Optical mapping of ground reaction force dynamics in freely behaving Drosophila melanogaster larvae
Figures
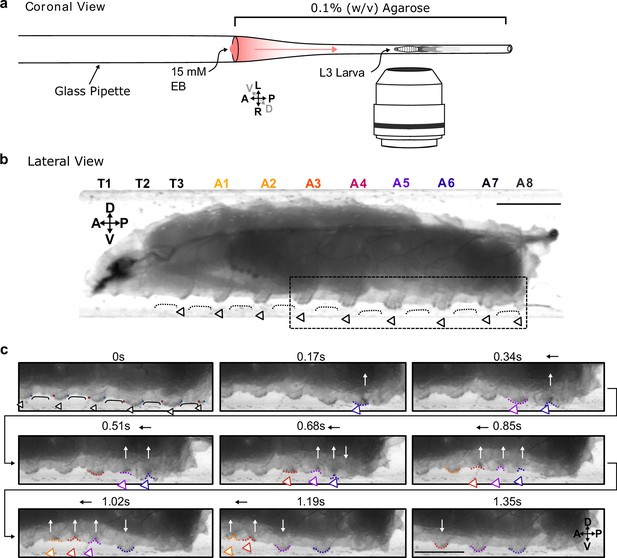
Protopodia protrusions in each segment are sequestered during swing phases of forward locomotion.
(a) Schematic of setup for lateral imaging of larvae, using confinement in Pasteur pipette pre-filled with 0.1% (w/v) agarose. To encourage forward crawling, 10 µL of 15 mM ethyl butanoate (EB) was placed as attractive odour at the end of the pipette. (b) Lateral brightfield image of third-instar larva showing convex areas of denticle bands (open arrowheads) protruding into the substrate, interdigitated by concave areas of naked cuticle (black line) not interacting with the substrate. Scale bar = 750 µm. (c) Time lapse of area marked by dotted box in (b) showing the swing periods and stance periods of protopodia (coloured open arrowheads and dotted lines) during a forward wave. Red and blue dots at 0 s denote anterior and posterior rows of denticles, respectively. As the posterior-most denticle row moved to meet the anterior row of the band, the medial row detached from the substrate via invagination (white arrows). The invaginated pocket is then moved forwards (black arrow) and subsequently replanted. This action repeats as the wave propagates. Scale bar = 500 µm. Images representative of three third-instar larvae.
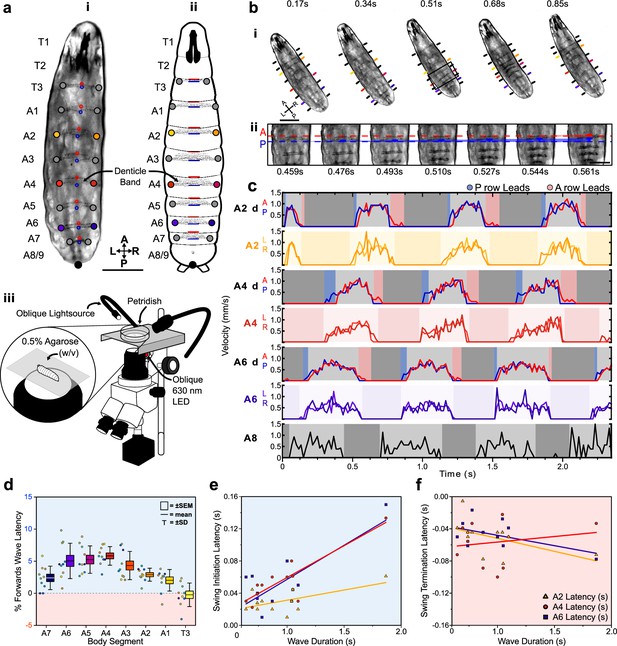
Protopodia kinematics follow ‘heel-toe’-like footfall dynamics.
(a) (i) Brightfield image and (ii) schematic of second-instar larvae showing ventral side denticle belts which reside upon the protopodia and (iii) schematic of the imaging setup used for kinematic tracking. Scale bar = 200 µm. (b) (i) As a forward wave travels through the animal, the distance between denticle bands decreases. Scale bar = 200 µm. (ii) At higher frame rate and magnification, changes in distance between the posterior and anterior-most denticle rows are resolved. The posterior-most row (P, blue) initiates movement first and moves until nearly reaching the anterior-most row (A, red) at 0.544 s, after which point, they move together (0.561 s). Scale bar = 100 µm. (c) Velocity of anterior- and posterior-most denticles rows (A2d A/P, A4d A/P, A6d A/P) and the left/right end of denticle bands (A2 L/R, A4 L/R, A6 L/R, and A8 L/R) over three representative forward waves, showing how the strategy observed in (b) is maintained across body segments. Background colours indicate swing initiation (SI, blue), swing period (SwP, light grey), swing termination (ST, pink), and stance period (StP, dark grey). (d) Forward wave latency for different animals and body segments. Positive values denote posterior row led latency. n = 10 animals, 30 waves. (e) SI latency scales with wave duration in the posterior abdomen (A6: R2 = 0.61, purple; A4: R2 = 0.78, red) but less so for the anterior abdomen (A2: R2 = 0.35, yellow). n = 12 animals with three latency periods per segment. (f) ST latencies do not scale with wave duration (A6: R2 = 0.26, A4: R2 = 0.26, A2: R2 = 0.03). n = 12 animals with three latency periods per segment.
