Annexin A6 mediates calcium-dependent exosome secretion during plasma membrane repair
Figures
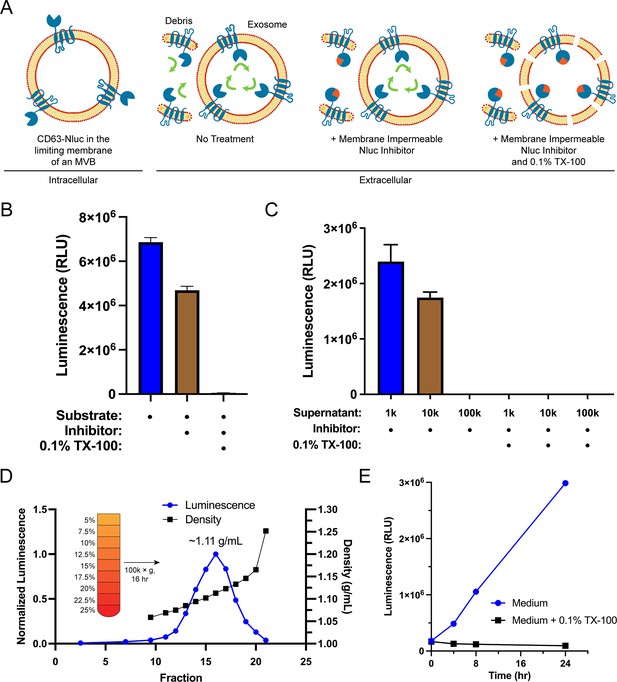
Endogenous CD63-nanoluciferase (Nluc) is a faithful reporter of exosome secretion.
(A) Schematic illustrating the topology of CD63-Nluc in different membranes and the cellular CD63-Nluc secretion assay. (B) Luminescence from the conditioned medium of CD63-Nluc cells with or without furimazine, the membrane-impermeable Nluc inhibitor, and 0.1% TX-100 are shown. (C) Luminescence derived from the supernatant fraction of CD63-Nluc conditioned medium subjected to differential centrifugation (1k, 10k, and 100k) with or without 0.1% TX-100 are shown. (D) Membrane-protected luminescence (in blue circles) and buoyant density (in black squares) of CD63-Nluc conditioned medium subjected to a high-resolution linear density gradient are shown. (E) Membrane-protected luminescence from CD63-Nluc conditioned medium collected over 24 hr with (blue circles) or without 0.1% TX-100 (black squares). Data plotted represent the means of three independent experiments, and error bars represent SDs. Note, for Figure 1D and E, the error bars are smaller than the dots in the image.
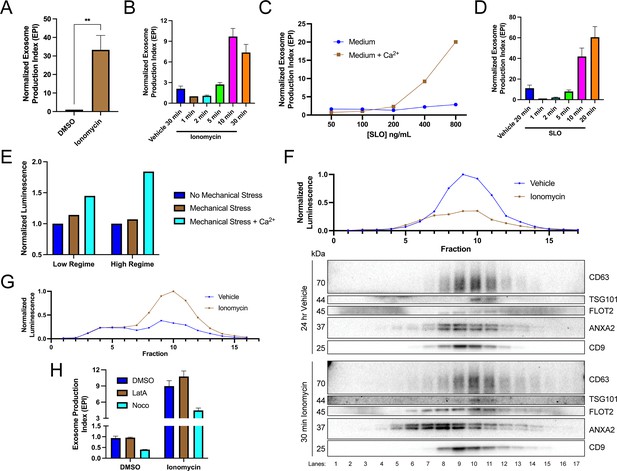
Elevation of cytosolic Ca2+ levels promotes exosome secretion.
