The zebrafish mutant dreammist implicates sodium homeostasis in sleep regulation
Figures
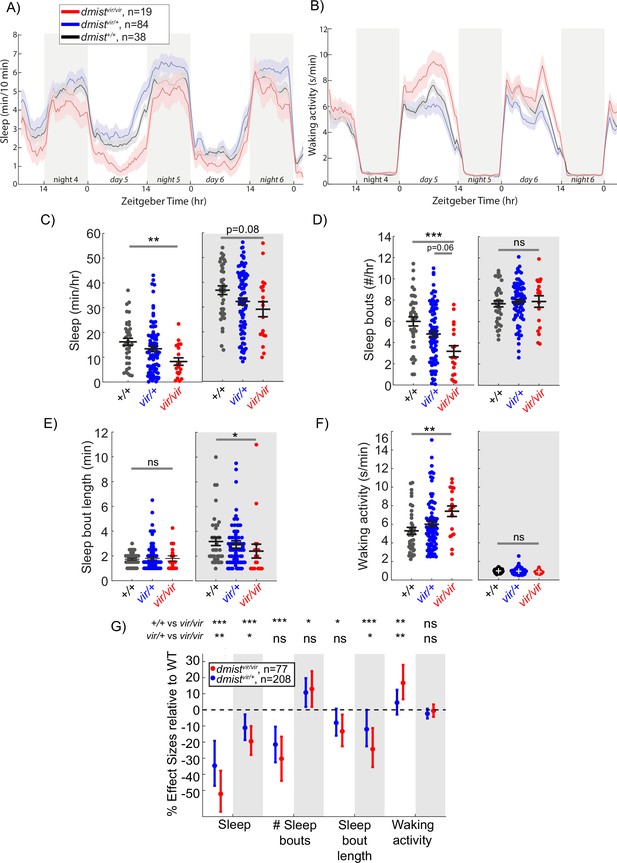
A viral insertion mini-screen identifies a short-sleeping mutant, dreammist.
(A, B) Mean ± SEM sleep (A) and waking activity (B) of progeny from dmistvir/+ in-cross from original screen. White blocks show day (lights on) and grey blocks show night (lights off). Data is combined from two independent experiments. n indicates the number of animals. (C–F) Analysis of sleep/wake architecture for the data shown in (A, B). (C) Quantification of total sleep across 2 d and nights shows decreased day and night sleep in dmistvir/vir. Analysis of sleep architecture reveals fewer sleep bouts during the day (D) and shorter sleep bouts at night (E) in dmistvir/vir compared with sibling controls. (F) Daytime waking activity is also increased in dmistvir/vir. The black lines show the mean ± SEM, except in (E), which labels the median ± SEM. *p<0.05, **p<0.01, ***p<0.001; ns p>0.05; one-way ANOVA, Tukey’s post hoc test. (G) Combining five independent experiments using a linear mixed effects model with genotype as a fixed effect and experiment as a random effect reveals dmistvir/vir larvae have decreased total sleep and changes to sleep architecture during both the day and night compared to dmist+/+ siblings. Plotted are the genotype effect sizes (95% confidence interval) for each parameter relative to wild type. Shading indicates day (white) and night (grey). p-Values are assigned by an F-test on the fixed effects coefficients from the linear mixed effects model. *p<0.05, **p<0.01, ***p<0.001, ns p>0.05. n indicates the number of animals.
-
Figure 1—source data 1
Gene selection for screening.
Annotations of genes selected in the screening pipeline.
- https://cdn.elifesciences.org/articles/87521/elife-87521-fig1-data1-v1.xlsx
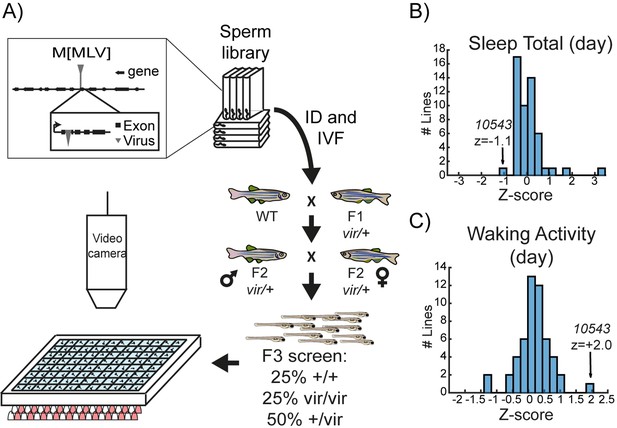
A viral insertion screen for sleep-wake regulators.