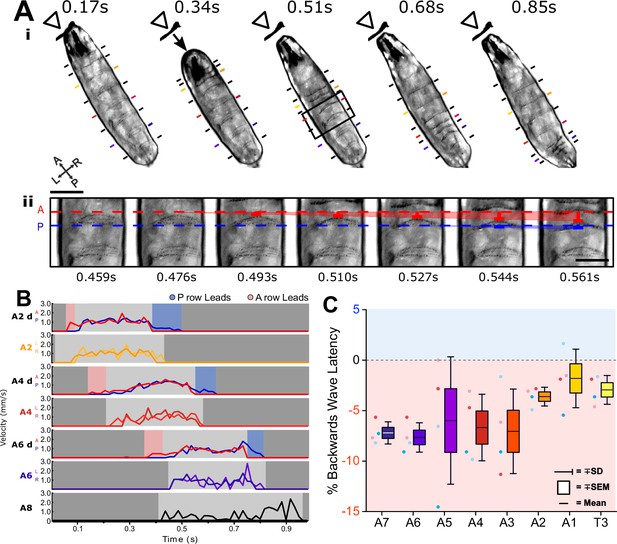
Backward waves show a reversed heel toe rule.
(A) Backward waves are characterised by sequential contractions moving from anterior to posterior, the reverse of forward waves (i). We observed that the anterior row of each denticle band moved to meet the posterior row before the whole protopodia began to move (ii). (B) We tracked the velocity of the lateral edge of each denticle band (A6, A4, A2) and the anterior and posterior rows of each denticle band (A6d, A4d, A2d). Similar to forward waves in Figure 2, we observed an anteroposterior latency between when each row moved relative to the other. However, this was the reverse of forward waves, with the swing initiation period being characterised by an anterior-led latency and the swing termination period being characterised by a posterior-led latency. (C) We found that this was relatively consistent across segments, where negative numbers represent anterior led latency, within a sample of four waves across four different animals.
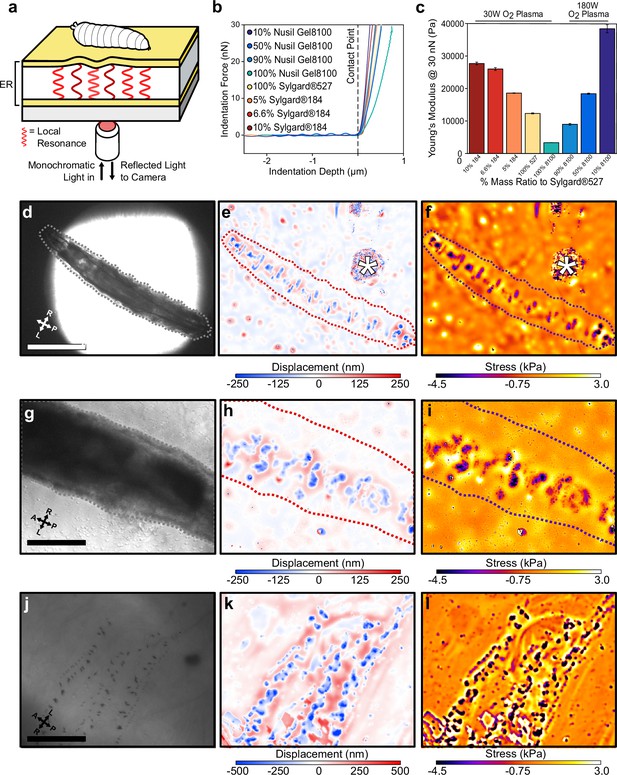
Elastic resonator interference stress microscopy (ERISM) maps mechanical substrate interactions in Drosophila larvae.