(A) Normalized exosome production from CD63-nanoluciferase (Nluc) cells treated with 5 µM ionomycin or DMSO (ionomycin vehicle) are shown. (B) Relative rate of exosome secretion over time is shown. CD63-Nluc cells were treated with DMSO or 5 µM ionomycin for the indicated times, and the normalized exosome production index was calculated. (C) Normalized exosome production from CD63-Nluc cells treated for 30 min with increasing concentrations of streptolysin O (SLO), with or without 1.8 mM extracellular Ca2+. Note, error bars are smaller than the dots in the image. (D) Relative rate of exosome secretion over time is shown. CD63-Nluc cells were treated with PBS or 250 ng/ml SLO for the indicated time points, and the normalized exosome production index was calculated. (E) Normalized luminescence derived from CD63-Nluc cells treated with a high or low dose of mechanical stress, with or without 1.8 mM extracellular Ca2+. (F) Iodixanol gradient fractionation of conditioned medium 100k × g pellet fraction is shown. Conditioned medium from cells treated for 30 min with 5 µM ionomycin or 24 hr vehicle is compared. Line graphs show distribution of CD63-Nluc luminescence (with membrane-impermeable inhibitor added) across the linear gradient. Immunoblots show distribution of several extracellular vesicle (EV) markers across the linear gradient. (G) Iodixanol gradient fractionation of conditioned medium 10k × g supernatant fractions are shown. Conditioned medium from cells treated for 4 hr with 5 µM ionomycin or 4 hr vehicle is compared. Line graphs show distribution of CD63-Nluc luminescence (with membrane-impermeable inhibitor added) across the linear gradient. (H) Normalized exosome production from 30 min of 5 µM ionomycin or DMSO vehicle, co-treated with DMSO vehicle, 1 µM latrunculin A (LatA), or 10 µM nocodazole (Noco) is shown. Data plotted represent the means from three independent experiments, and error bars represent SDs. Statistical significance was performed using a Student’s T-test (**p<0.01).
-
Figure 2—source data 1
Uncropped immunoblot images corresponding to Figure 2.
- https://cdn.elifesciences.org/articles/86556/elife-86556-fig2-data1-v2.zip
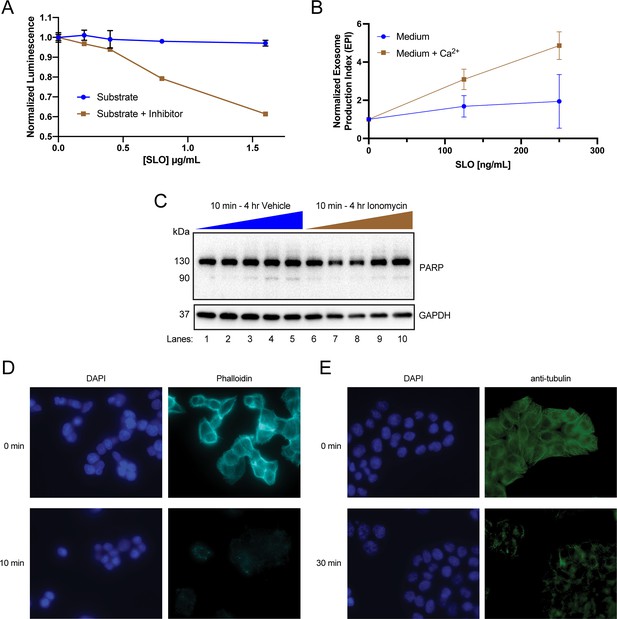
Elevation of cytosolic Ca2+ levels promotes exosome secretion.
(A) Normalized luminescence derived from the 10k × g conditioned medium supernatant from CD63-nanoluciferase (Nluc) cells with or without the addition of the membrane-impermeable Nluc inhibitor, as a function of increasing streptolysin O (SLO) concentrations, is shown. (B) Normalized exosome production from HEK293T FLAG-Nluc-CD63 cells treated for 20 min with increasing concentrations of SLO, with or without 1.8 mM extracellular Ca2+. (C) PARP and GAPDH immunoblots from HCT116 CD63-Nluc cells during a 4 hr ionomycin (5 µM) time course. (D) Phalloidin staining after treatment with 1 µM latrunculin A or (E) tubulin immunofluorescence after treatment with 10 µM nocodazole. Data plotted represent the means from three independent experiments, and error bars represent SDs.
