, 18%, and 39% decrease observed in France | Bara et al., 2022 | |
| A proportion of COVID-19 infected individuals in the community receiving azithromycin | [0–0.20]testing between 0% and 20% | Tsay et al., 2022; Wittman et al., 2023 | |
| Pathogenicity (invasive pneumococcal disease risk): | |||
| A reduction factor for the risk of developing an invasive pneumococcal disease (IPD) due to the absence of influenza-like-illnesses (ILIs) after lockdown implementation | 1 (pre-lockdown) 0.2 (lockdown) 0.4 (post-lockdown) for an average of 0.5 in 2020 | Shaw et al., 2023 | |