(A) Schematic of screening strategy. Candidate genes were selected from a list of 904 mammalian genes encoding protein classes most often linked to behavioural regulation, including (1) genes previously implicated in sleep and circadian rhythms; (2) G-protein coupled receptors; (3) neuropeptide ligands; (4) channels; and (5) proteins involved in post-translational regulation, such as de-ubiquitinating enzymes (Figure 1—source data 1). tBLASTN of the human protein sequences identified 1162 zebrafish orthologs (Zv6), of which 702 (60.4%) had viral insertions mapped in the ‘Zenemark’ zebrafish viral insertion library (Varshney et al., 2013). Sperm harbouring viral insertions in 26 loci were successfully used for in vitro fertilisation and propagated to the F3 generation for screening. F3 larvae from single family F2 in-crosses were monitored on a 14 hr:10 hr light:dark cycle from 4 to 7 dpf using videography and genotyped at the end of the experiment. (B, C) Histogram of total daytime sleep (B) and average daytime waking activity (C) normalised as standard deviations from the mean (Z-score) of all the viral-insertion lines tested (including heterozygous vir/+ and homozygous vir/vir). Line 10543 (renamed dreammist) exhibited decreased daytime sleep and increased daytime waking activity.
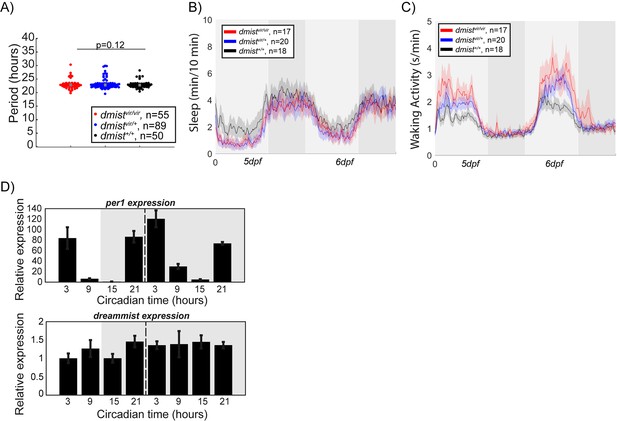
dmistvir/vir fish are hyperactive and have normal circadian rhythms.
(A) Free-running circadian period length of the locomotor activity of larvae from a dmistvir/+ in-cross following the transition at 5 dpf from a 14 hr:10 hr light:dark cycle to constant dark conditions. The data is quantified for 48 hr after the shift to darkness and shows no difference in period between dmistvir/vir larvae and their sibling controls. Data is from three independent experiments. p>0.05, one-way ANOVA, Tukey’s post hoc test. (B, C) Representative mean ± SEM sleep (B) and waking activity (C) traces of animals used to calculate circadian period length in (A). Light and dark grey blocks show subjective day and night, respectively. (D) RT-qPCR time-course before (light) and after (grey) transfer into constant dark demonstrates that dmist mRNA levels do not oscillate with a circadian period, unlike per1 mRNA which does. n = 3 replicates per timepoint. Expression is normalised to circadian time 3. Data are mean ± SEM.
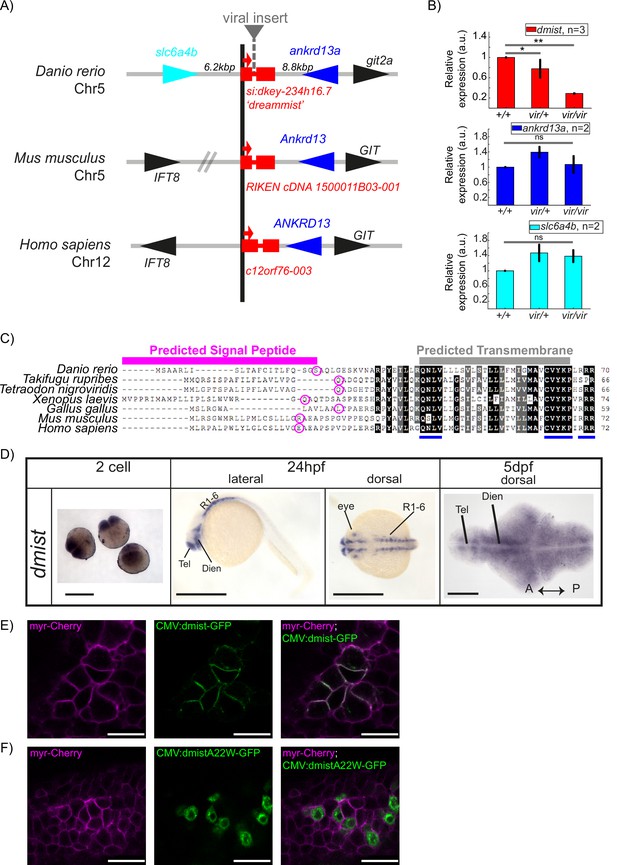
dmist encodes a conserved vertebrate single-pass transmembrane protein.
