Specific modulation of CRISPR transcriptional activators through RNA-sensing guide RNAs in mammalian cells and zebrafish embryos
Figures
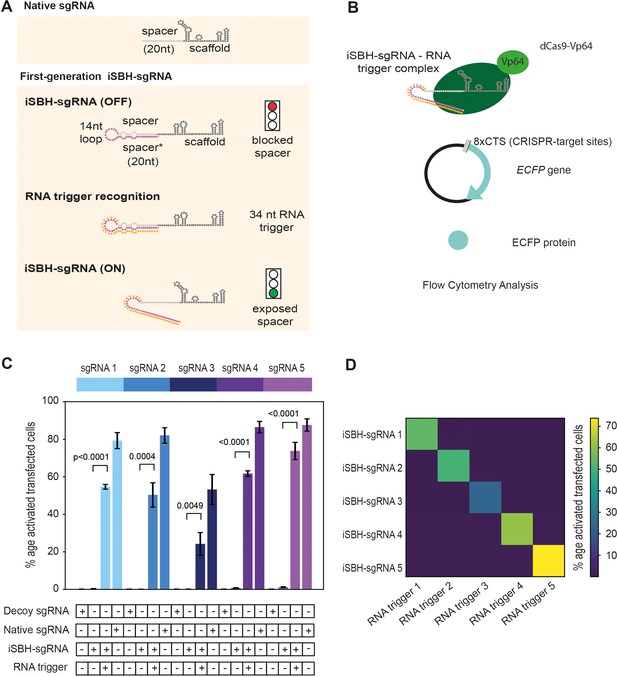
First-generation iSBH-sgRNAs detect short RNA triggers in HEK293T cells.
(A) Native sgRNA sequences are composed of spacer and scaffold sequences (Jinek et al., 2012). iSBH-sgRNAs fold into complex secondary structures that interfere with the Cas9 ability to recognise target DNA sequences (OFF-state, Ferry et al., 2017). iSBH-sgRNAs were designed by extending the 5’ end of the spacer sequence with a 14 nt loop and a spacer* sequence partially complementary with the spacer. Bulges were also introduced within the iSBH-sgRNA sequence in order to ensure that the interaction between the spacer* and RNA trigger is more energetically favourable. In the ON-state, iSBH-sgRNAs recognise complementary RNA triggers and become activated, enabling Cas9 to perform its function. Short RNA triggers are complementary with the iSBH-sgRNA loop and spacer* sequence. (B) Inside cells, RNA triggers are expected to bind to complementary iSBH-sgRNAs, inducing iSBH-sgRNA activation. Activated iSBH-sgRNAs are recognised by CRISPRa effectors and drive ECFP production from a fluorescent reporter. In this particular example, activated iSBH-sgRNAs interact with dCas9-Vp64 (Maeder et al., 2013) and drive ECFP production from an 8xCTS-ECFP reporter (Nissim et al., 2014). Following reporter induction, ECFP production could be monitored by Flow Cytometry. (C) Starting from five different sgRNA spacer sequences, we designed five different iSBH-sgRNA sequences. For each iSBH-sgRNA, corresponding RNA triggers and 8xCTS-ECFP reporters were also designed. Ability of first-generation iSBH-sgRNA designs to drive expression of the ECFP reporter was assessed in the absence or presence of complementary RNA triggers. Experiments were carried out using dCas9-Vp64 and 8xCTS-ECFP reporters. (D) An orthogonality test was performed, in which the five iSBH-sgRNA designs were tested against all five RNA triggers. Activation is only detected in the presence of matching iSBH-sgRNA and RNA trigger pairs. Figure shows mean ± standard deviation values measured for three biological replicates. Values above bars represent fold turn-on values for iSBH-sgRNA activation (blue) and p-values (black) determined through unpaired t-tests.
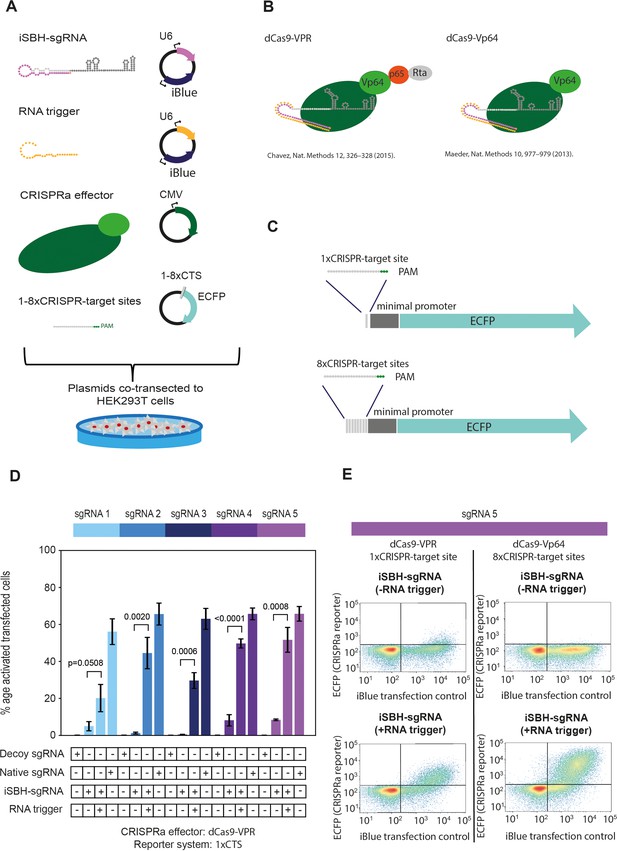
First-generation iSBH-sgRNAs detect short RNA triggers in HEK293T cells.