-
Figure 2—figure supplement 1—source data 1
Uncropped immunoblot images corresponding to Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/86556/elife-86556-fig2-figsupp1-data1-v2.zip
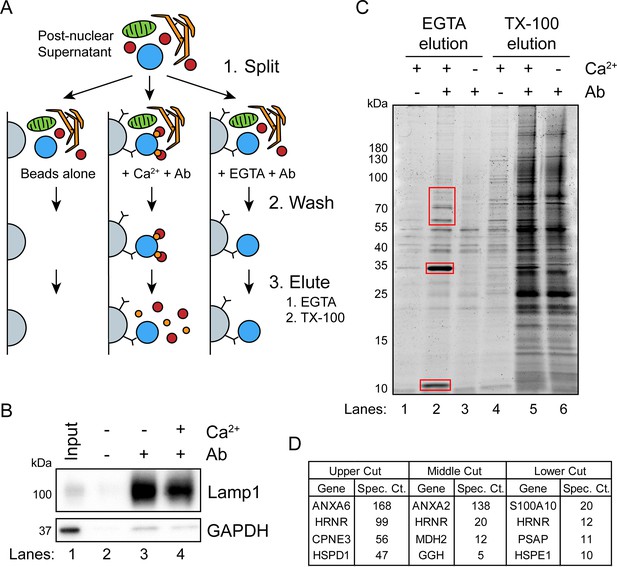
A targeted proteomics approach identifies genes important for Ca2+-dependent exosome secretion.
(A) Schematic illustrating the isolation of Ca2+-dependent multivesicular body (MVB)/lysosome binding proteins (Ab: antibody; gray-beads, blue-MVB, green-mitochondria, orange-ER, red-proteins, and gold-Ca2+). (B) Immunoblot analysis of LAMP1 and GAPDH from the TX-100 elutions, relative to the input, is shown. (C) Total protein gel (Sypro Ruby stained) of eluted fractions is shown. Red boxes indicate gel cuts sent for proteomic analysis. (D) Table depicting the top four proteomic hits from each gel cut are shown, excluding keratin family proteins.
-
Figure 3—source data 1
Uncropped immunoblot and gel images corresponding to Figure 3.
- https://cdn.elifesciences.org/articles/86556/elife-86556-fig3-data1-v2.zip
-
Figure 3—source data 2
Mass spectrometry analysis of proteins recruited to multivesicular bodies (MVBs) in the presence of Ca2+.
- https://cdn.elifesciences.org/articles/86556/elife-86556-fig3-data2-v2.xlsx
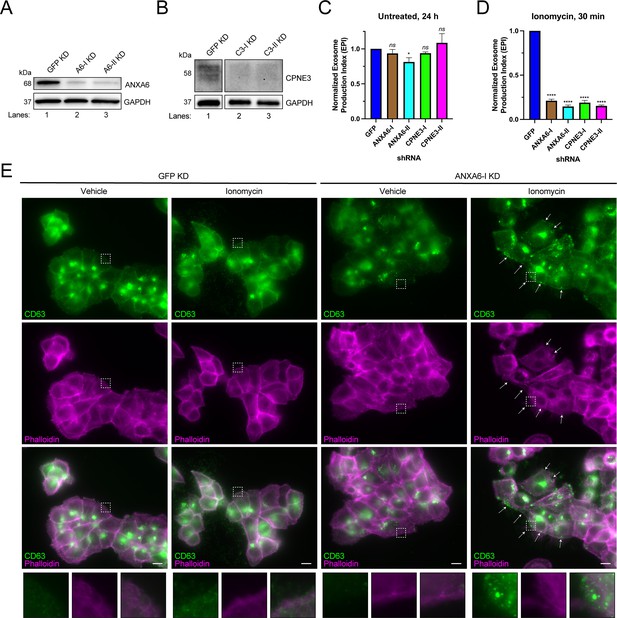
ANXA6 depletion blocks ionomycin-mediated exosome secretion and stalls multivesicular bodies (MVBs) at the cell periphery.
