A Pvr–AP-1–Mmp1 signaling pathway is activated in astrocytes upon traumatic brain injury
Figures

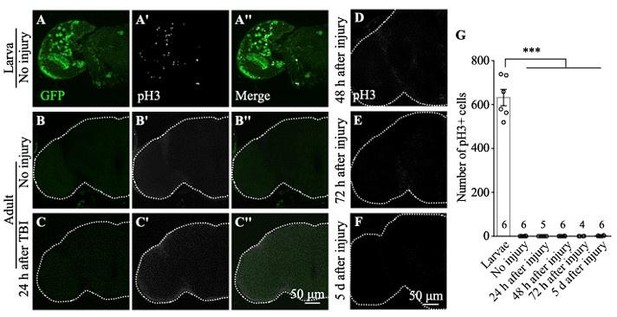
Traumatic brain injury (TBI) is induced in adult flies by a high-impact trauma (HIT) device.
(A) MI24 is the mortality index calculated from the percentage of dead over total flies. Increasing the number of strikes from 4 to 6 increases the MI24 value. Error bars denote standard error of the mean (SEM; ***p<0.001, Student’s t-test). (B–E) The intactness of blood–eye barrier (BEB) and blood–brain barrier (BBB) before or after six strikes is indicated by the accumulated fluorescence intensity at the border of the eye (B, D) and brain (C, E), respectively. The examination was carried out at 2 hr after fluorescent dye injection. Arrows indicate the hemolymph exclusion line. Scale bar = 100 μm. (F) The percentage of permeable BEB with or without TBI (***p<0.001). (G) The percentage of permeable BBB following different numbers of strikes. Error bars denote SEM (**p <0.01 and ***p <0.001, Student’s t-test). (H) Histogram showing mRNA expression levels of innate immune response genes of the AMP family (Attc, DiptB, and Mtk) in the adult brain. Expression was increased in all cases following treatment. The RPL28 gene served as an internal reference. Results are means ± SEM (***p <0.001, Student’s t-test). (I, J) The number of Dcp-1+ puncta (green) increased in injured adult brains 24 hr after TBI. Scale bar = 50 μm. (K) Quantification of Dcp-1+ puncta. Error bars denote SEM (**p<0.01, Student’s t-tests). Results are means ± SEM. (L–M'') Apoptotic debris labeled with anti-Dcp-1 antibodies (Red). Glial and neuronal nuclei are labeled with anti-Repo (L) and anti-Elav (M) antibodies (blue), respectively. Scale bar = 20 μm.

Traumatic brain injury (TBI) alters glial morphology and upregulates Mmp1 expression.
(A–C) Representative images of astrocyte membranes labeled with mCD8::GFP driven by the astrocyte driver R86E01-Gal4. Co-expression of UAS-Redstinger labels astrocyte nuclei. GFP intensities are increased at 4 hr (B) and 24 hr (C) after injury. The rectangle defined by white dash lines denotes antennal lobes. Scale bars = 100 μm. (D) Quantification of GFP intensity at different timepoints after TBI. Results are means ± SEM (**p<0.01, Student’s t-test). (E, F) MARCM (y w UAS-CD8::GFP hs-FLP; tubP-Gal80 FRT-40A/CyO; alrm-Gal4 UAS-CD8::GFP UAS-Dcr2/TM6 Tb Hu cross with FRT-40A flies) analysis reveals single astrocytes with evenly distributed GFP+ cellular processes without injury (E) and an increased number of GFP+ puncta at 24 hr after injury (F). Arrows denote GFP+ accumulations in astrocyte processes. White dots demarcate neuropils. Anti-Repo antibodies labeled glial nuclei. TO-PRO labeled all nuclei. Insets show the morphology of a glial soma before and after injury. Scale bar = 10 μm. (G) Quantification of GFP+ puncta in astrocyte processes before and after injury. (H) Quantification of the astrocyte soma diameter surrounding antennal lobes (ALs) after injury. Results are means ± SEM (***p<0.001, Student’s t-test). (I–J'', M–N'') Representative images of ALs co-labeled with GFP and Mmp1 (magenta) in uninjured and injured adult brains. (J–J'') GFP-labeled astrocyte membranes (green) overlapped with Mmp1 (magenta) after injury. (N–N'') After injury, Mmp1 co-localizes with GFP-labeled ensheathing glia (ENG) (R56F03-Gal4) membranes (green). Arrows show Mmp1-GFP colocalization in (L'') and (P''). Scale bar = 20 μm. (K, L) Graph showing fluorescence intensity per pixel in each channel for the inset in (I'') and (J'') of the astrocyte membrane and Mmp1 along a line drawn through a cell body. (O, P) Graph showing fluorescence intensity per pixel in each channel for the inset in (M'') and (N'') of the ENG membrane and Mmp1 along a line drawn through the process of ENG. (Q) Quantification of Mmp1 intensity in (I–J'') and (M–N''). Ast is the short of astrocytes. Results are means ± SEM (**p <0.01, Student’s t-test).