(A) iSBH-sgRNAs and RNA triggers were cloned in mammalian U6 expression vectors. One or eight CRISPR-target sequences (CTSs) were also cloned upstream of an ECFP fluorescent reporter. We co-transfected the three cloned plasmids as well as CRISPRa effectors into HEK293T cells. Fluorescence outputs were measured by Flow Cytometry. (B) Effectors used include dCas9-VPR (Chavez et al., 2015) and dCas9-Vp64 (Maeder et al., 2013). In contrast with dCas9-Vp64, dCas9-VPR is a stronger transcriptional activator due to the presence of the additional p65 and Rta domains. (C) 1xCTS (CRISPR-target sequence) reporters contain a single Cas9-binding region upstream from the ECFP reporter. 8xCTS reporters (Nissim et al., 2014) contain eight repeats of the Cas9-binding region upstream from the ECFP reporter. For promoting expression of the ECFP reporters, minimal adenovirus major late promoters were also introduced within these cassettes. (D) Starting from five different sgRNA spacer sequences, we designed five different iSBH-sgRNA sequences. For each iSBH-sgRNA, corresponding RNA triggers and 1xCTS-ECFP reporters were also designed. Ability of first-generation iSBH-sgRNA designs to drive expression of the ECFP reporter was assessed in the absence or presence of complementary RNA triggers. Experiments were carried out using dCas9-VPR and 1xCTS-ECFP reporters. (E) Plots compare ECFP fluorescence observed for iSBH-sgRNA 5 activation. In the left, OFF-state and ON-state activity is displayed using CRISPRa assays consisting of the 1xCTS reporters and dCas9-VPR. In the right, OFF-state and ON-state activity is displayed using CRISPRa assays consisting of the 8xCTS reporters and dCas9-Vp64. As iBlue fluorescent gene is encoded in plasmids encoding both iSBH-sgRNAs and RNA triggers, this marker serves as a transfection control. Figure shows mean ± standard deviation values measured for three biological replicates. Values above bars represent fold turn-on values for iSBH-sgRNA activation (blue) and p-values (black) determined through unpaired t-tests.
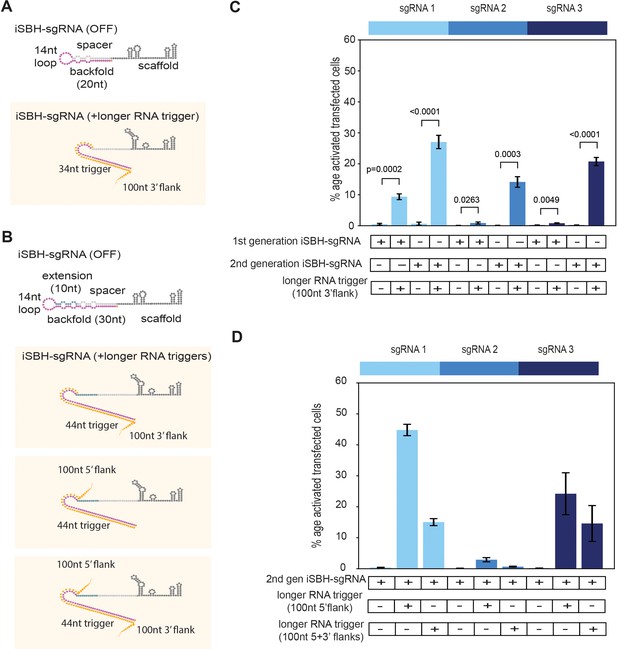
Second-generation iSBH-sgRNAs detect longer RNA triggers in HEK293T cells.
(A) Longer RNA triggers complementary with first-generation iSBH-sgRNAs have a 34 nt sequence complementary with the loop and spacer* iSBH-sgRNA sequences. Triggers also have a 100 nt flanking sequence immediately downstream from the iSBH-sgRNA complementary region. (B) Second-generation designs contain a longer hairpin structure. A 10 nt extension region was inserted between the spacer and loop sequences. This enabled increasing the size of the backfold sequence to 30 nt. Longer RNA triggers complementary with second-generation iSBH-sgRNAs were designed, including triggers with 100 nt 3’ flanks, 100 nt 5’ flanks as well as 100 nt 5’ and 3’ flanks. All trigger designs contain 44 nt sequences complementary with the loops and backfold of the second-generation iSBH-sgRNAs. (C) Ability of first-generation and second-generation iSBH-sgRNAs to sense 100 nt 3’ flank triggers was assessed. (D) Ability of second-generation iSBH-sgRNAs to detect different triggers with 100 nt 5’ flanks and 100 nt 5’ and 3’ flanks was assessed. Figure shows mean ± standard deviation values measured for three biological replicates. Values above bars represent fold turn-on values for iSBH-sgRNA activation (blue) and p-values (black) determined through unpaired t-tests.