(A) Immunoblot analysis of ANXA6 and GAPDH expression from GFP, ANXA6-I, and ANXA6-II shRNA CD63-nanoluciferase (Nluc) cells is shown. (B) Immunoblot analysis of CPNE3 and GAPDH expression from GFP, CPNE3-I, and CPNE3-II shRNA CD63-Nluc cells are shown. (C) Normalized exosome production derived from GFP, ANXA6-I, ANXA6-II, CPNE3-I, and CPNE3-II shRNA CD63-Nluc cells grown in conditioned medium for 24 hr are shown. (D) Normalized exosome production derived from GFP, ANXA6-I, ANXA6-II, CPNE3-I, and CPNE3-II shRNA CD63-Nluc cells treated with 5 µM ionomycin for 30 min are shown. Data plotted represent the means from three independent experiments, and error bars represent SDs. Statistical significance was performed using an ANOVA (*p<0.05, ****p<0.0001, and ns = not significant). (E) CD63 immunofluorescence and phalloidin staining of GFP or ANXA6-I shRNA CD63-Nluc cells after 30 min of DMSO or 5 µM ionomycin treatment are shown. White arrows indicate peripheral CD63 puncta. Scale bars: 10 µm.
-
Figure 4—source data 1
Uncropped immunoblot images corresponding to Figure 4.
- https://cdn.elifesciences.org/articles/86556/elife-86556-fig4-data1-v2.zip
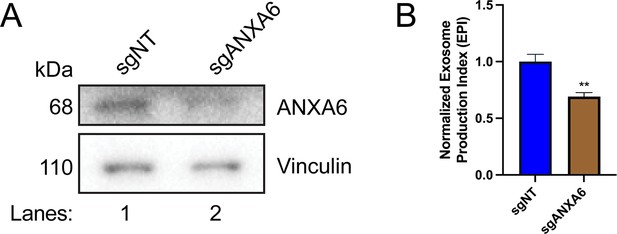
Exosome secretion from polyclonal ANXA6 KO cells.
(A) Immunoblot analysis of ANXA6 and vinculin expression from sgNT and sgANXA6 CD63-Nluc cells are shown. (B) Normalized exosome production derived from sgNT and sgANXA6 CD63-Nluc cells treated with 5 µM ionomycin for 30 min is shown. Data plotted represent the means from three independent experiments, and error bars represent SDs. Statistical significance was performed using a Student’s T-test (**p<0.01).
-
Figure 4—figure supplement 1—source data 1
Uncropped immunoblot images corresponding to Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/86556/elife-86556-fig4-figsupp1-data1-v2.zip
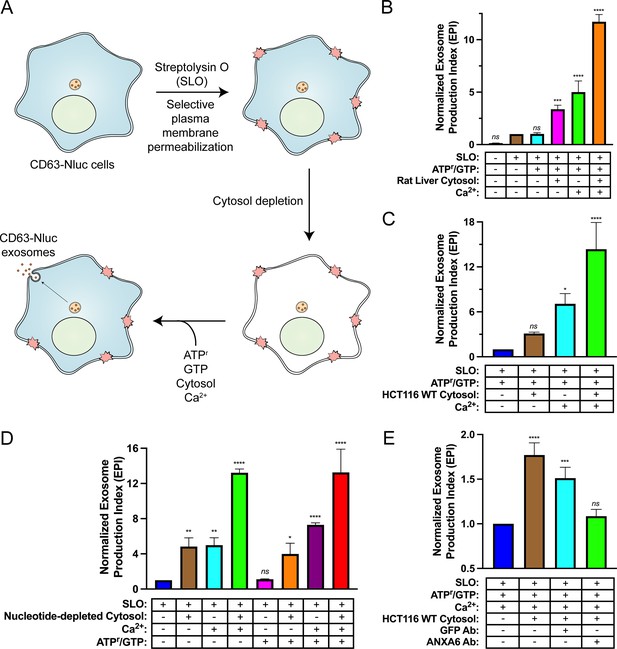
Biochemical reconstitution of Ca2+- and ANXA6-dependent exosome secretion in permeabilized cells.