(a) Schematic of setup for ERISM with Drosophila larva on an optical microcavity. Maps of local cavity deformation (displacement) due to indentation forces are generated by analysing cavity resonances. (b) Force distance relationship measured by atomic force microscopy (AFM) and (c) mechanical stiffnesses (Young’s moduli) for microcavities produced by mixing different elastomers at different ratios and applying different plasma conditions. (d, g, j) Brightfield images of anaesthetised second-instar larvae recorded at low, medium, and high magnification. (e, h, k) Corresponding maps of microcavity displacement. (* denotes contamination on cavity surface from handling the larva.) (f, i, l) Corresponding maps of mechanical stress obtained by finite element analysis of displacement maps, showing the stress on the substrate due to passive interaction between larvae and substrate. Scale bar = 500 µm (d), 250 µm (g), and 50 µm (j). Images representative of four separate second-instar larvae. Microcavities in (d–i) used 30 W O2 10% Sylgard184 design, and (j–l) used a 30 W O2 5% Sylgard184 design.
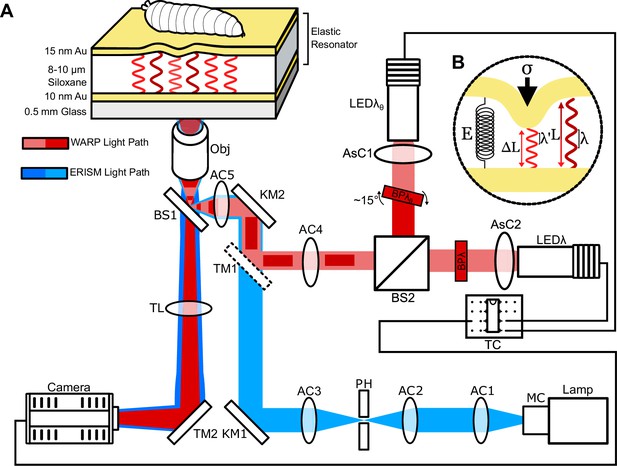
Optical setup for elastic resonator interference stress microscopy (ERISM) and wavelength alternating resonance pressure (WARP) experiments.
(A) Optical light path used to record resonance from incident light on elastic resonators. For ERISM (blue), light originates from a halogen lamp (Lamp) and is spectrally scanned by a monochromator (MC) before being collimated by an achromatic doublet lens (AC1) and focused by another achromatic doublet lens (AC2) through a pinhole (PH). Light emerging from PH is then recollimated by AC3 into a kinematic mirror (KM1), directing it to KM2 which then directs the light under the nosewheel of a Nikon Ti2 inverted microscope. Under the nosewheel, the light is focused by an achromatic doublet lens (AC5) to a 50:50 RT beamsplitter plate (BS1), which directs the focused light to the back aperture of the objective (Obj). Light is then introduced onto the elastic resonator via Obj, whereupon it either enters the cavity, should it meet the resonance condition for the given cavity thickness, or is reflected, should it not meet this condition. Reflected light is then collected by Obj, focused by the tube lens (TL) and directed by the microscope turning mirror (TM2) then recorded by a camera (Camera). Though depicted as blue, ERISM typically scans through a spectral band from 550 to 750 nm. For WARP (red), light is generated by two 625 nm red LEDs (LEDλ and LEDλθ). For both LEDs, the light is collimated by aspheric condenser lenses (AsC1 and AsC2) and is then filtered by 633 nm bandpass filters (BPλ and BPλθ). BPλθ, is rotated roughly 15° such that the filter pass band is blue-shifted and the resultant transmitted light is approximately 90° out of phase (in terms of the resonances of the elastic cavity) relative to light passing through BPλ. These light paths are combined by a beamsplitter cube (BS2) and collimated using AC4 into the KM2, BS1, AC5, Obj common light path by a dielectric turning mirror (TM1) only present when using WARP. The LEDs are then triggered in an alternating pattern by a trigger circuit decade counter (TC) which is controlled by the trigger out of the sCMOS camera. (B) Working principle within the elastic cavity. When under stress (σ), the elastic cavity deforms from its resting length (L) to its strained length (ΔL). The change in cavity length causes a change in the wavelengths that fulfil the resonance condition of the cavity. The amount of strain under a given stress is a direct consequence of the Young’s modulus (E) of the elastic material.
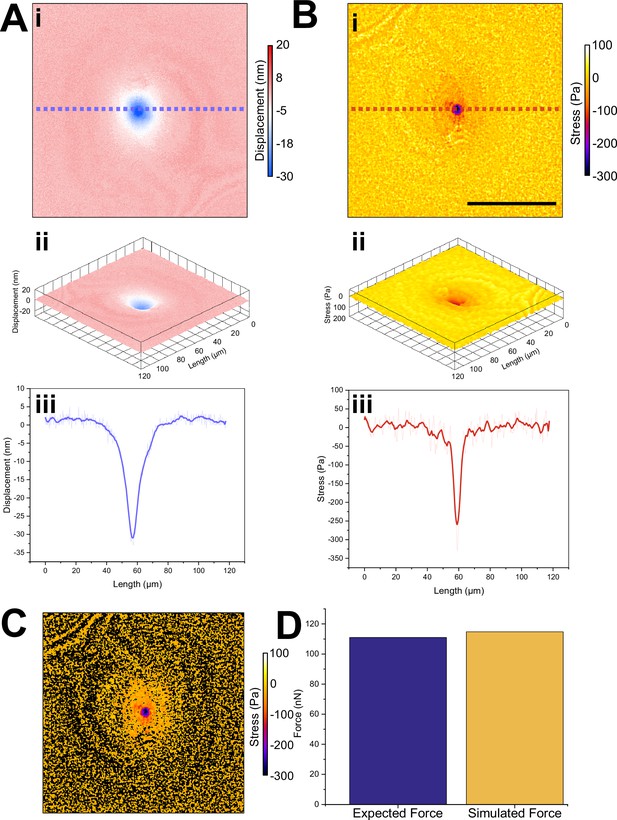
Confirmation of finite element method (FEM) simulation accuracy.