(A) dmist mutants harbour a viral insertion in the first intron of si:key-234h16.7. dmist is syntenic with Ankrd13 and GIT orthologs in mouse, human, and zebrafish. (B) RT-qPCR of dmist (red) show reduced expression of dmist and not the 5′ and 3′ flanking zebrafish genes, slc6a4b (cyan) and ankrd13a (blue), in dmistvir/vir larvae compared to dmistvir/+ and dmist+/+ siblings. **p<0.01, *p<0.05; ns p>0.05; one-way ANOVA, Tukey’s post hoc test. Data shows mean ± SEM normalised to the wild-type mean. (C) dmist_Dr contains an open-reading frame encoding a 70 amino acid protein that is conserved across vertebrates. All identified homologues have a predicted signal peptide sequence (magenta line), signal peptide cleavage site (magenta circle), and predicted transmembrane domain (grey), with additional highly conserved C-terminal motifs (blue lines). Identical amino acids in all species are shown in black; similar amino acids (80–99% conserved across species) are shown in grey. (D) In situ hybridisation using a dmist antisense probe reveals dmist is maternally deposited as it is detected at the two-cell stage. At 24 hpf, expression is restricted to regions containing neuronal precursors, and at 5 dpf expression is widespread throughout the brain. Tel, telencephalon; Dien, diencephalon; R1-6, rhombomeres 1–6; A, anterior; P, posterior. Scale bars = 0.5 mm (two-cell and 24 hpf), 0.1 mm (5 dpf). (E, F) Representative confocal image of 90% epiboly embryo co-injected at the one-cell stage with mRNA encoding membrane-RFP (magenta) and a plasmid encoding either C-terminal tagged Dmist-GFP (E, green) or DmistA22W-GFP (F, green). Scale bar = 25 μm.
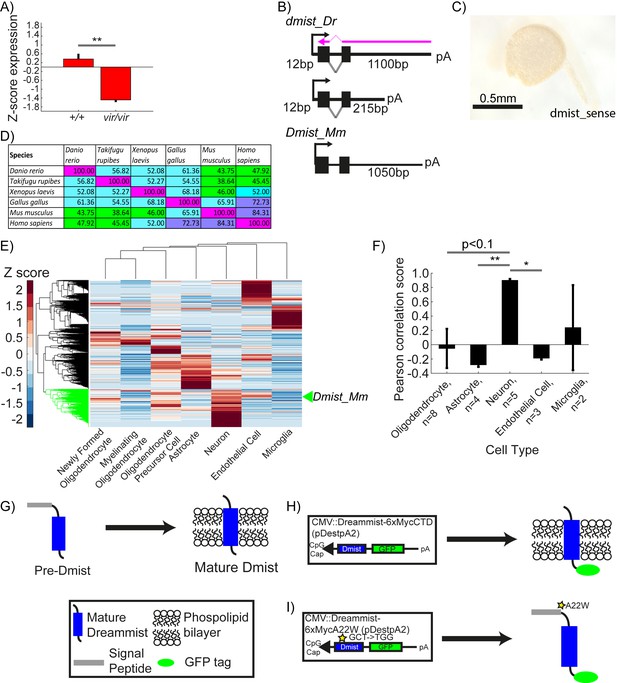
dmist is enriched in neurons and requires the signal peptide cleavage site for membrane localisation.
(A) Relative expression level of dmist transcript from RNA sequencing of 6 dpf dmistvir/vir and dmist+/+ siblings. Z-scores were calculated by subtracting mean expression and normalising by the standard deviation across all expressed transcripts (27,243 transcripts). Data show mean ± SEM from three independent biological replicates. **p<0.01 Student’s t-test. (B) 3′ and 5′ RACE identify a long (1100 bp) and short (215 bp) 3′UTR variant in dmist_Dr, and a long 3′UTR (1050 bp) in Dmist_Mm. The purple arrow indicates the ISH probe used in Figure 2D. (C) dmist_Dr sense probe negative control at 24 hpf shows no detectable expression. (D) Percentage identity matrix comparing Dmist homologues across six vertebrate species (100% = magenta; >70% = purple; >50% = cyan; <50% = green). (E) Hierarchical clustering of RNAseq dataset of six different cell types isolated from the developing (E13.5) mouse brain (Zhang et al., 2014) and post hoc identification of Dmist_Mm. Data was standardised by subtracting the mean expression and normalising by the standard deviation across all expressed transcripts in each cell type (column). Dmist_Mm (green arrow) co-clusters with genes highly expressed in neurons (green shaded branches).(F) Pearson rank correlation of canonical cell-type markers with Dmist_Mm shows high co-expression with neuronal markers compared to astroglial and endothelial cell markers. Data are mean ± SEM. *p<0.05, **p<0.01; Kruskal–Wallis, Dunn–Sidak post hoc test. (G–I) Predicted processing of Dmist to its mature form in the plasma membrane (G). C-terminal GFP fusion to Dmist is predicted to localise to the membrane (H). However, a mutation (A22W) at the signal peptide cleavage site (I) is predicted to inhibit signal peptide cleavage and so prevent proper subcellular localisation of the mature protein.