(A) Schematic illustrating the permeabilized cell exosome secretion assay. (B) Permeabilized cell exosome secretion reactions with or without SLO, ATP regeneration system (ATPr)/GTP, rat liver cytosol, and Ca2+ are indicated. (C) Permeabilized exosome secretion assays with or without HCT116 WT cytosol and Ca2+ are shown. (D) ATP requirements for the permeabilized exosome secretion assay. Reactions with or without nucleotide-depleted rat liver cytosol, Ca2+, and ATPr/GTP are indicated. (E) Requirement of ANXA6 in the permeabilized exosome secretion assay. Reactions with or without HCT116 WT cytosol, an anti-GFP rabbit IgG antibody, and an anti-ANXA6 rabbit IgG antibody are depicted. Data plotted represent the means from three independent experiments, and error bars represent SDs. Statistical significance was performed using an ANOVA (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, and ns = not significant).
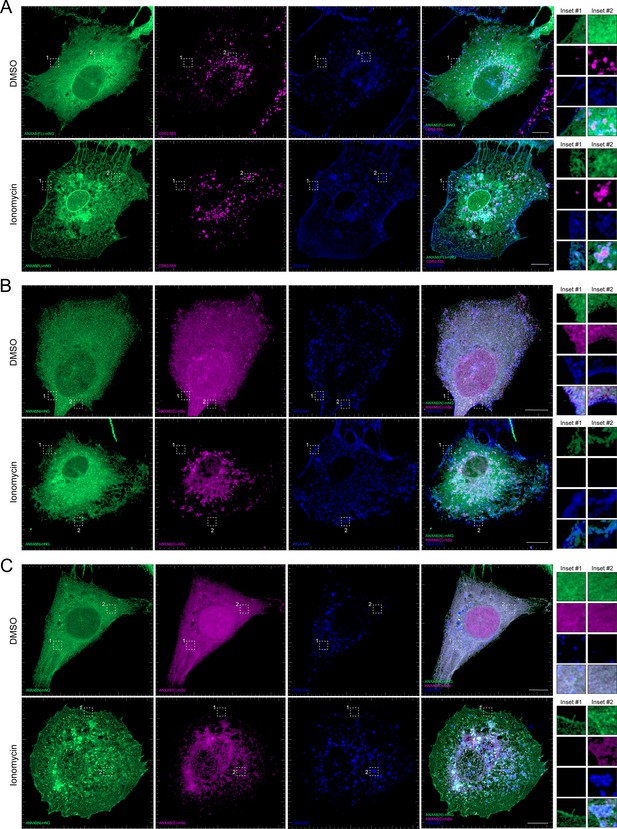
Localization of full-length and truncated ANXA6 constructs with or without ionomycin treatment.
(A) Airyscan 3D projection of U-2 OS cells expressing ANXA6(FL)-mNG with endogenous CD63 and wheat germ agglutinin (WGA) counterstain upon treatment with DMSO or 1 µM ionomycin for 10 min. Green: ANXA6(FL)-mNG; magenta: endogenous CD63 immunofluorescence; blue: WGA CF640R conjugate. Scale bars: 10 µm. (B) Airyscan 3D projection of U-2 OS cells expressing ANXA6(N)-mNG and ANXA6(C)-mSc with WGA counterstain upon treatment with DMSO or 1 µM ionomycin for 10 min. Green: ANXA6(N)-mNG; magenta: ANXA6(C)-mSc; blue: WGA CF640R conjugate. Scale bars: 10 µm. (C) Airyscan 3D projection of U-2 OS cells expressing ANXA6(N)-mNG and ANXA6(C)-mSc with endogenous CD63 upon treatment with DMSO or 1 µM ionomycin for 10 min. Green: ANXA6(N)-mNG; magenta: ANXA6(C)-mSc; blue: endogenous CD63 immunofluorescence. Scale bars: 10 µm.
Rotating 3D projection of DMSO-treated U-2 OS merge in Figure 6A.
Green: ANXA6(FL)-mNG; magenta: endogenous CD63 immunofluorescence; blue: WGA CF640R conjugate.
Rotating 3D projection of ionomycin-treated U-2 OS merge in Figure 6A.
Green: ANXA6(FL)-mNG; magenta: endogenous CD63 immunofluorescence; blue: wheat germ agglutinin (WGA) CF640R conjugate.
Rotating 3D projection of DMSO-treated U-2 OS merge in Figure 6B.
