Differential increase of hippocampal subfield volume after socio-affective mental training relates to reductions in diurnal cortisol
Figures
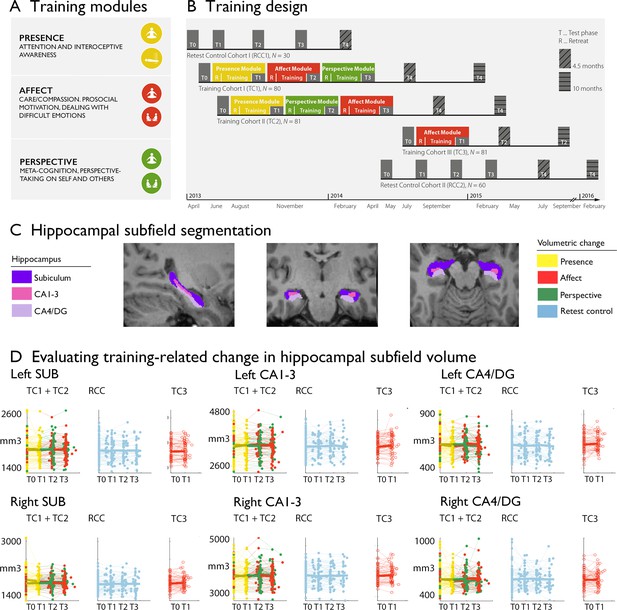
Training induced plasticity of hippocampal subfield volume.
(A) Training modules; (B) Training design; (C) Subfield volumes in left and right hemispheres across individuals and timepoints; (D) Scatterplot of subfield volumes as a function of timepoints and training cohorts. Panels A and B are reproduced from Figure 1A, B of Valk et al., 2023a.
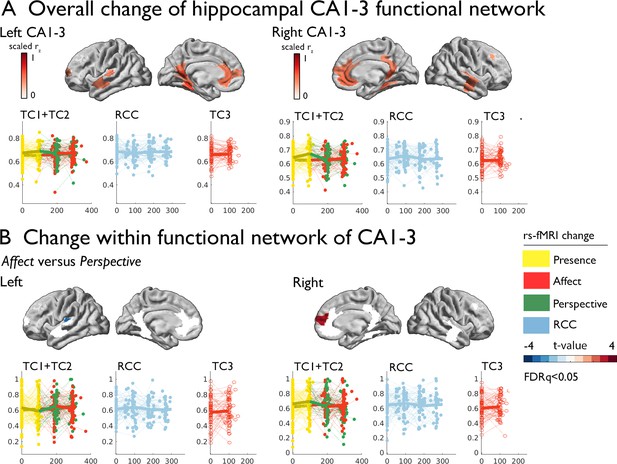
Training induced plasticity of CA1-3 functional connectivity.
(A) upper: CA1-3 functional connectivity at baseline, top 10% of regions representing the CA1-3 functional network; lower: scatter plot visualizing change within the CA1-3 network across timepoints and groups; networks and scatters of SUB and CA4/DG are available in the supplements; (B) Regional change within CA1-3 functional network Affect versus Perspective (FDRq <0.05); right: scatter plot visualizing mean change within the CA1-3, FDRq <0.05 regions across timepoints and groups.
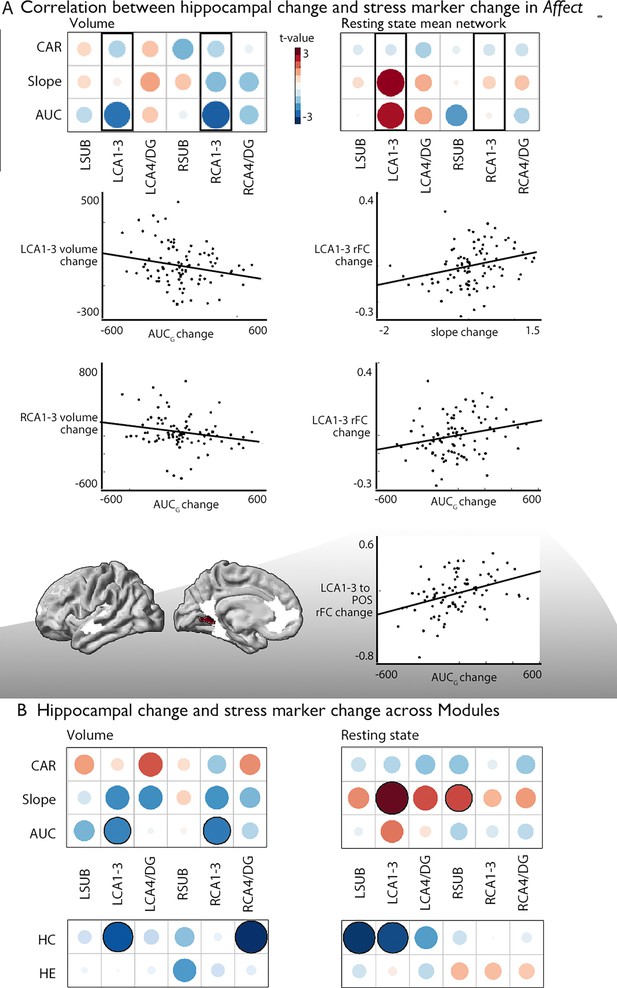
Associations between changes in structure and function of hippocampal subfield volume and markers of stress change.