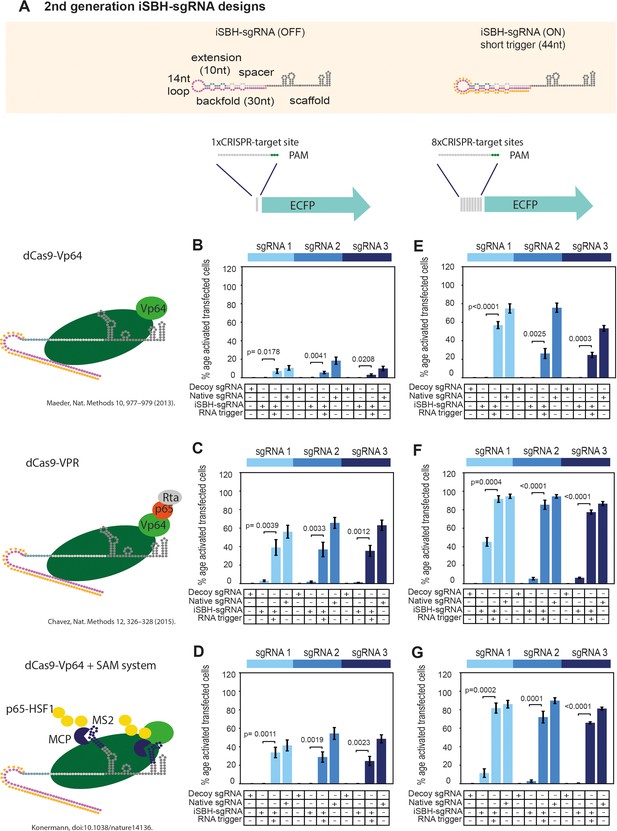
CRISPRa reporters of choice influence ON/OFF ratios of second generation iSBH-sgRNA designs while detecting short RNA triggers.
(A) Second-generation iSBH-sgRNA designs and corresponding short RNA triggers. (B) Testing second-generation iSBH-sgRNAs using dCas9-VP64 (Maeder et al., 2013) and 1xCTS-ECFP reporters. (C) Testing second-generation iSBH-sgRNAs using dCas9-VPR (Chavez et al., 2015) and 1xCTS-ECFP reporters. (D) Testing second-generation iSBH-sgRNAs using dCas9-VP64, the SAM booster system (Konermann et al., 2015), and 1xCTS-ECFP reporters. (E) Testing second-generation iSBH-sgRNAs using dCas9-VP64 (Maeder et al., 2013) and 8xCTS-ECFP reporters (Nissim et al., 2014). (F) Testing second-generation iSBH-sgRNAs using dCas9-VPR (Chavez et al., 2015) and 8xCTS-ECFP reporters (Nissim et al., 2014). (G) Testing second-generation iSBH-sgRNAs using dCas9-VP64, the SAM booster system (Konermann et al., 2015) and 8xCTS-ECFP reporters (Nissim et al., 2014). Figure shows mean ± standard deviation values measured for three biological replicates. Values above bars represent fold turn-on values for iSBH-sgRNA activation (blue) and p-values (black) determined through unpaired t-tests.
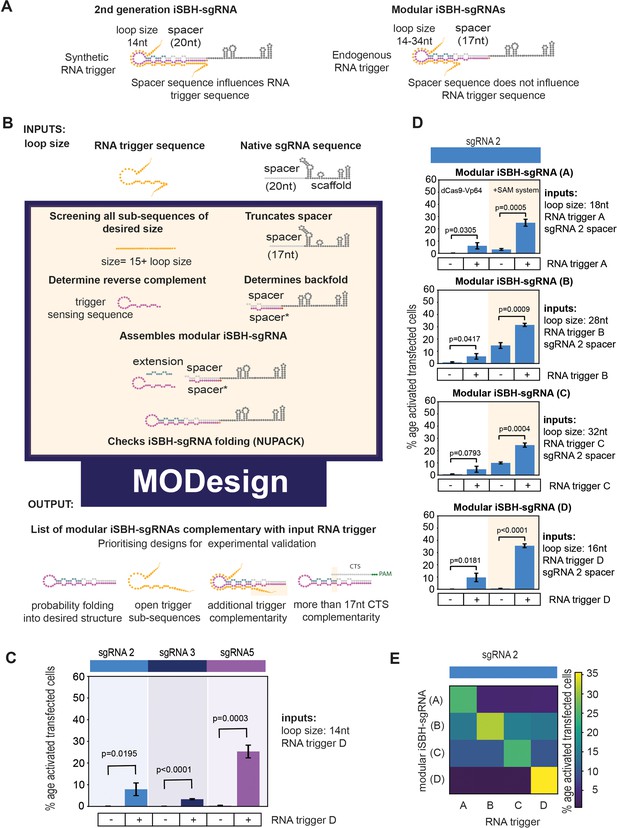
Modular iSBH-sgRNA designs enable spatial separation of spacer and trigger-sensing sequences.