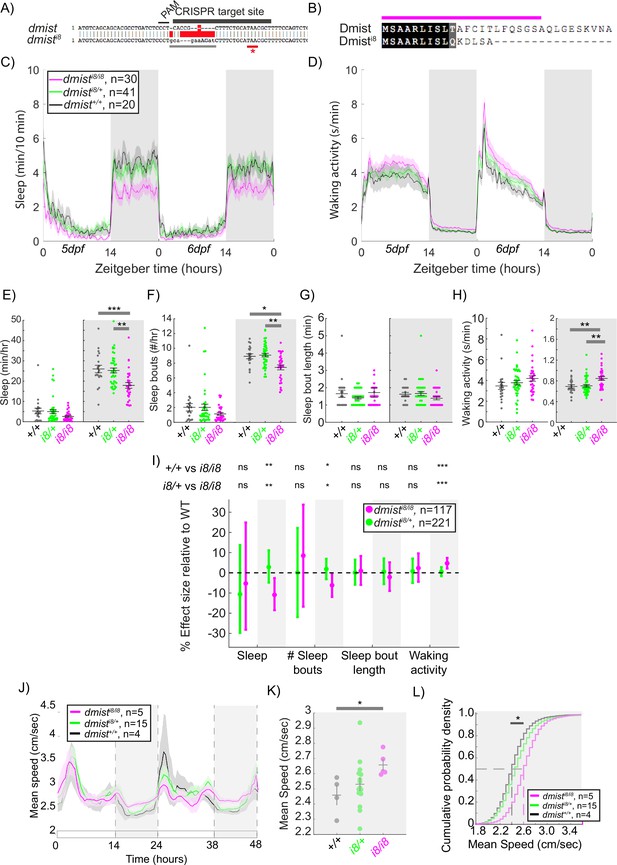
CRISPR-generated dmist mutants sleep less and are hyperactive at night.
(A) CRISPR/Cas9 targeting of the first exon of dmist resulted in an 8 bp insertion (dmisti8) (grey line) within the coding sequence, leading to an early stop codon (red line with *). Guide RNA target sequence and PAM sequence are shown as black bars. The sequence that is deleted in the mutant is indicated with a red bar. (B) Predicted Dmisti8 peptide sequence lacks most of the N-terminal signal peptide sequence (magenta) and the full C-terminus. (C, D) Representative 48 hr traces of mean ± SEM sleep (C) and waking activity (D) shows decreased sleep and increased waking activity at night for dmisti8/i8 fish compared to dmisti8/+ and dmist+/+ siblings. n = number of fish. (E–H) Analysis of sleep/wake architecture of the experiment depicted in (C, D) indicates that dmisti8/i8 larvae sleep less at night (E) due to fewer sleep bouts (F). Sleep bout length is unchanged (G). Waking activity is also increased in dmisti8/i8 fish (H). The black line represents the mean ± SEM except for (G), which is the median ± SEM. *p<0.05, **p<0.01, ***p<0.001; one-way ANOVA, Tukey’s post hoc test. (I) Combining five independent experiments with a linear mixed effects model reveals dmisti8/i8 fish sleep less at night due to fewer sleep bouts and also show increased waking activity at night. Plotted are the genotype effect sizes (95% confidence interval) for each parameter relative to wild type. Shading indicates day (white) and night (grey). p-Values are assigned by an F-test on the fixed effects coefficients from the linear mixed effects model. *p<0.05, **p<0.01, ***p<0.001, ns p>0.05. (J) Adult dmisti8/i8 fish have a higher mean swim speed compared to their wild-type siblings at night. Data in (J) is quantified at night in (K). (J, K) show mean ± SEM. *p<0.05, one-way ANOVA. (L) Cumulative probability distribution of all night-time swim bout speeds in adult fish. The dashed lines show the half max (0.5 probability) for each curve. *p<0.05 for dmisti8/i8 fish compared to wild-type siblings; Kolmogorov–Smirnov test.
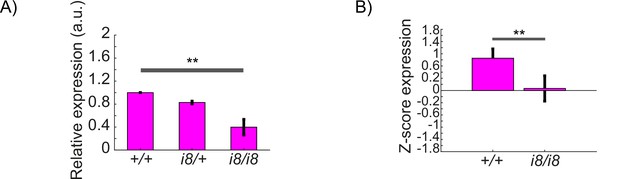
CRISPR-generated dmist mutants have reduced dmist transcript levels.
(A) RT-qPCR shows dmisti8/i8 larvae have reduced dmist mRNA levels, suggesting that dmisti8 transcripts undergo nonsense mediated decay. Data are mean ± SEM of three biological replicates. **p<0.01; one-way ANOVA, Tukey’s post hoc test. (B) Relative expression level of dmist transcript from RNA sequencing of 6 dpf dmisti8/i8 and dmist+/+ siblings. Z-score calculated by subtracting mean expression and normalising by the standard deviation across all expressed transcripts. Data are mean ± SEM for three independent biological replicates. **p<0.01, Student’s t-test.
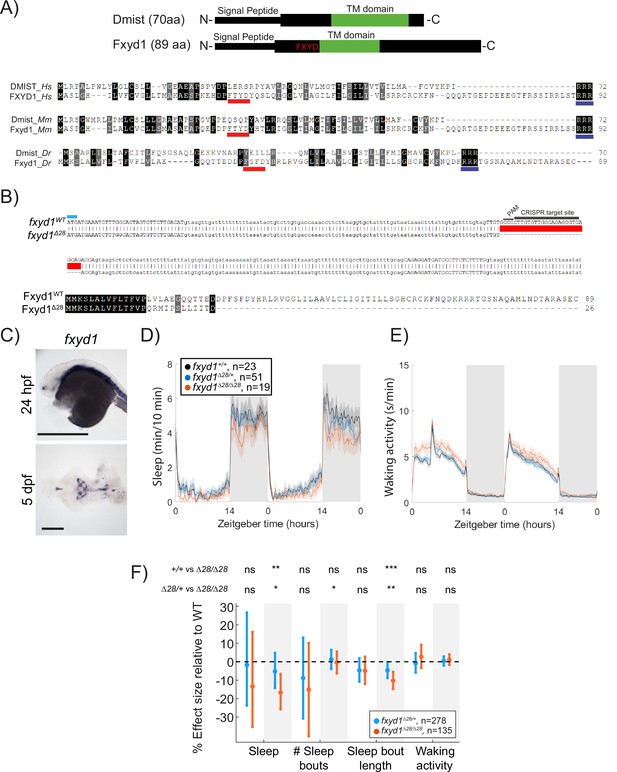
Mutation of the dmist-related gene fxyd1 causes reduced sleep at night.