(A) Using atomic force microscopy (AFM), we indented into an elastic resonator made within the same batch as those used for videorate stress mapping. (i) False colour map and (ii) 3D projection of displacement smoothed by 10 points. (iii) Profile along blue dotted line in (i) showed that a 111 nN indentation force resulted in a roughly 32 nm peak indentation; data averaged by 10 points, raw data shown in a lighter colour. (B) Using FEM, we calculated a stress map from the displacement map, using the Young’s modulus of the bulk material, previously recorded as 16,450 Pa by AFM. (i) False colour and (ii) 3D projection of stress experienced by the resonator smoothed by 10 points (ii). Profile along red dotted line in (i) showing the peak stress produced by 111 nN of force approximately 320 Pa; data averaged by 10 points, raw data shown in a lighter colour. (C) We thresholded the resultant simulation to remove all cavity displacements >0 nm. (D) Integration of stress in (C) gives a prediction of the total applied force as determined from displacement map and FEM model, without prior knowledge of the indentation force. Comparing this simulated force to the applied force of 111 nN, we found the relative difference to be only 3.4%, with our simulation estimating a total applied force of 114.8 nN. Scale bar in (B) denotes 50 µm.
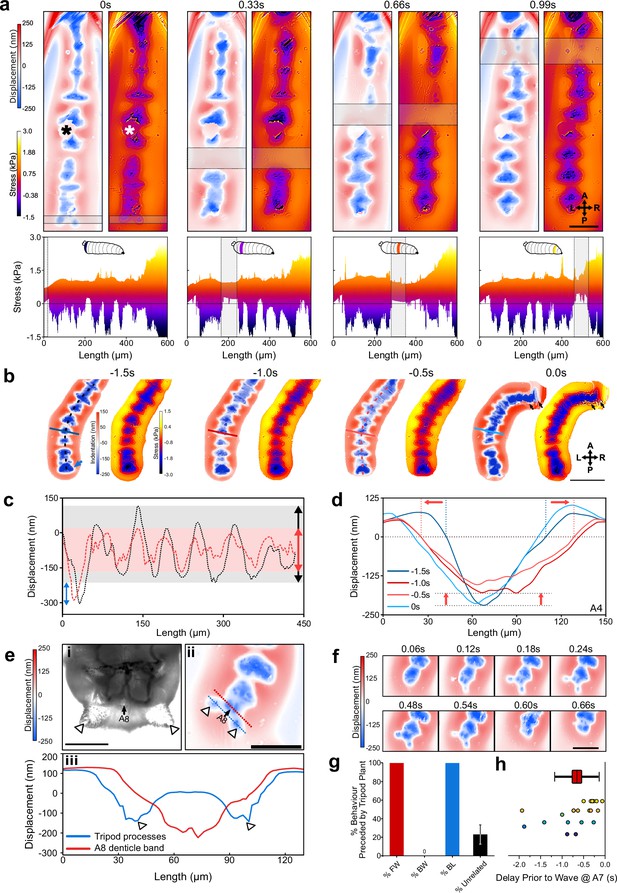
Wavelength alternating resonance pressure (WARP) imaging reveals dynamics of substrate interactions during larval movement.