(A) In second-generation iSBH-sgRNAs, RNA triggers are complementary with the iSBH-sgRNA backfolds, thus sgRNA spacers influence RNA trigger sequences. In modular iSBH-sgRNAs, design constraints were eliminated as triggers are only complementary with the iSBH-sgRNA loop and first 15 nt of the backfold. To increase affinity between iSBH-sgRNAs and RNA triggers, we increased loop sizes. Separation between trigger-sensing and spacer sequences was also achieved by reducing the complementary between the spacer sequence and CTS from 20 to 17 nt. (B) MODesign enables users to design modular iSBH-sgRNAs starting from input RNA triggers, sgRNA spacers, and loop sizes. MODesign calculates the size of trigger-sensing sequences and creates a list of trigger sub-sequences having that size. Script determines the reverse complement of these sequences that could act as trigger-sensing sequences. iSBH-sgRNAs are assembled through adding spacer*, trigger-sensing sequences, extension, spacer, and scaffold sequences. Extension sequences are engineered to be partially complementary with trigger-sensing sequences. Before producing a list of output sequences, iSBH-sgRNA folding is checked using NuPACK (Allouche, 2011). Simulations could result in multiple modular iSBH-sgRNA designs. Designs chosen for experimental validation were selected based on the probability of folding into the iSBH-sgRNA structure and lack of trigger secondary structures in the iSBH-sgRNA complementary region. Priority was also given to iSBH-sgRNAs that, by chance, displayed extra complementarity between RNA triggers and the last 15 nt of the backfold or more than 17 nt complementarity with the CTS. (C) MODesign simulations were carried out for designing iSBH-sgRNAs capable of sensing trigger RNA D (146 nt eRNA sequence). In each simulation, a different sgRNA sequence was used and a desired loop size of 14 nt was kept constant between simulations. Selected designs were transfected to HEK293T cells together with the RNA trigger D sequence (expressed from a U6 promoter). Tests were carried out using dCas9-Vp64 and 8xCTS-ECFP reporters. (D) MODesign simulations were run for designing iSBH-sgRNAs capable of sensing trigger RNA A (146 nt repetitive RNA sequence), trigger RNA B (267 nt repetitive RNA sequence), trigger RNA C (268 nt repetitive RNA sequence), and trigger RNA D (146 nt eRNA sequence). Tests were performed using different CRISPRa effectors. (E) Four modular iSBH-sgRNAs (A–D) were co-transfected to HEK293T cells and all iSBH-sgRNA: RNA trigger combinations were tested. Figure shows mean ± standard deviation values measured for three biological replicates. Values above bars represent fold turn-on values for iSBH-sgRNA activation (blue) and p-values (black) determined through unpaired t-tests.
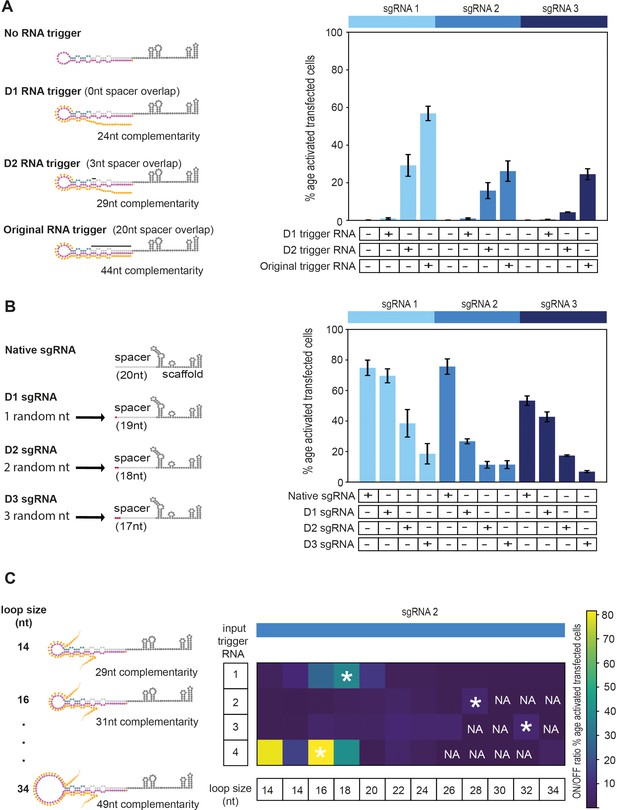
Modular iSBH-sgRNA designs enable spatial separation of spacer and trigger-sensing sequences.