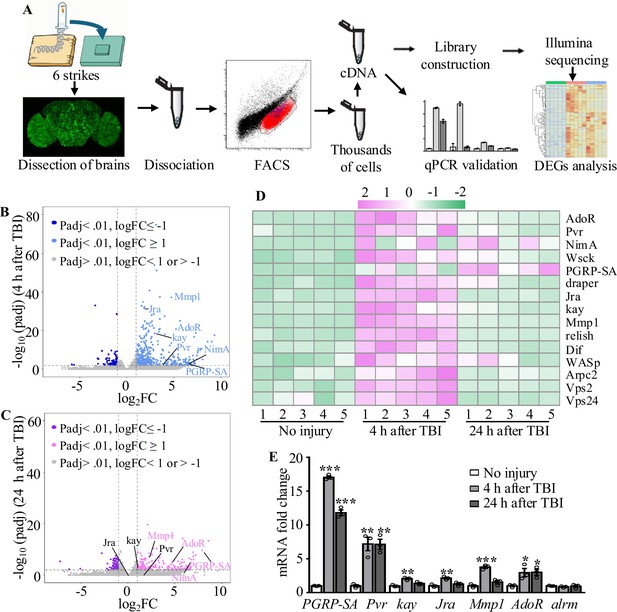
Transcriptome analysis of astrocytes reveals upregulation of injury-related and innate immunity genes.
(A) After dissection, Drosophila brains were disrupted and the cell suspension was subjected to fluorescence-activated cell sorting (FACS). Astrocyte cDNAs were amplified by the Smart-seq2 method and a cDNA library was constructed. A subset of upregulated genes from RNA-seq screening was validated by quantitative real-time PCR. (B, C) Volcano plots of the gene expression analysis; 522 differentially expressed genes (416 upregulated, 106 downregulated) in astrocytes were detected at 4 hr after injury (B), and 357 differentially expressed genes (229 upregulated, 129 downregulated) in astrocytes were detected at 24 hr after injury (C). The threshold for differential expression was set at log2FC = 1 with an adj. p<0.01. FC = fold change; adj. = adjusted (for multiple comparisons by the Benjamin–Hochberg procedure). (D) Heatmap of the expression of a small set of representative genes, including draper, Jra, and kay, previously identified as essential factors during glial clearance of severed axons. (E) Quantitative real-time PCR validation of a subset of upregulated genes from RNA-seq screening. Error bars represent SEM. Results are means ± SEM (*p<0.05, **p<0.01, and ***p<0.001 by Student’s t-tests).
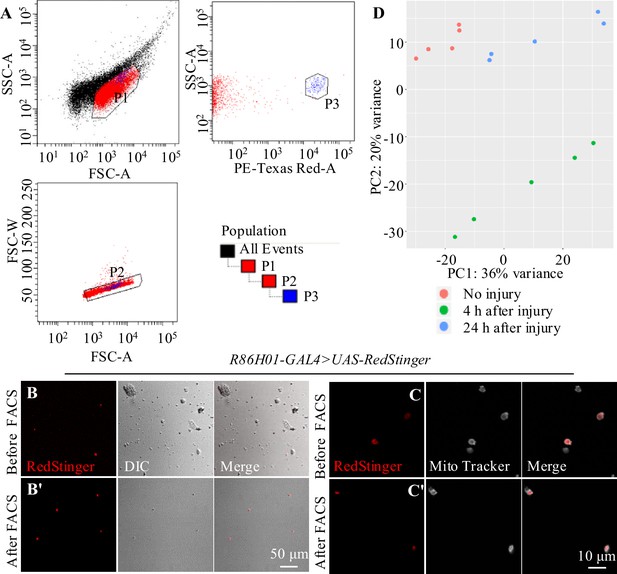
Sorting of adult astrocytes by fluorescence-activated cell sorting (FACS).