(A) Schematic of zebrafish Dmist and Fxyd1 protein domains and alignments comparing human, mouse, and zebrafish Dmist and FXYD1 protein sequences. Black and grey shading indicate amino acid identity and similarity, respectively. The FXYD domain is indicated with a red line and the RRR motif in the C-terminus is indicated with a dark blue line. (B) CRISPR-Cas9 targeting of the third exon of fxyd1 created a 28 bp deletion, resulting in a predicted truncated protein. The start codon is marked by a cyan line. Guide RNA target sequence and PAM sequence are shown as black bars. The mutant deleted sequence is indicated with a red bar.(C) In situ hybridisation of fxyd1 at 24 hpf (whole animal) and 5 dpf brain (ventral view). Anterior is to the left. Scale bar = 0.5 mm (24 hpf); 0.1 mm (5 dpf). (D, E) Representative behavioural experiment showing fxyd1Δ28 mutants have decreased night-time sleep (D) but normal waking activity at night (E). (F) Combining five independent experiments with a linear mixed effects model reveals fxyd1Δ28/ Δ28 larvae sleep significantly less at night due to shorter sleep bouts compared to fxyd1+/+ siblings. Plotted are the genotype effect sizes (95% confidence interval) on each parameter relative to wild type. Shading indicates day (white) and night (grey). p-Values are assigned by an F-test on the fixed effects coefficients from the linear mixed effects model. *p<0.05, **p<0.01, ***p<0.001, ns p>0.05.
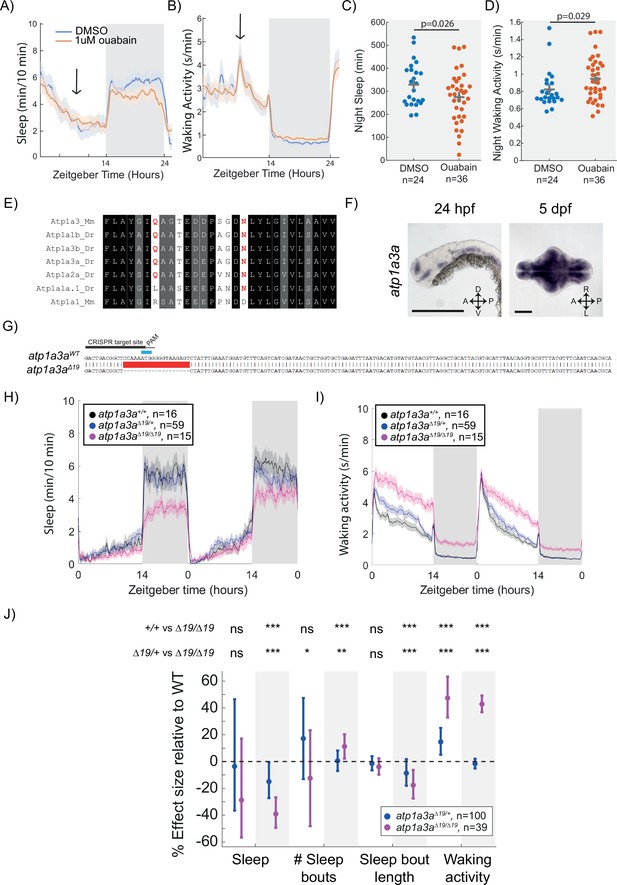
Mutation of the Na+/K+ pump alpha subunit atp1a3a reduces sleep at night.
(A, B) Sleep and waking activity traces (± SEM) of wild-type larvae following exposure to 1 µM ouabain. Arrows indicate time the drug was added. (C, D) At night, sleep is significantly reduced and waking activity is significantly increased after ouabain exposure. Student’s t-test, one-tailed. (E) Alignments of Na+/K+ pump alpha subunits around the ouabain binding sites. Red indicates residues that are critical for higher sensitivity to ouabain, both of which are present in mouse Atp1a3 but not Atp1a1. (F) In situ hybridisation of atp1a3a at 24 hpf (whole animal) and 5 dpf brain (ventral view). Anterior is to the left. Scale bar = 0.5 mm (24 hpf); 0.1 mm (5 dpf). A, anterior; P, posterior; D, dorsal; V, ventral (G) CRISPR-Cas9 targeting of the atp1a3a resulted in a 19 bp deletion that eliminates the start codon (blue) and splice junction. Guide RNA target sequence and PAM sequence are shown as black bars. Sequence that is deleted in the mutant is indicated with a red bar. (H, I) Representative behavioural experiment showing atp1a3aΔ19/Δ19 fish are hyperactive throughout the day-night cycle and have decreased sleep at night. Mean ± SEM are shown. (J) atp1a3aΔ19/ Δ19 larvae sleep less at night due to shorter sleep bouts. Plotted are the genotype effect sizes (95% confidence interval) on each parameter relative to wild type. Shading indicates day (white) and night (grey). p-Values are assigned by an F-test on the fixed effects coefficients from the linear mixed effects model. *p<0.05, **p<0.01, ***p<0.001, ns p>0.05.