(A) Trigger RNA truncation experiments. In the original RNA trigger design, the trigger sequence was influenced by the 20 nt sgRNA spacer sequence. A way of avoiding this involves truncating the trigger sequence, so that there is little to no overlap with the spacer sequence. In D1 design, the 20 nt spacer sgRNA sequence does not influence at all the sequence of the RNA trigger. In D2 design, the first 3 nt of the spacer are still influencing the sequence of the RNA trigger. (B) Spacer RNA randomisation experiments. Here, we reduced the complementary between the sgRNA spacer sequence and the CTS. This experiment was carried out to accommodate D2 RNA trigger designs and to completely abolish the dependency between the sgRNA spacer and trigger sequences. Native sgRNAs that matched 19, 18, and 17 nt of the CTS were designed and tested. (C) Optimising modular iSBH-sgRNA designs by changing loop length. Multiple MODesign simulations were run for designing iSBH-sgRNAs capable of sensing trigger RNA A (146 nt repetitive RNA sequence), trigger RNA B (267 nt repetitive RNA sequence), trigger RNA C (268 nt repetitive RNA sequence), and trigger RNA D (146 nt eRNA sequence). Each simulation contained different loop size specifications and sgRNA 2 sequence was kept constant between simulations. Selected iSBH-sgRNA designs were transfected to HEK293T cells together with corresponding RNA trigger sequences expressed from U6 promoters. For a loop size of 14 nt, two different modular iSBH-sgRNAs were tested for each trigger. These iSBH-sgRNAs hybridised with different trigger sub-sequences. NA represents conditions where the RNA trigger sequence did not allow designing of iSBH-sgRNAs with a particular loop size. iSBH-sgRNA designs with best ON/OFF ratios observed for different RNA triggers are marked with *. Figure shows mean ± standard deviation values measured for three biological replicates. Fold turn-on values for iSBH-sgRNA activation are also displayed in blue.
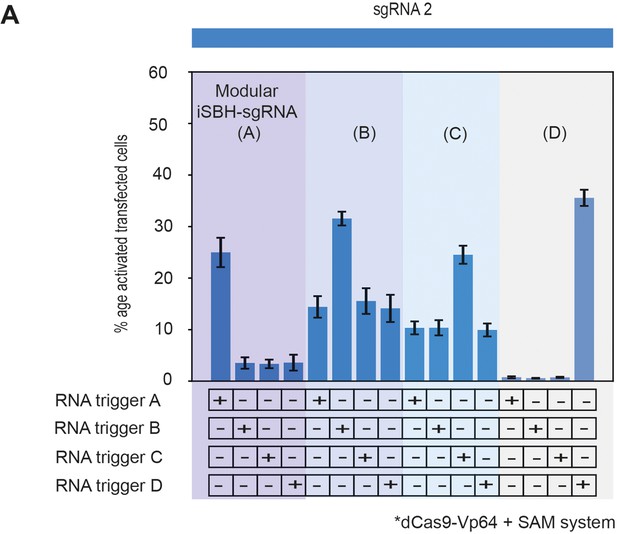
This supplementary figure reinterprets the data presented in Figure 3E using bar plots for enhanced clarity and comparison.
It depicts the results of co-transfecting HEK293T cells with four modular iSBH-sgRNAs (A–D) and examines all combinations of iSBH-sgRNA: RNA trigger pairings. The bar plots provide a visual representation of mean values, with error bars indicating the standard deviation, based on three biological replicates.
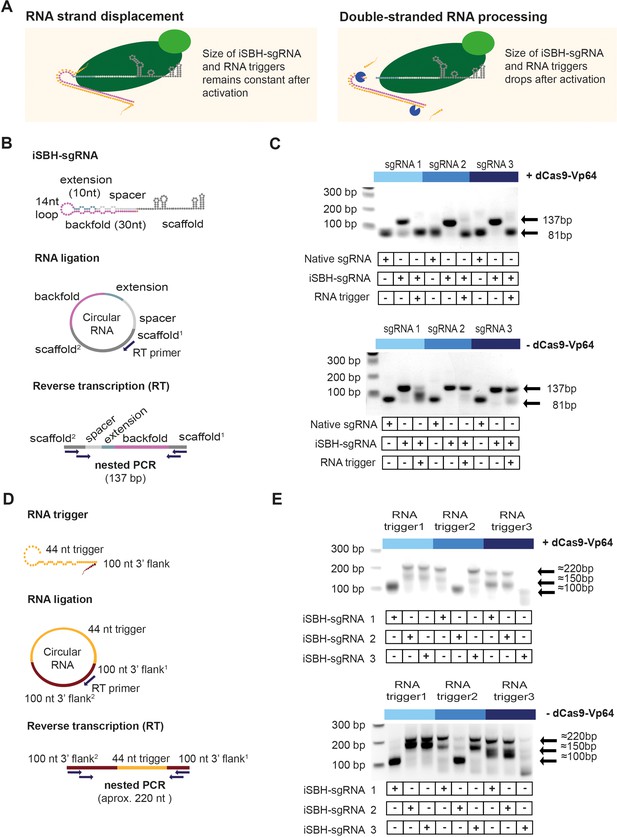
Insights into the mechanism of iSBH-sgRNA activation.