(a) WARP image sequence of displacement and stress maps (top) for a freely behaving second-instar larva during forward locomotion. (* denotes dust artefact.) Lateral projections of stress maps (bottom) showing individual protopodia interdigitated by naked cuticle. As a contractile wave (grey box) progressed through the animal, protopodia were lifted off the substrate. Scale bar = 100 µm. (b) WARP image sequence of larva prior to (–1.5 s to –0.5 s) and engaging in (0 s) a headsweep (representative of two animals and three turns). Note the large posterior displacement (blue arrow; images cropped around the animal). Scale bar = 200 µm. (c) Profiles of cavity displacement along anteroposterior (A-P) axis in resting state (black dotted line at –1.5 s in b) and pre-headsweep (red dotted line at –0.5 s in b), showing that peak displacement decreased across all segments from the resting state (grey box) to pre-headsweep (pink box). (d) Bilateral displacement profile across the mediolateral (ML) axis of the A4 protopodium (solid lines in b) at different times prior to the headsweep, showing that the width of the contact increases from the resting state (–1.5 s) to the pre-headsweep state (–0.5 s) and partially reduces again immediately after head movement. (e, i) Brightfield image (third-instar larva) and (ii) displacement map (second-instar larva) of the posterior-most body segment, showing how two cuticular protrusions (white arrowheads) and the terminal protopodium (A8) generate a tripod-shaped substrate displacement. (iii) Profiles along blue and red dotted lines in (ii). Scale bar = 200 µm (i) and 100 µm (ii). (f) Sequence of displacement maps of tripod structure before the start of a forward wave (<0.24 s) and the removal of tripods upon beginning of peristalsis (>0.48 s). Scale bar = 100 µm. (g) Percentage of forward waves (FW), bilateralisms (BL), backward waves (BW) preceded by tripod contact, and tripod deployments without any observed locomotor behaviour (unrelated). (h) Time delay between tripod deployment and initiation of movement at A7. Points colour-coded by animal, n = 6. Line = mean, box = ±1 standard error of the mean, whiskers = ±1 standard deviation.
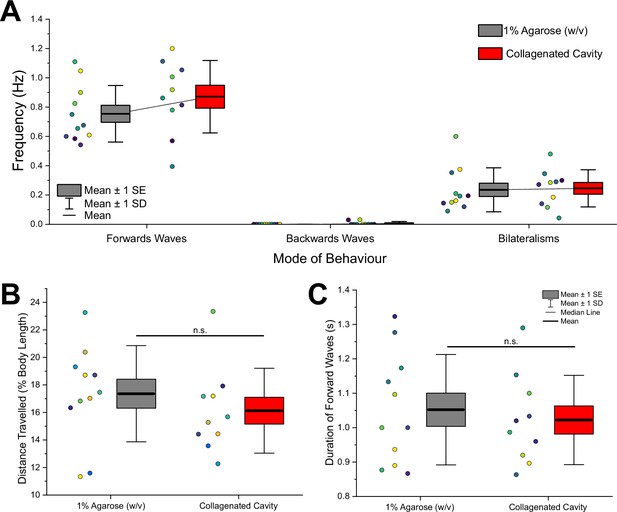
Ordinary larval behaviour is maintained on collagen-treated microcavities compared to commonly used agarose substrates.
(A) Total number of behaviours per second is not significantly different between agarose substrates and elastic cavities with collagen coating according to a two-sample t-test (t(18) = -1.24, p=0.23). Data for forward waves, backward waves, and headsweep bilateralisms are shown. (B) Distance travelled as % of body length was not significantly different between the two substrates according to a two-sample t-test (t(18) = 1.34, p=0.20). Each data point represents the mean of five waves from a single animal. (C) The mean duration of five forward waves was not significantly different on microcavities compared to agarose according to a two-sample t-test (t(18) = 0.62, p=0.54). Data taken from 10 animals. Colour of data point indicates data from an individual animal. All tested data were found to be normally distributed according to a Shapiro–Wilk test (p>0.05).
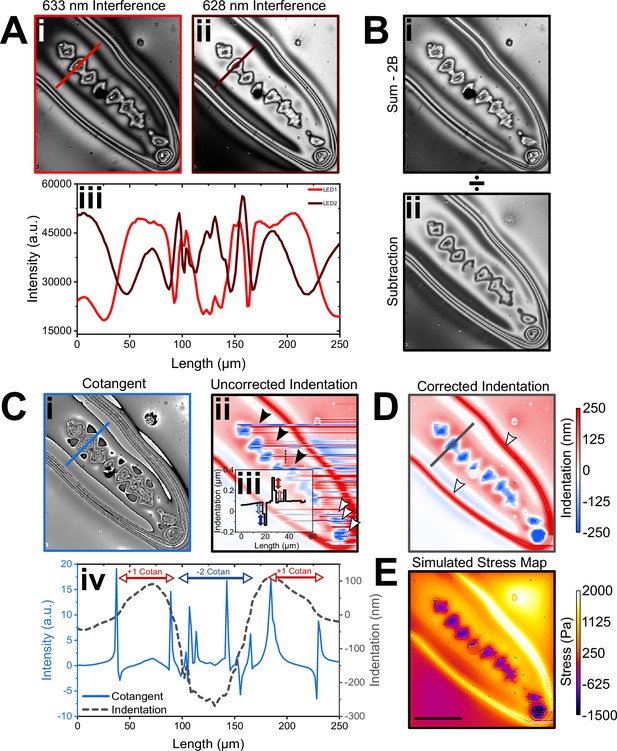
Wavelength alternating resonance pressure (WARP) computation pipeline.