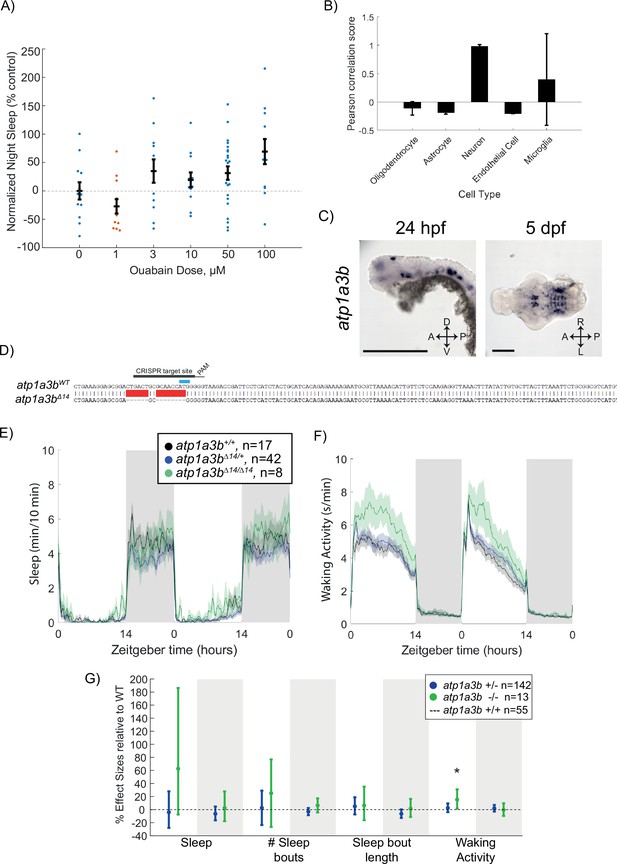
Ouabain dose curve and effects of atp1a3b mutation on behaviour.
(A) Dose–response curve of ouabain’s effects on sleep at night, shown as mean ± SEM and normalised to the DMSO control. Each data point represents a single fish. (B) Pearson rank correlation of canonical cell-type markers with Atp1a3a_Mm shows high co-expression with neuronal markers compared to astroglial and endothelial cell markers. Data are mean ± SEM. (C) In situ hybridisation of atp1a3a at 24 hpf (whole animal) and 5 dpf brain (ventral view). Anterior is to the left. Scale bar = 0.5 mm (24 hpf); 0.1 mm (5 dpf). (D) CRISPR-Cas9 targeting of atp1a3b resulted in a 14 bp deletion that eliminates the start codon (blue). Guide RNA target sequence and PAM sequence are shown as black bars. The sequence that is deleted in the mutant is indicated with a red bar. (E, F) Representative single behavioural experiment showing atp1a3bΔ14/Δ14 mutants have increased daytime waking activity but normal sleep patterns. (G) Data from two independent experiments combined with a linear mixed effects model. Plotted are the genotype effect sizes (95% confidence interval) for each parameter relative to wild type (dotted line) for each genotype. Shading indicates day (white) and night (grey). n indicates the number of animals. p-Values are assigned by an F-test on the fixed effects coefficients from the linear mixed effects model relative to atp1a3b+/+ animals. *p<0.05.
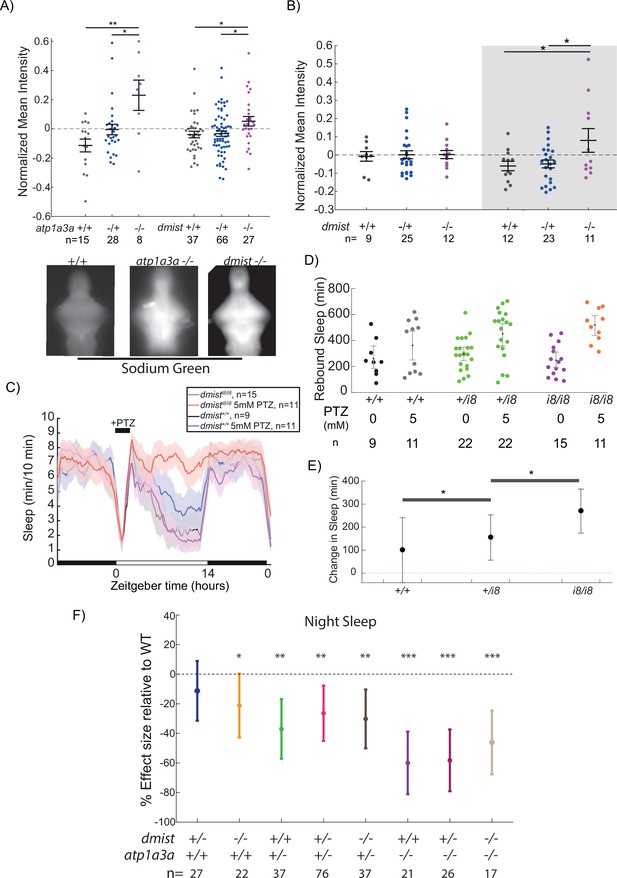
dmist mutants have altered sodium homeostasis.