(A) Interaction between iSBH-sgRNAs and RNA trigger leads to the formation of long double-stranded RNA structures. A potential activation mechanism might involve RNA strand displacement and formation of stable molecular complexes between the iSBH-sgRNA and the RNA trigger sequence. Supposing this scenario is correct, the size of the iSBH-sgRNA and RNA triggers is expected to remain constant after activation. A second scenario involves double-stranded RNA processing. If this is correct, iSBH-sgRNAs and RNA trigger sequences are expected to be truncated. (B) iSBH-sgRNA circularisation assay. Cells were transfected with system components, followed by RNA extraction and ligation. Reverse transcription (RT) was performed on circular RNAs by using RT primers complementary with the sgRNA scaffold. The size of the RT products was determined by two sequential PCR reactions. PCR primers annealed with the scaffold2 and scaffold1 sequences, which are the scaffold sequences found downstream and upstream from the RT primer. For a full-length iSBH-sgRNA sequence, a second PCR product of 137 bp is expected, while for a non-engineered native sgRNA, an 81 nt product is expected. (C) Determining the size of the iSBH-sgRNA after activation. Assays were performed in the presence or absence of complementary 44 nt, short RNA triggers and dCas9-Vp64. Non-engineered, native sgRNA controls were also included. (D) RNA trigger circularisation assay. After transfection, RNA extraction, and RT, RNA trigger size was determined by nested PCR. PCR primers annealed with the 100 nt 3’ flank2 and 100 nt 3’ flank1 sequences, which are the flank sequences downstream and upstream from the RT primer. For full-length RNA triggers, 220 bp PCR bands are expected. (E) Determining the size of the RNA triggers after activation. Assays were performed in the presence or absence of a complementary iSBH-sgRNAs and dCas9-Vp64.
-
Figure 4—source data 1
Uncropped raw gels for Figure 4C and E.
- https://cdn.elifesciences.org/articles/87722/elife-87722-fig4-data1-v1.zip
-
Figure 4—source data 2
Uncropped gels with relevant bands labelled for Figure 4C and E.
- https://cdn.elifesciences.org/articles/87722/elife-87722-fig4-data2-v1.zip
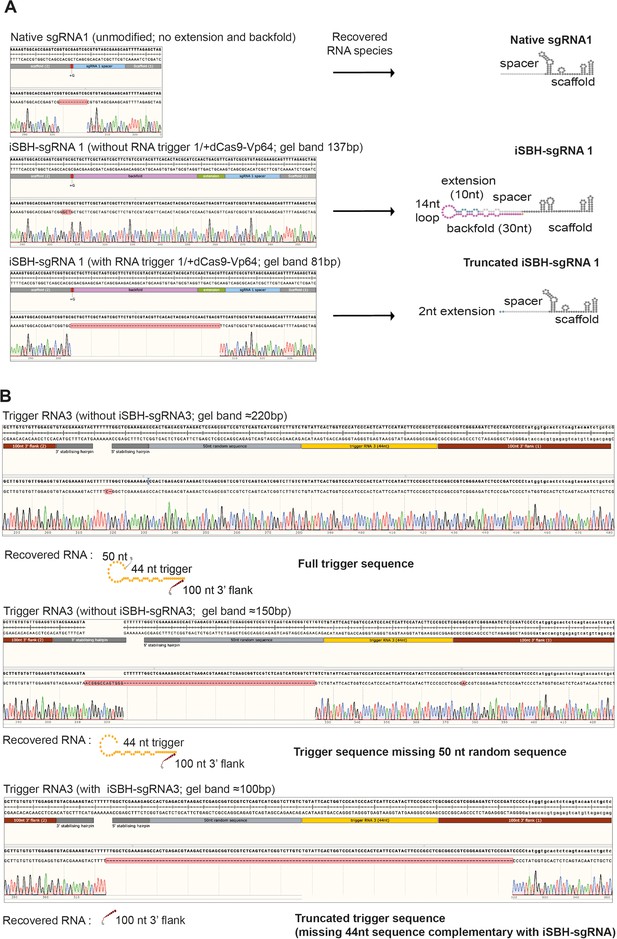
Sequencing results for iSBH-sgRNA circularisation assays.