(A). Upper left: Correlation between hippocampal subfield volume change in Affect and CAR, slope, and AUC markers of stress change, Upper right: Correlation between hippocampal subfield intrinsic functional change in Affect and CAR, slope, and AUC markers of stress change, middle: Scatter plots visualize the correlation between volume change and cortisol marker change (below p<0.05), bottom: region level change within left CA1-3, FDRq <0.05. CA1-3 is the focus of this analysis based on our group-level findings and highlighted with boxes in A; (B). Upper: Overall impact of diurnal cortisol markers on hippocampal subfield volume and function over Presence, Affect and Perspective; Lower: Overall impact of hair cortisol markers on hippocampal subfield volume and function over Presence, Affect and Perspective.
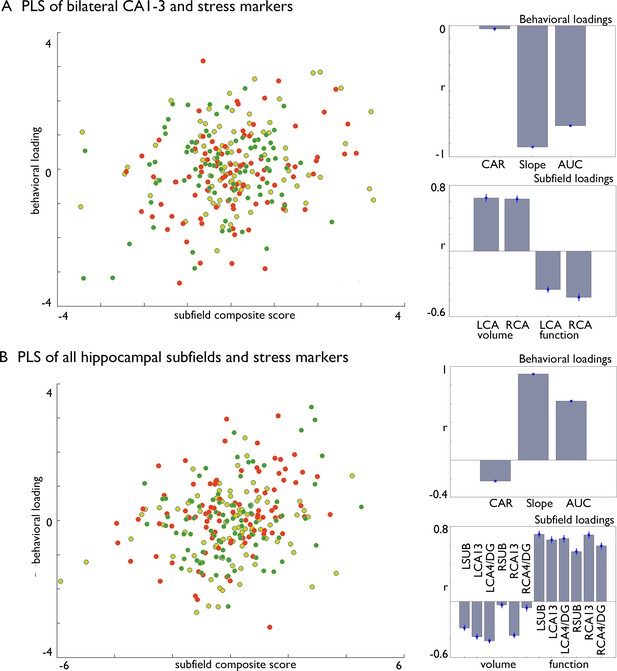
Multivariate associations between changes in structure and function of hippocampal subfield volume and markers of stress change in Affect.
(A). Multivariate associations between bilateral CA1-3 volume and intrinsic function and stress markers. Left: Scatter of loadings, colored by Training Module; Right upper: individual correlations of stress markers; Right lower: individual correlation of subfields; (B). Multivariate associations between all subfields’ volume and intrinsic function and stress markers. Left: Scatter of loadings, colored by Training Module; Right upper: individual correlations of stress markers; Right lower: individual correlation of subfields.
Tables
Sample size per timepoint.
| Structural MRI data | Structural and Functional MRI data | |
|---|---|---|
| T0 | 288 (TC3:71) | 258 (TC3: 70) |
| T1 | 272 (TC3:68) | 238 (TC3: 64) |
| T2 | 193 | 172 |
| T3 | 190 | 181 |
Reason for missing data across the study duration.
MR incidental findings are based on T0 radiological evaluations; participants who did not meet MRI quality control criteria refers to movement and/or artefacts in the T1-weighted MRI; dropout details can be found in Singer et al., 2016; no MRT: due to illness / scheduling issues / discomfort in scanner; other: non-disclosed; functional MRI missing: no complete functional MRI; functional MRI quality:>0.3 mm movement (low quality in volume +surface).
| Reason for dropout(TC1, TC2, RCC: N=251) | T0 | T1 | T2 | T3 |
|---|---|---|---|---|
| Structural MR incidental finding | 5 | (5 based on T0) | (5 based on T0) | (5 based on T0) |
| Structural MRI quality control | 7 | 6 | 4 | 2 |
| Dropout | 2 | 7 (2 based on T0) | 9 (7 based on T01) | 16 (9 based on T012) |
| Medical reasons | 1 | 7 (1 based on T0) | 8 (7 based on T01) | 15 (8 based on T012) |
| Other | 4 | 10 | 7 | 7 |
| Functional MRI missing/low QC | 29 | 30 | 21 | 9 |
| Hippocampal QC | 15 | 12 | 25 | 16 |
Reason for missing data across the study duration.