(A) To discriminate between healthy and damaged or dying cells, side scatter (SSC-A, log) and forward scatter (FSC-A, log) were plotted. Higher SSC-A signals indicate increased granularity and therefore likely dying cells. Thus, one should define the sorting gate as the population with the lower SSC-A signal (P1). (P2) Exclude cell clusters by sorting only events with a low FSC width (FSC-W) versus similar area (FSC-A) signal to get exact single cell. (P3) To gate for RFP+ cells, plotted the signal from the excitation of cells with the 568-nm laser (PE-Texas Red-A, log) against the signal from the SSC-A. The population with the higher PE-Texas Red-A signal was the candidate cells cluster. The strategy for FACS is shown on the lower-right corner. (B–C') A population of cells with strong RFP signal was purified from adult flies of R86E01-Gal4>UAS-Redstinger. (B, B') Confocal images of representative astrocytes before and after sorting by FACS. Scale bar = 50 μm. (C, C') As cell-permeant MitoTracker labels mitochondria of live cells, cells after FACS both labeled by RFP and MitoTracker. Scale bar = 10 μm. (D) A principal component analysis of all biological replicates (uninjured versus injured) revealing genes expressed at absolute value of logarithmic fold change (log2FC) > 1.0.
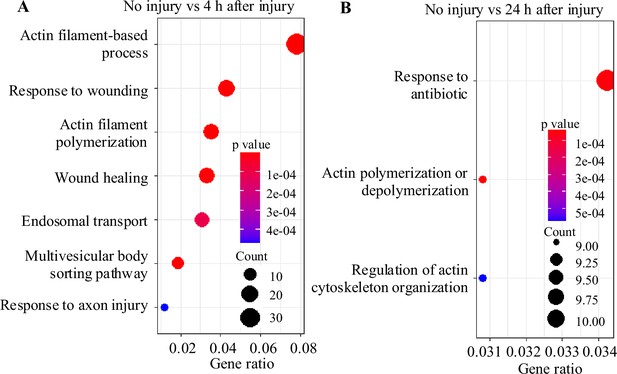
Gene Ontology (GO) analysis of differentially expressed genes at 4 hr and 24 hr after injury.
Scatterplot showing GO functional enrichment results for differentially expressed genes of astrocytes in the brain at 4 hr (A) and 24 hr (B) after injury, p<0.01.
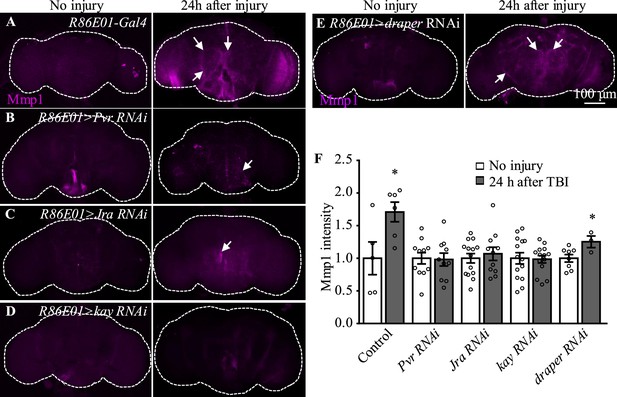
Pvr, Jra, and kay are required for Mmp1 upregulation after traumatic brain injury (TBI).
(A–F) Knockdown of Pvr, Jra, or kay by R86E01-Gal4-driven RNAi in astrocytes suppresses Mmp1 upregulation after TBI. Scale bar = 100 μm. All images are projections of 25–30 μm immunostaining slices (one image per 1 μm slice). Arrows show Mmp1 signals. (F) Quantification of Mmp1 intensity in different genotypes. Results are means ± SEM (*p<0.05 by Student’s t-test).