PCR products were submitted for Sanger sequencing. Data displays sequencing results obtained for iSBH-sgRNA 1 in the presence of dCas9-Vp64. Top sequencing trace was recovered from cells transfected with non-engineered, native sgRNA. Middle and lower sequencing traces resulted from cells transfected with iSBH-sgRNAs in the absence or presence of complementary RNA triggers. (B) Sequencing results for RNA trigger circularisation assay. PCR products obtained for RNA trigger 3 were submitted for Sanger Sequencing. Top and middle traces represent RNA trigger sequences recovered in the absence of complementary iSBH-sgRNA (≈220 and 150 bp bands), while bottom trace was recovered in the presence of complementary iSBH-sgRNAs.
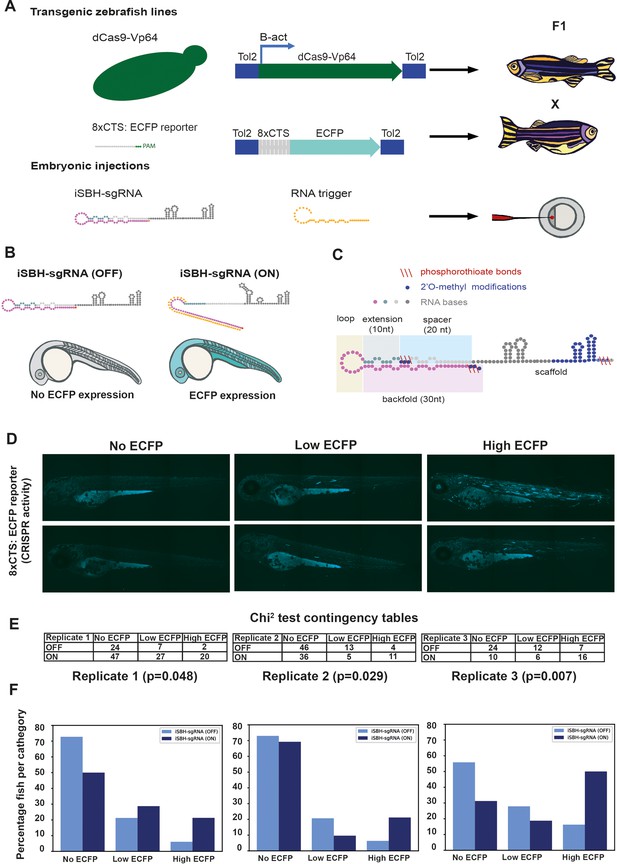
Testing the ability of second-generation iSBH-sgRNA designs to detect short RNA triggers in vivo.
(A) Transgenic lines encoding dCas9-Vp64 and 8xCTS-ECFP reporters were created. Embryos resulting from in-crossing first-generation (F1) transgenics were injected with second-generation chemically synthesised iSBH-sgRNAs and RNA triggers. (B) Second-generation iSBH-sgRNAs were injected into transgenic zebrafish embryos with or without corresponding short RNA triggers. In the absence of RNA triggers (iSBH-sgRNA OFF), embryos are expected to display no ECFP signals, while trigger presence (iSBH-sgRNA ON) should promote ECFP expression. (C) Figure presents our second strategy for chemically modifying iSBH-sgRNAs. This strategy involved protecting the iSBH-sgRNA 5’ end as well as the 5’ end of the sgRNA spacer. These modifications were used together with sgRNA scaffold modifications. (D) In order to quantify the impact of RNA triggers on iSBH-sgRNA activation, we grouped fish according to the intensity of ECFP signals. At 3 days post-fertilisation, embryos displaying no, low, or high ECFP expression were counted. (E) Embryos injected with iSBH-sgRNAs and non-complementary (iSBH-sgRNA OFF) of complementary RNA triggers (iSBH-sgRNA ON) were scored according to their ECFP intensity. Row number counts determined for three experimental replicates are displayed as part of χ2 contingency tables. p-Values displayed were determined using χ2 test. (F) Figure shows percentage of embryos recovered in each category for the three experimental replicates. Percentage of embryos with no ECFP expression varied between the three experimental replicates. This was due to the fact that both 8xCTS-ECFP and dCas9-VP64 transgenes are necessary for successfully expressing ECFP. These alleles segregate in a Mendelian fashion and our adult transgenic fish encode variable copy numbers of the transgene. For each individual replicate, we used embryos with identical genetic backgrounds for testing the iSBH-sgRNA (OFF) and iSBH-sgRNA (ON) conditions. Nevertheless, genetic backgrounds were different between the three experimental replicates.
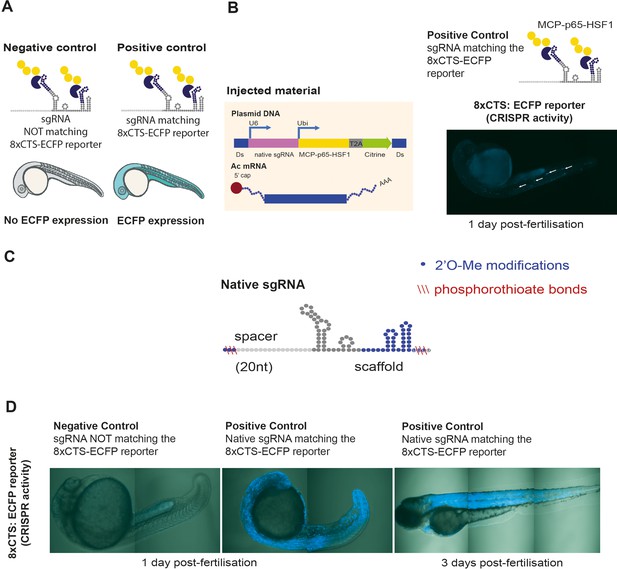
Optimising sgRNA delivery to zebrafish embryos.