MR incidental findings are based on T0 radiological evaluations; participants who did not survive MRI quality control refers to movement and/or artefacts in the T1-weighted MRI; dropout details can be found in Singer et al., 2016; no MRT: due to illness / scheduling issues / discomfort in scanner; other: non-disclosed.
| Reason for dropout (TC3, N=81) | T0 | T1 |
|---|---|---|
| MR incidental finding | 3 | (3 based on T0) |
| MRI quality control | 0 | 0 |
| Dropout | 0 | 3 |
| Medical reasons | 1 | 2 |
| Other | 5 | 3 |
| Functional MRI missing | 1 | 4 |
| Hippocampal QC | 1 | 2 |
Changes in mean CA1-3 functional network between training and active control cohorts [T0-T1] and [T1-T3].
| Affect TC3 vs Presence | LCA1-3 | RCA1-3 |
|---|---|---|
| t-value | 0.366 | –0.411 |
| p- and q-value | p>0.1, q>0.1 | p>0.1, q>0.1 |
| Cohens D | 0.052 | –0.058 |
| Affect vs Perspective | ||
| t-value | 0.137 | 2.420 |
| p- and q-value | p=0.891, q>0.1 | p=0.016, q=0.032 |
| Cohens D | 0.016 | 0.289 |
Correlating change in CA1-3 subfield volume and diurnal cortisol indices in Affect.
| LCA1-3 | RCA1-3 | |
|---|---|---|
| CAR | –0,355, p>0.1 | –1,543, p>0.1 |
| Slope | –0,878, p>0.1 | –1,245, p>0.1 |
| AUCg | –2,237, p=0.028, q=0.056 | –2,283, p=0.025, q=0.05 |
Correlating change in CA1-3 subfield functional network and diurnal cortisol indices in Affect.
| LCA1-3 | RCA1-3 | |
|---|---|---|
| CAR | –0,476, p>0.1 | –0,425, p>0.1 |
| Slope | 2,653, p=0.009, q=0.018 | 0,773, p>0.1 |
| AUCg | 2,261, p=0.026, q=0.052 | 0,024, p>0.1 |
Multivariate PLS analyses linking cortisol markers to hippocampal subfield volume and function.
| LC1 | Overall | Presence | Affect | Perspective | |
|---|---|---|---|---|---|
| CA1-3 | p<0.01, 67% | r=0.20 | r=0.17 | r=0.27 | r=0.16 |
| all | p<0.01, 71% | r=0.24 | r=0.16 | r=0.30 | r=0.26 |
Additional files
-
Supplementary file 1
Supplementary analyses, descriptive statistics and supplementary tables.
(a) Descriptive statistics T0-T1. (b) Descriptive statistics T1-T3. (c) T0-T1 change statistics. (d) T1-T3 change statistics. (e) T1-T3 change statistics – Training cohort 1 and 2 Affect versus Perspective. (f) T1-T2 change. (g) T2-T3 change. (h) Subfield-specific changes following the Training Modules, controlling for the other two ipsilateral subfields. (i) Overall change in subfield volume. (j) Sex differences (female versus male) in hippocampal subfield volumes. (k) Descriptive statistics mean subfield functional network change T0-T1. (l) Descriptive statistics mean subfield functional network change T1-T3. (m) Functional connectivity network change T0-T1. (n) Functional connectivity network change T1-T3. (o) Functional connectivity network change T1-T3: Training cohort 1 and 2 Affect versus Perspective. (p) Functional connectivity network change T1-T2. (q) Functional connectivity network change T2-T3. (r) Correlating change in subfield volume and diurnal cortisol indices in Affect. (s) Association between stress-markers and within functional network sub-regions in Affect and Perspective. (t) Correlating change in subfield functional network and diurnal cortisol indices in Affect. (u) Correlating change in subfield volume and diurnal cortisol indices in Presence. (v) Correlating change in subfield volume and diurnal cortisol indices in Perspective. (w) Correlating change in subfield function and diurnal cortisol indices in Presence. (x) Correlating change in subfield function and diurnal cortisol indices in Perspective. (y) Overall effects of cortisol markers on hippocampal volume in Presence, Affect, and Perspective. (z) Overall effects of cortisol markers on hippocampal function in Presence, Affect, and Perspective. (za) Effects of hair cortisol markers on hippocampal subfield volume in Presence, Affect, and Perspective. (zb) Effects of hair cortisol markers on hippocampal subfield function in Presence, Affect, and Perspective.
- https://cdn.elifesciences.org/articles/87634/elife-87634-supp1-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/87634/elife-87634-mdarchecklist1-v1.docx