Green: ANXA6(N)-mNG; magenta: ANXA6(C)-mSc; blue: wheat germ agglutinin (WGA) CF640R conjugate.
Rotating 3D projection of ionomycin-treated U-2 OS merge in Figure 6B.
Green: ANXA6(N)-mNG; magenta: ANXA6(C)-mSc; blue: wheat germ agglutinin (WGA) CF640R conjugate.
Rotating 3D projection of DMSO-treated U-2 OS merge in Figure 6C.
Green: ANXA6(N)-mNG; magenta: ANXA6(C)-mSc; blue: endogenous CD63 immunofluorescence.
Rotating 3D projection of ionomycin-treated U-2 OS merge in Figure 6C.
Green: ANXA6(N)-mNG; magenta: ANXA6(C)-mSc; blue: endogenous CD63 immunofluorescence.
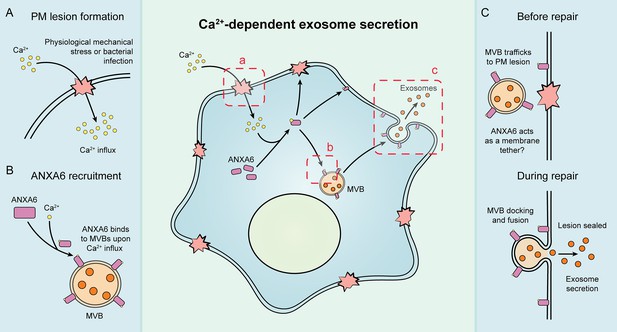
Schematic depicting the current model of Ca2+- and ANXA6-dependent exosome secretion.
(A) Upon physiological mechanical stress or bacterial infection, plasma membrane lesions form. This results in the flow of Ca2+ from the extracellular space into the cytoplasm. (B) This influx of Ca2+ mediates the recruitment of ANXA6 to multivesicular bodies (MVBs), which are then transported on microtubules to a plasma membrane lesion. (C) The MVBs then dock at the plasma membrane (with ANXA6 serving as a putative membrane tether) and undergo fusion, resulting in plasma membrane repair and exosome secretion.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Homo sapiens) | ANXA6 | Addgene | RRID: Addgene_29509 | N/A |
| Gene (Homo sapiens) | CPNE3 | N/A | N/A | N/A |
| Antibody | Anti-ANXA2 (rabbit monoclonal) | Abcam | Catalog #: ab178677 | WB: (1:1000) |
| Antibody | Anti-ANXA6 (rabbit monoclonal) | Abcam | Catalog #: ab201024 | WB: (1:1000), see Materials and methods for further details. |
| Antibody | Anti-CD9 (rabbit monoclonal) | Cell Signaling | RRID: AB_2798139 | WB: (1:1000) |
| Antibody | Anti-CD63 (mouse monoclonal) | BD Biosciences | RRID: AB_396297 | WB: (1:1000), IF: (1:250) |
| Antibody | Anti-CPNE3 (rabbit polyclonal) | Sigma | RRID: AB_10600703 | WB: (1:1000) |
| Antibody | Anti-Flotillin-2 (mouse monoclonal) | BD Biosciences | RRID: AB_397766 | WB: (1:1000) |
| Antibody | Anti-GAPDH (rabbit monoclonal) | Cell Signaling | RRID: AB_561053 | WB: (1:1000) |
| Antibody | Anti-GFP (rabbit polyclonal) | Torrey Pines Biolabs | RRID: AB_10013661 | See Materials and methods for further details. |
| Antibody | Anti-LAMP1 (rabbit monoclonal) | Cell Signaling | RRID: AB_2687579 | WB: (1:1000) |
| Antibody | Anti-Nluc (mouse monoclonal) | Promega | Catalog #: N7000 | See Materials and methods for further details. |
| Antibody | Anti-Tubulin (mouse monoclonal) | Abcam | RRID: AB_2241126 | IF: (1:250) |
| Antibody | Anti-TSG101 (mouse monoclonal) | GeneTex | RRID: AB_373239 | WB: (1:1000) |
| Antibody | Anti-Vinculin (rabbit monoclonal) | Abcam | RRID: AB_11144129 | WB: (1:1000) |
| Cell line (Homo sapiens) | HCT116 CD63Nluc-KI #17 | Hikita et al., 2018 | N/A | Dr. Chitose Oneyama (Aichi Cancer Center Research Institute) |