(A) Validation controls. Embryos were injected with native sgRNAs (without the iSBH-sgRNA scaffold) matching or not matching the 8xCTS-ECFP reporter. Only embryos injected with matching sgRNA controls are expected to express ECFP. (B) Embryos resulted from crossing dCas9-Vp64 and 8xCTS-ECFP lines were injected with control sgRNA sequences. sgRNAs were expressed under the control of a fish U6 promoter and contained the modified sgRNA scaffold compatible with the SAM amplification system. SAM effector proteins (MCP-p63-HSF1, Konermann et al., 2015) were also encoded into the same vector, together with a Citrine fluorescent protein. This construct was expressed under the control of the ubiquitin promoter. Vectors were co-injected with Ac transposase mRNA (Chong-Morrison et al., 2018). Resulting embryos display mosaic ECFP expression. (C) sgRNA chemical modifications were designed and synthesised by IDT, comprising 2’O-methyl (Me) modifications, as well as phosphorothioate (PS) bonds. (D) Delivery of chemically modified sgRNAs. Embryos were injected with negative and positive control sgRNAs. For positive control, images are taken at 1 day as well as 3 days post-fertilisation. Resulting embryos display homogeneous ECFP expression across tissues.
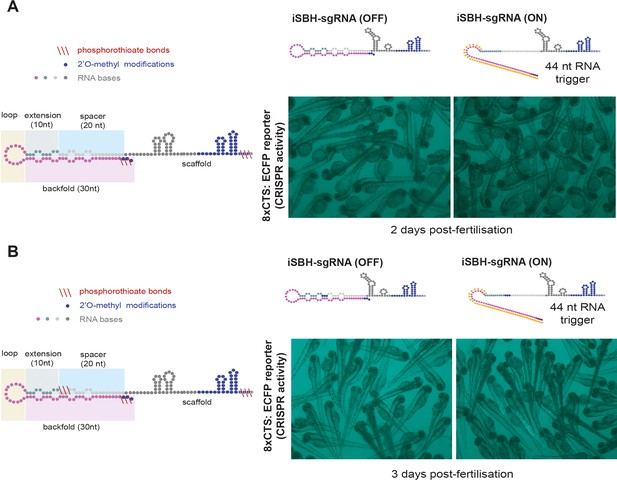
Testing different iSBH-sgRNA chemical modifications in vivo.
(A) An initial strategy for chemically modifying iSBH-sgRNAs involved protecting the iSBH-sgRNA 5’ end. These modifications were used together with sgRNA scaffold modifications used in the native sgRNA designs (Figure 5—figure supplement 1C). Chemically modified iSBH-sgRNAs were co-injected together with either non-complementary (iSBH-sgRNA OFF) or complementary (iSBH-sgRNA ON) RNA triggers. (B) A second strategy for chemically modifying iSBH-sgRNAs involved protecting the iSBH-sgRNA 5’ end as well as the 5’ end of the sgRNA spacer. These modifications were used together with sgRNA scaffold modifications used in the native sgRNA designs (Figure 5—figure supplement 1C). Chemically modified iSBH-sgRNAs were co-injected together with either non-complementary (iSBH-sgRNA OFF) or complementary (iSBH-sgRNA ON) RNA triggers.
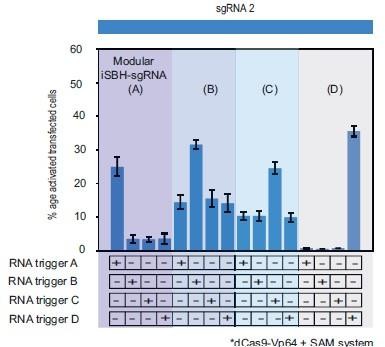
This supplementary figure reinterprets the data presented in Figure 3.
E. using bar plots for enhanced clarity and comparison. It depicts the results of cotransfecting HEK293T cells with four modular iSBH-sgRNAs (A, B, C, and D) and examines all combinations of iSBH-sgRNA: RNA trigger pairings. The bar plots provide a visual representation of mean values with error bars indicating the standard deviation, based on three biological replicates.
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/87722/elife-87722-mdarchecklist1-v1.pdf
-
Supplementary file 1
Supplementary Materials – relevant RNA and primer sequences.
- https://cdn.elifesciences.org/articles/87722/elife-87722-supp1-v1.pdf