(A) Interference images were taken at 633 nm (i) and 628 nm (ii) in quick alternation. Profile plot (iii) across the lines in (i) and (ii). Note that interference pattern is approximately 90° out of phase relative to the other as a result of the specific wavelength difference and total cavity thickness chosen here. (B) Images were then added together (i) with a background correction (2B) and then divided by the same two images subtracted from each other (ii). (C) The resultant images are referred to as cotangent images as pixel intensity changes approximately as the cotangent of the cavity thickness in these (i). A cotangent lookup table was then used to convert 16-bit greyscale values in the cotangent images to local cavity length. Raw and uncorrected displacement map computed by subtracting a linear plane of mean cavity thickness (ii). Profile plot (iii) along the thin dashed line in (ii), clearly showing discontinuity artefacts (indicated by blue and red double arrows). These linear artefacts correspond to step heights amounting to jumps by one free spectral range, which was 112 nm in this instance. (D) Corrected displacement map where the linear artefacts across the image were corrected by applying a continuity condition. (E) Profile plots of cotangent signal and corrected displacement along the thick lines in (Ci and D). (F) Stress maps were calculated from the displacement map by finite element method (FEM) using the known mechanical properties of the substrate. Scale bar = 200 µm.
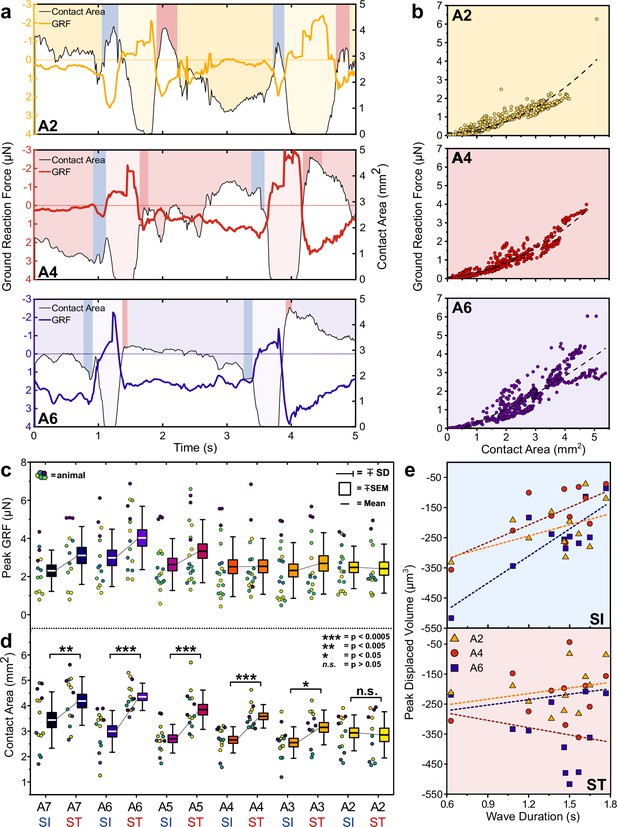
Protopodia produce ground reaction forces (GRFs) in the micronewton range and show complex spatiotemporal dynamics.
(a) GRF (coloured line) and protopodial contact areas (white area under black line) during forward crawling for A2, A4, and A6 protopodia, showing progression of waves through animal (light-coloured boxes). Blue (SI) and pink (ST) boxes denote characteristic troughs in GRF immediately prior to protopodia leaving the substrate and returning to the substrate, respectively. (b) GRFs exerted by different protopodia show a second-order polynomial relationship (dashed line) with the contact area of that protopodium (A6: Adj. R2 = 0.77, A4: Adj. R2 = 0.92, A2: Adj. R2 = 0.79). (c) Peak GRFs and (d) peak contact area during SI and ST across body segments. Data points denote single events, colours indicate different animals. n = 5, 15 waves. Contact areas were compared by a two-way repeated-measures ANOVA (*<0.05, **<0.005, ***<0.0005, n.s. = not significant). (e) During SI, peak displaced volume scaled with wave duration for larger abdominal segments (A6: R2 = 0.69; A4: R2 = 0.48) but not for smaller anterior segments (A2: R2 = 0.24). During ST, displaced volume did not scale with wave duration regardless of the segment (A6: R2 = 0.05; A4: R2 = 0.05; A2: R2 = 0.08). n = 4, 11 waves.