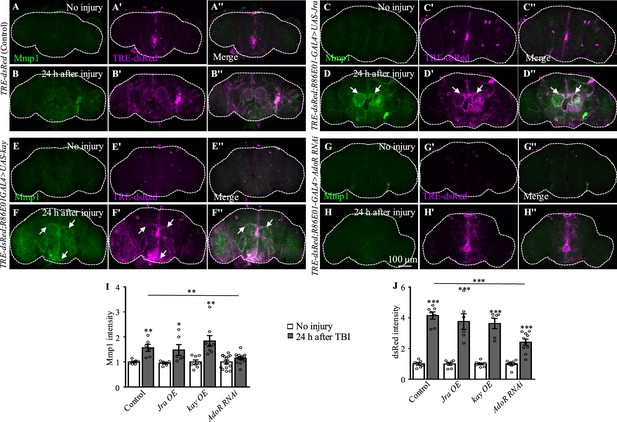
Overexpression of Jra or kay does not enhance the expression of TRE-RFP and Mmp1 in adult brain after traumatic brain injury (TBI).
(A, B'') Fly brains showing TRE-RFP expression before and 24 hr after TBI. (C–F'') overexpression of Jra or kay in astrocytes shows no apparent changes of RFP and Mmp1 staining signals compared with control flies with or without brain injury (TRE-dsRed/UAS-Jra or kay; R86E01-Gal4/+). Scale bar = 100 μm. (G, H) Knockdown of AdoR in astrocytes inhibits the expression of Mmp1 after TBI. However, Knockdown of AdoR in astrocytes does not inhibit the expression of dsRed. (I, J) Quantification of Mmp1 and dsRed intensity before and after injury in different genotypes. Data are present as means ± SEM; *p<0.05, **p<0.01, ***p<0.001 by Student’s t-tests.
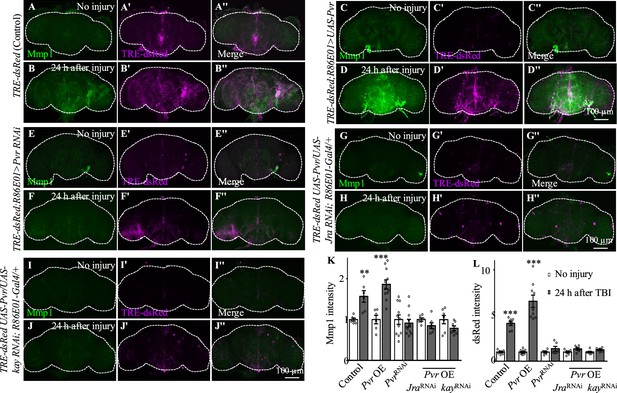
Pvr is required for AP-1 upregulation after traumatic brain injury (TBI).
(A−B'') Adult brains showing increased TRE-dsRed (an AP-1 reporter) expression at 24 hr after TBI. (C−D'') Overexpression of Pvr in astrocytes leads to upregulated dsREd expression compared with control flies. Scale bar = 100 μm. (E−F'') Knockdown of Pvr in astrocytes causes downregulation of dsRed expression compared with control flies after TBI. (G−H'') Overexpression of Pvr and knockdown of Jra at the same time in astrocytes causes downregulation of dsRed and Mmp1 after TBI compared with controls. (I−J'') Overexpression of Pvr and knockdown of kay in astrocytes at the same time causes downregulation of dsRed and Mmp1 after TBI compared with controls. (K, L) Quantification of Mmp1 (K) and dsRed (L) intensity. Results are means ± SEM. **p<0.01, ***p<0.001, Student’s t-tests.