| Cell Line (Homo sapiens) | HCT116 | Other | N/A | Cell culture facility at UC Berkeley |
| Cell line (Homo sapiens) | HEK293T | Other | N/A | Cell culture facility at UC Berkeley |
| Cell line (Homo sapiens) | HEK293T FLAG-Nluc-CD63 | This study | N/A | To assess exosome secretion in HEK293T cells |
| Cell line (Homo sapiens) | U-2 OS | Other | N/A | Cell culture facility at UC Berkeley |
| Chemical compound and drug | Ionomycin | Cayman Chemical | Catalog #: 10004974 | See Materials and Methods for further details. |
| Recombinant DNA reagent | pLKO.1_Hygro GFP shRNA | This study | N/A | shRNA target sequence: ACAACAGCCACAACGTCTAT |
| Recombinant DNA reagent | pLKO.1_Hygro ANXA2-1 shRNA | This study | N/A | shRNA target sequence: ACTTTAGAAACACGTACTTTG |
| Recombinant DNA reagent | pLKO.1_Hygro ANXA2-2 shRNA | This study | N/A | shRNA target sequence: TGAGGGTGACGTTAGCATTAC |
| Recombinant DNA reagent | pLKO.1_Hygro ANXA6-1 shRNA | This study | N/A | shRNA target sequence: AGTTGGACATGCTCGACATTC |
| Recombinant DNA reagent | pLKO.1_Hygro ANXA6-2 shRNA | This study | N/A | shRNA target sequence: CGAAGACACAATCATCGATAT |
| Recombinant DNA reagent | pLKO.1_Hygro CPNE3-1 shRNA | This study | N/A | shRNA target sequence: ACTCTATGGACCAACTAATTT |
| Recombinant DNA reagent | pLKO.1_Hygro CPNE3-2 shRNA | This study | N/A | shRNA target sequence: AGCATTCTTTCTAGGTTATTT |
| Recombinant DNA reagent | lentiCRISPR v2-Blast sgNT (non-targeting) | This study | N/A | Protospacer sequence: GCCCCGCCGCCCTCCCCTCC |
| Recombinant DNA reagent | lentiCRISPR v2-Blast sgANXA6 | This study | N/A | Protospacer sequence: AGCCTCCAGGTCCCGCTCGT |
| Recombinant DNA reagent | pLJM1-L30-FLAG-Nluc-CD63-hPGK-BlastR | This study | N/A | To express FLAG-Nluc-CD63 in various cell lines under control of the low-expression L30 promoter |
| Recombinant DNA reagent | pN1-mNeonGreen (mNG) | This study | N/A | To assess the localization of mNeonGreen-tagged proteins |
| Recombinant DNA reagent | pN1-mScarlet-i (mSc) | This study | N/A | To assess the localization of mScarlet-i-tagged proteins |
| Recombinant DNA reagent | pN1_ANXA6(FL; aa1-673)-mNG | This study | N/A | To assess localization of full-length ANXA6 |
| Recombinant DNA reagent | pN1_ANXA6(N; aa1-322)-mNG | This study | N/A | To assess localization of N-terminal truncation of ANXA6 |
| Recombinant DNA reagent | pN1_ANXA6(C; aa323-673)-mSc | This study | N/A | To assess localization of C-terminal truncation of ANXA6 |
| Software, algorithm | Prism 9 | GraphPad | RRID:SCR_002798 | N/A |
| Peptide, recombinant protein | Alexa Fluor 680 Phalloidin | Invitrogen | N/A | IF: (1:400) |
| Peptide, recombinant protein | CF640R Wheat Germ Agglutinin Conjugate | Biotium | N/A | IF: (5 µg/ml) |
| Peptide, recombinant protein | Streptolysin O (SLO) | Sigma-Aldrich | N/A | See Materials and methods for further details. |