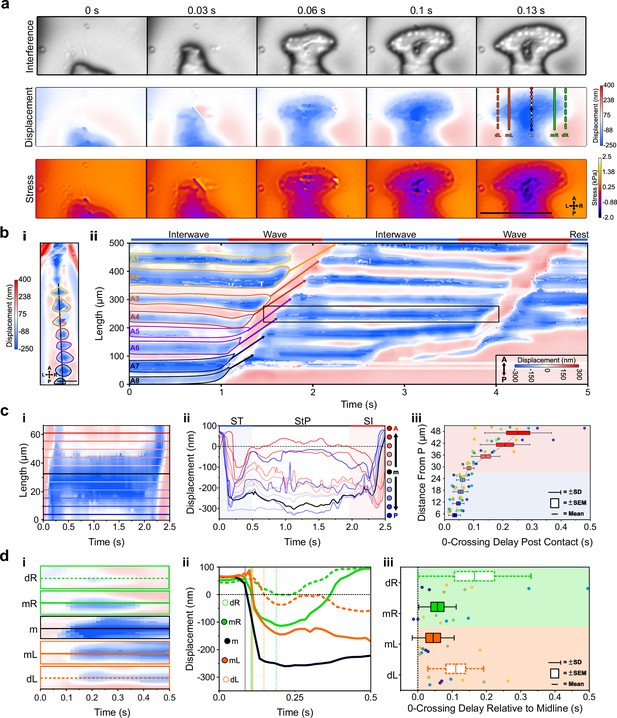
Sub-protopodial force dynamics reveal sub-step processes and functional substrate-interfacing domains in each step.
(a) Wavelength alternating resonance pressure (WARP) imaging of protopodial landing during swing termination (ST) of an A6 protopodium. Raw interference images from WARP acquisition show footprints of individual denticles as white dots. Displacement and stress maps show how landing starts with posterior denticle rows before spreading out along the anteroposterior (AP) and mediolateral (ML) axes. Scale bar = 100 µm. (b, i) Displacement map of whole animal. (ii) Kymograph of displacement along AP axis (black line in i) over two forward waves. Bands of red and blue correspond to naked cuticle and protopodia, respectively. Scale bar = 100 µm. (c, i) Kymograph of displacement along the AP axis of an A6 protopodium (box in b). (ii) Profiles across kymograph at different positions along the AP axis of protopodium (lines in i). (iii) Latency of substrate indentation (displacement <0 nm) during ST along the AP axis, relative to the extreme posterior of protopodium. Compared to the posterior half of protopodium (light blue area), the anterior half shows larger latencies and variations in latency (light red area). n = 4 animals, eight ST events. (d) Kymograph of displacement along AP axis during ST for the distal left (dL), medial left (mL), midline (m), medial right (mR), and distal right (dR) section of the A6 protopodium. Height of each kymograph, 66.42 µm. (ii) Profiles across the central AP line of each kymograph in (i). Vertical lines indicate times when midline, medial right/left, and distal right/left indentation starts (displacement <0 nm). (iii) Latency of substrate indentation during ST relative to the midline for medial right/left and distal right/left locations. n = 4, eight swing termination events.
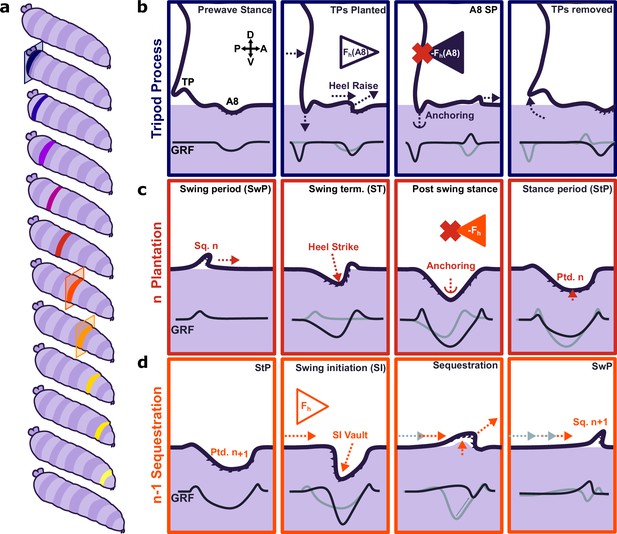
Proposed model for protopodia–substrate interactions during Drosophila larval locomotion.
(a) Schematic illustration of forward wave propagating from posterior (blue) to anterior (yellow). (b) At the start of a forward wave, animals contract the posterior-most abdominal segment (A8), producing an anterograde horizontal force Fh (A8). Due to Newton’s third law, there is an equal but opposite reaction force -Fh (A8). To counteract this force, tripod processes (TPs) deploy onto the substrate and generate a temporary anchor, allowing the A8 protopodium to swing forward. (c) During swing termination (ST) at the end of the swing period (SwP) of segment n, the corresponding sequestered protopodium (Sq. n) strikes the substrate with its posterior most denticle row, then gradually unfolds into the substrate along its entire anteroposterior extent. During the stance period (StP), this planted segment n (Ptd. n) forms an anchor to mitigate the retrograde reaction force due to the subsequent contraction of segment n-1. (d) In time with anchoring of protopodium n, protopodium n-1 performs swing initiation (SI) by removing denticles from the substrate and sequestering into an invagination pocket, which reduces friction during the subsequent SwP. The contraction of segment n-1 then leads to an anterograde force (Fh) that is balanced by the anchoring of protopodium n as illustrated in (c).