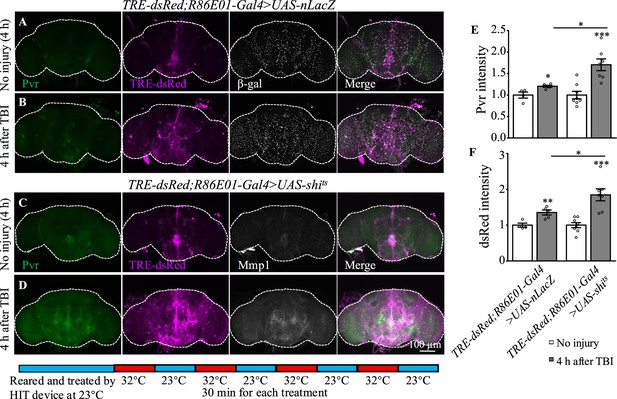
Inhibition of dynamin activity increases the expression levels of Pvr and dsRed after traumatic brain injury (TBI).
(A, B) Representative Pvr immunostaining (green), dsRed (magenta), and β-gal intensity (gray) in control animals (TRE-dsRed;R86E01-Gal4>UAS-nLacZ) at 4 hr after TBI. All images are projections of 25–30 μm immunostaining slices (one image per 1 μm slice). (C, D) Representative Pvr immunostaining (green), dsRed (magenta), and Mmp1 intensity (gray) in shits-expressing animals (TRE-dsRed;R86E01-Gal4>UAS-shits) at 4 hr after TBI. For thermogenetic inactivation of dynamin, flies after TBI were incubated at intermittent temperature at 32°C (32°C for 30 min, and then back to 23°C for 30 min, for four rounds, 4 hr in total) until dissection. (E, F) Quantification of Pvr and dsRed intensity in different genotypes. Results are means ± SEM (*p<0.05).

Knockdown of endocytic trafficking-related genes up-regulates expression of AP-1 and Mmp1 upon traumatic brain injury (TBI).
(A, H) Representative Mmp1 immunostaining (magenta) and dsRed intensity (magenta) in control animals (R86E01-Gal4) at 24 hr after TBI. (B–D, I–K) Knockdown of Arpc2, cpa, and cpb in astrocytes results in further upregulation of Mmp1 and dsRed upon TBI. All images are projections of 25–30 μm immunostaining slices (one image per 1 μm slice). (E–G, L–N) Knockdown of Stam, Chmp1, or Vps24, components of endosomal sorting complex required for transport (ESCRT), in astrocytes had no influence on Mmp1 and dsRed upregulation compared with control after TBI (A, H). All images are projections of 25–30 μm immunostaining slices (one image per 1 μm slice). (O, P) Quantification of Mmp1 and dsRed intensity in different genotypes. Results are means ± SEM (*p<0.05, **p<0.01, ***p<0.001, Student’s t-tests). (Q) Schematic diagram of Pvr–AP-1–MMP1 signaling activated by TBI in astrocytes. AP-1 transcription factor complex is composed of Jra and kay.
Tables
Upregulated genes related to receptors and endocytosis.
| Genes in Drosophila | Conserved genes in mouse | Conserved genes in human | 4 hr after injury(FC) | 24 hr after injury (FC) |
|---|---|---|---|---|
| Pvr | pdgfr/vegfr | VEGFR | 13.83 | 2.79 |
| AdoR | Adora2a | ADORA2B | 37.53 | 7.73 |
| Mmp1 | Mmp14 | MMP14 | 26.35 | 4.79 |
| Jra | Jun | JUN | 3.58 | 1.18 |
| kay | FOSL2 | FOS | 6.23 | 1.77 |
| cpa | Capza2 | CAPZA1 | 3.43 | 1.72 |
| cpb | Capzb | CAPZB | 2.01 | 1.25 |
| Stam | Stam2 | STAM | 2.14 | 1.46 |
| Chmp1 | Chmp1b | CHMP1B | 2.16 | 0.92 |
| Vps24 | Chmp3 | CHMP3 | 2.11 | 0.87 |
-
FC: fold change.
Additional files
-
Supplementary file 1
Primer sequences for the RT-qPCR analysis.
- https://cdn.elifesciences.org/articles/87258/elife-87258-supp1-v1.xlsx
-
Supplementary file 2
DEGs after TBI.
- https://cdn.elifesciences.org/articles/87258/elife-87258-supp2-v1.xlsx
-
Supplementary file 3
Upregulated gene list after TBI.
- https://cdn.elifesciences.org/articles/87258/elife-87258-supp3-v1.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/87258/elife-87258-mdarchecklist1-v1.docx