(A) Brain sodium levels are significantly elevated after exposure to pentylenetetrazol (PTZ) in both atp1a3aΔ19/Δ19 (two independent experiments) and dmisti8/i8 (four independent experiments) fish relative to wild type and heterozygous mutant siblings, as measured by fluorescence intensity of Sodium Green, normalised to the sample mean intensity. Crosses show mean ± SEM. n indicates the number of animals. Below are example images of brains stained with Sodium Green. *p<0.05, **p<0.01, one-way ANOVA, Tukey’s post hoc test. (B) Under baseline conditions, brain sodium levels are significantly elevated in dmisti8/i8 fish at night but not during the day, as measured by fluorescence intensity with Sodium Green. Crosses show mean ± SEM. *p<0.05, **p<0.01, one-way ANOVA, Tukey’s post hoc test. (C) dmisti8/i8 larvae have increased rebound sleep compared to wild-type siblings following exposure to 5 mM PTZ. Representative sleep traces of dmist+/+ (no drug, water vehicle controls in black; PTZ exposed in blue) and dmisti8/i8 (no drug in purple; PTZ exposed in red) following 1 hr exposure to 5 mM PTZ (black bar) in the morning. Data are mean ± SEM. dmisti8/+ animals are not plotted for clarity but are included in panel (D). (D) Rebound sleep after exposure to 5 mM PTZ, calculated from the experiment in (C). Each dot represents a single fish, grey lines show mean ± SEM. (E) Effect size of change in sleep after 1 hr treatment with 5 mM PTZ (and washout) compared to vehicle-treated controls (error bars show 95% confidence intervals). *p<0.05, one-way ANOVA, Tukey’s post hoc test. (F) Effect sizes (and 95% confidence interval) relative to wild types (dotted line) on sleep at night in larvae from dmist+/-; atp1a3a+/- in-crosses from three independent experiments. p-Values are assigned by an F-test on the fixed effects coefficients from the linear mixed effects model relative to dmist+/+; atp1a3a+/+ animals. For all sleep-wake parameters, see Figure 6—figure supplement 1. *p<0.05, **p<0.01, ***p<0.0001, ns p>0.05.

Sleep effects in dmist-/-; atp1a3a-/- double mutants are non-additive.
Combining three independent experiments with a linear mixed effects model reveals that the effects of loss-of-function dmist and atp1a3a mutations are non-additive. Plotted are the genotype effect sizes (95% confidence interval) for each parameter relative to wild type for each genotype. Shading indicates day (white) and night (grey). n indicates the number of animals.
Tables
Zebrafish lines used in the article.
Strain designation | Allele number | Gene identifier | Additional information |
|---|---|---|---|
| 10543/dmistvir | la015577Tg | ENSDARG00000095754 | Maintained at UCL |
| dmisti8 | u505 | ENSDARG00000095754 | Maintained at UCL |
| fxyd1Δ28 | u504 | ENSDARG00000099014 | Maintained at UCL |
| atp1a3aΔ19 | u513 | ENSDARG00000018259 | Maintained at UCL |
| atp1a3b Δ14 | u514 | ENSDARG00000104139 | Maintained at UCL |
Primer sequences used in the article.