Videos
Lateral view crawing.
Video showing the sequestration and planting of protopodia during locomotion from a lateral view.
Kinematic tracking of forward and backward peristaltic waves.
Manual tracking of 33 points across the body during forward and backward peristalses.
Wavelength alternating resonance pressure (WARP) imaging during forward peristalses.
Video showing high frame rate displacement maps produced by a freely behaving Drosophila larva. Displacement maps were high-pass Fourier filtered to make denticulated cuticle more readily visible and projected in 3D to show the effects of substrate interaction. Details of the Fourier filtering procedure are described in a previous study (Kronenberg et al., 2017b).
Interference mapping of body mass redistribution during anterior bilateral behaviours.
Video showing the raw reflection data during the preparatory phase of bilateral behaviours.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Biological sample (Drosophila melanogaster) | Canton S (wildtype) | Bloomingtons Stock Center | FBsn0000274 ID 64349 | |
| Software, algorithm | OriginPro 2019b | OriginLab Corporation | Statistical analysis and plotting | |
| Software, algorithm | COMSOL Multiphysics | COMSOL Inc | Finite element method simulation resolving stress maps | |
| Software, algorithm | Python 3.0 and 2.0 | Anaconda Inc | Cavity length map computation | |
| Software, algorithm | Inkscape v.1.01 | Inkscape Organisation | Vector figure making | |
| Software, algorithm | FIJI | National Institutes of Health/SciJava | 1.52p | Image analysis and manual tracking |
| Chemical compound, drug | Phosphate-buffered saline | Gibco | 10010023 | Collagen coating |
| Chemical compound, drug | Hydrochloric acid 5 M | Sigma-Aldrich | 10605882 | Collagen coating |
| Chemical compound, drug | Acetic acid 95% | VWR | 84528.290 | Collagen coating |
| Chemical compound, drug | Collagen-I | Millipore | L7220 | Collagen coating |
| Other | Gold grains (99.99%) | Kurt J. Lesker Company | EVMAU40SHOT | Microcavity fabrication |
| Other | Chromium 99.95% | Kurt J. Lesker Company | EVMCR35 EJTCRXX351 | Microcavity fabrication |
| Other | Silicon Dioxide Fused quartz target | Kurt J. Lesker Company | EJUSIO2451 | Microcavity fabrication |
| Chemical compound, drug | NusilGel8100 | Nusil | GEL-8100 | Microcavity fabrication |
| Chemical compound, drug | Sygard527 | Dowsil | 2270030 | Microcavity fabrication |
| Chemical compound, drug | Sylgard184 | Dowsil | 1673921 | Microcavity fabrication |
| Chemical compound, drug | Ethyl butanoate | VWR | ACRO118182500 | Retaining animals within field of view |
| Chemical compound, drug | Mineral (Paraffin) oil | VWR | 31911.D9 | Suspension of ethyl butanoate |
| Other | 24 mm2 glass substrate | ORSAtec | 2.01.03.0167.59.16.1 | Microcavity fabrication |
| Other | FlexAFM | Nanosurf | Atomic force microscope | |
| Other | uniqprobe Cantilevers | Nanosensors | qp-CONT | Stiffness calibration by atomic force microscopy |
| Other | CM110 Monochromator | Spectral Products | Monochromator for scanning wavelength ERISM | |
| Other | Optical cage system components | Thorlabs | Cage system for ERISM and WARP, see supplementary information | |
| Other | EMS 6000 Photoresist Spincoater | Electronic Microsystems | EMS 6000 | Microcavity fabrication |
| Other | Ultra high vacuum deposition chamber | Ångstrom Engineering | Microcavity fabrication | |
| Other | Andor Zyla 4.2 10-Tap | Andor Technology | WARP and ERISM image acquisition | |
| Other | iCube CMOS | NET GmbH | NS4203BU | Brightfield image acquisition |
| Other | XIMEA CMOS | XIMEA GmbH | MQ013MG-E2 | Behavioural image acquisition |
| Chemical compound, drug | CHAPS, 3-[(3-cholamidopropyl)dimethyl ammonio]–1-propane sulfonate | Acros Organics | 10834531 | Electrostatic buffer for atomic force microscopy measurements |
Additional files
-
Supplementary file 1
Measured effective Young’s modulus per elastomer mixture post plasma treatment.
- https://cdn.elifesciences.org/articles/87746/elife-87746-supp1-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/87746/elife-87746-mdarchecklist1-v1.docx