| Oligo name | Sequence (5' -> 3') | Anneal temperature (°C) | Application | |
|---|---|---|---|---|
| 1 | dmist_vir_fw | CACAGGGATGTGATGCCGGTTAAC | 55 | dmistvir genotyping |
| 2 | dmist_vir_rev | GTAGACACATACTGCCATACCAATC | 55 | dmistvir genotyping |
| 3 | vir_fw | CACCAGCTGAAGCCTATAGAGTACGAGC- | 55 | dmistvir genotyping |
| 4 | dmist_Dr_5RACE_fw | CGTTTCGCCACAATGTCAGCA | 55–65 | dmist_Dr 5'RACE |
| 5 | dmist_Dr_5RACE_rev_outer | AATGTTCAACTCCAGGCGTC | 55–65 | dmist_Dr 5'RACE |
| 6 | dmist_Dr_5RACE_rev_inner | AATGTTCAACTCCAGGCGTC | 55–65 | dmist_Dr 5'RACE |
| 7 | dmist_Dr_3RACE_fw_inner | GACGCCTGGAGTTGAACATT | 55–65 | dmist_Dr 3'RACE |
| 8 | dmist_Dr_3RACE_fw_outer | GGTATGGCAGTATGTGTCTACA | 55–65 | dmist_Dr 3'RACE |
| 9 | Dmist_Mm_3RACE_outer | GCTGGTGACTGTCCTCCTTATG | 55–65 | dmist_Mm 3'RACE |
| 10 | Dmist_Mm_3RACE_inner | GTGTCTACAAGCCCATCCGTC | 55–65 | dmist_Mm 3'RACE |
| 11 | dmist_Dr_fw | TTTCGCCACAATGTCAGCAGC | 56 | dmist_Dr probe |
| 12 | dmist_Dr_rev | CGACTTTCATTTATTAGTTCAGACATGTC | 56 | dmist_Dr probe |
| 13 | qPCR_dmist_fw | ACGCCAGACCTTATGAAATCC | 60 | RT-qPCR |
| 14 | qPCR_dmist_rev | TGCGTCGGAGAGGTTTGTAG | 60 | RT-qPCR |
| 15 | qPCR_ankrd13a_fw | TGGTGGCGTTCCAGAGTTAC | 60 | RT-qPCR |
| 16 | qPCR_ankrd13a_rev | GGACACGAGAGGAATCCAGC | 60 | RT-qPCR |
| 17 | qPCR_slc6a4b_fw | ACATGGTTGGGTCGACGTTT | 60 | RT-qPCR |
| 18 | qPCR_slc6a4b_rev | TCCAACCCACCAAAAGTGCT | 60 | RT-qPCR |
| 19 | ef1alpha_fw | TGCTGTGCGTGACATGAGGCAG | 60 | RT-qPCR |
| 20 | ef1alpha_rev | CCGCAACCTTTGGAACGGTGT | 60 | RT-qPCR |
| 21 | SP6dmist_sgRNA | ATTTAGGTGACACTATAGCGTTATGCAGAAAGCGGTGGTTTTAGAGCTAGAAATAGCAAG | n/a | CRISPR |
| 22 | T7atp1a3a_sgRNA | TAATACGACTCACTATAGACTGACGGCTCCAAAATGGGTTTTAGAGCTAGAAATAGCAAG | n/a | CRISPR |
| 23 | SP6fxyd1_sgRNA | ATTTAGGTGACACTATAGGACCCTCTGCCAACACAAGGTTTTAGAGCTAGAAATAGCAAG | n/a | CRISPR |
| 24 | SP6atp1a3b_sgRNA | ATTTAGGTGACACTATAGGACTGACTGCGCAACCATGGTTTTAGAGCTAGAAATAGCAAG | n/a | CRISPR |
| 25 | HRM_dmist_fw | GCCACAATGTCAGCAGCACG | 59 | HRM |
| 26 | HRM_dmist_rev | GCGTTCACTTTAGACTCTCCCAGC | 59 | HRM |
| 27 | HRM_atp1a3a_fw | TGACAGACTGAAGAAACAGC | 55 | HRM |
| 28 | HRM_atp1a3a_rev | TTAAATCTCAGCACCAGCAG5 | 55 | HRM |
| 29 | HRM_fxyd1_fw | TGACCAAACCTTCTTAAGGTGC | 58 | HRM |
| 30 | HRM_fxyd1_rev | AAATTGAGAAGACTTACTGGTCTGC | 58 | HRM |
| 31 | HRM_atp1a3b_fw | AAAGGCTGTCACTTTCTCCATCAC5 | 58 | HRM |
| 32 | HRM_atp1a3b_rev | TGCAGTAGATGAGGAATCGGTC | 58 | HRM |
| 33 | MiSeq_dmist_fw | TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGTATAACTTACGTGTGGACGGACTC | 58 | MiSeq |
| 34 | MiSeq_dmist_rev | GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGTTGCCTCAGCAGGATTTCATAAG | 58 | MiSeq |
| 35 | MiSeq_atp1a3a_fw | TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGTCGTTATCCGTGCAAGAGCTTC | 58 | MiSeq |
| 36 | MiSeq_atp1a3a_rev | GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGTTCTCAGCACCAGCAGTTATCG | 58 | MiSeq |
| 37 | MiSeq_atp1a3b_fw MiSeq_atp1a3b_rev | TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGTGACTGACATTCTCTCTTCGTG | 68 | MiSeq |
| 38 | GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGTTCTCTGTGATGCAGTAGATGAGG | 68 | MiSeq | |
| 39 | MiSeq_fxyd1_fw | TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGAAATACTGTCTTGTGACCAAACC | 57 | MiSeq |
| 40 | MiSeq_fxyd1_rev | GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGTTCATCCTCTGCTGCAAAATGC | 57 | MiSeq |
| 41 | attB1-dreammist forward primer | GGGGACAAGTTTGTACAAAAAAGCAGGCTTCACCATGTCAGCAGCACGCCTGATCTCC | 55–60 | Gateway |
| 42 | attB3-dreammist reverse primer | GGGGACCACTTTGTACAAGAAAGCTGGGTATCACCTGCGTCGGAGAGGTTTGTAG | 55–60 | Gateway |
| 43 | Dmist-GFPA22WFw | GCTTTTCCAGTCTGGGAGTTGGCAGCTGGGAGAGTCTAAAG | 66 | SDM |
| 44 | Dmist-GFPA22WRev | CTTTAGACTCTCCCAGCTGCCAACTCCCAGACTGGAAAAGC | 66 | SDM |





