Free volume theory explains the unusual behavior of viscosity in a non-confluent tissue during morphogenesis
Figures
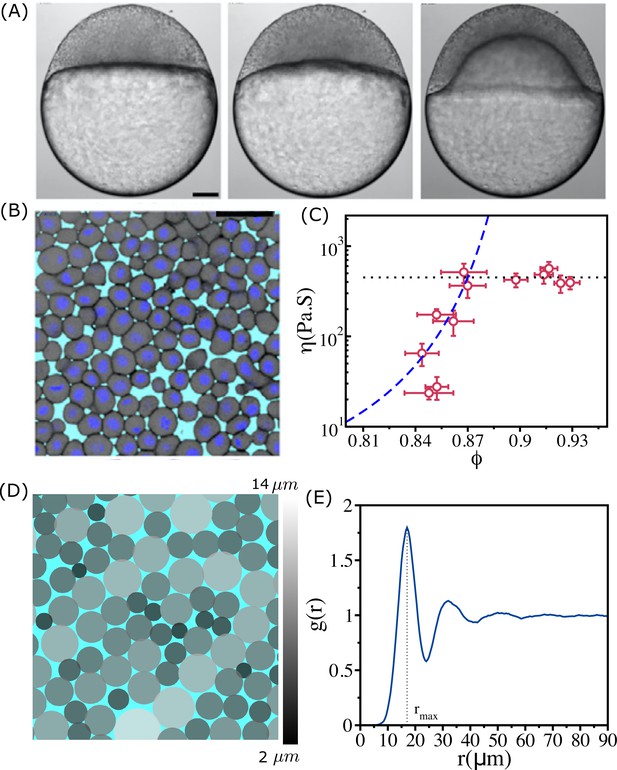
Structure and viscosity of non-confluent tissues.
(A) Bright-field single-plane images of an exemplary embryo of zebrafish before ( min), at the onset ( min), and after blastoderm spreading ( min). (B) Snapshot of 2D confocal sections at the 1st–2nd deep-cell layer of the blastoderm at min. (A) and (B) are taken from Petridou et al., 2021. (C) Viscosity of zebrafish blastoderm as a function of in a log-linear scale using the data from Petridou et al., 2021. The dashed line is the fit to Vogel–Fulcher–Tammann (VFT) equation. Note that does not change significantly beyond . (D) A typical snapshot taken from cell-based simulations for . Cells are colored according to their radii (in µm) (color bar shown on the right). (E) The pair correlation function, , as a function of for . The vertical dashed line is the position of the first peak (). The pair correlation function does not exhibit signs of long-range order. Scale bars in (A) is 100 µm and (B) is 50 µm.
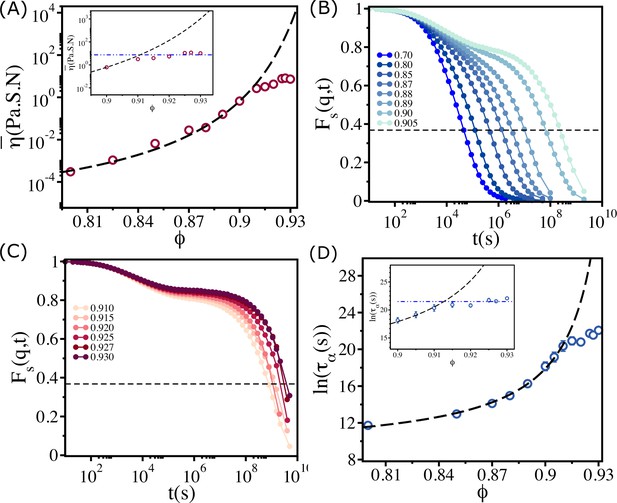
Saturation in viscosity and relaxation time.
(A) Effective viscosity as a function of , with the solid line being the fit to Vogel–Fulcher–Tammann (VFT) equation. The inset shows at high . The dashed line in the inset is the expected behavior assuming that the VFT relation holds at all . (B) The self-intermediate scattering function as a function of for . The dashed line corresponds to . (C) A similar plot for . (D) The logarithm of the relaxation time as a function of . The VFT fit is given by the dashed line. The inset shows a zoomed-in view for . The error bars in (D) are calculated using the standard deviation of for 24 independent simulations.
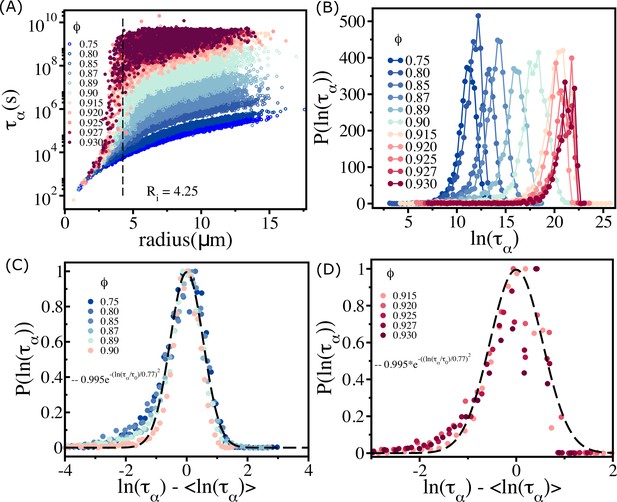
Spectrum of relaxation times.
(A) Scatter plot of relaxation times as a function of cell radius. From top to bottom, the plot corresponds to decreasing . The vertical dashed line is for , beyond which the changes sharply at high packing fractions. (B) Histogram as a function of . Beyond (), the histogram peaks do not shift substantially towards a high values. (C) For (scaled by ) falls on a master curve, as described in the main text. (D) Same as (C) except the results are for . The data deviates from the Gaussian fit, shown by the dashed line.

Density-dependent cell–cell overlap.
(A) Probability of overlap () between two cells, , for various values.The peak in the distribution function shifts to higher values as increases. (B) Mean as a function of . Inset shows a pictorial illustration of between two cells with radii and at a distance .
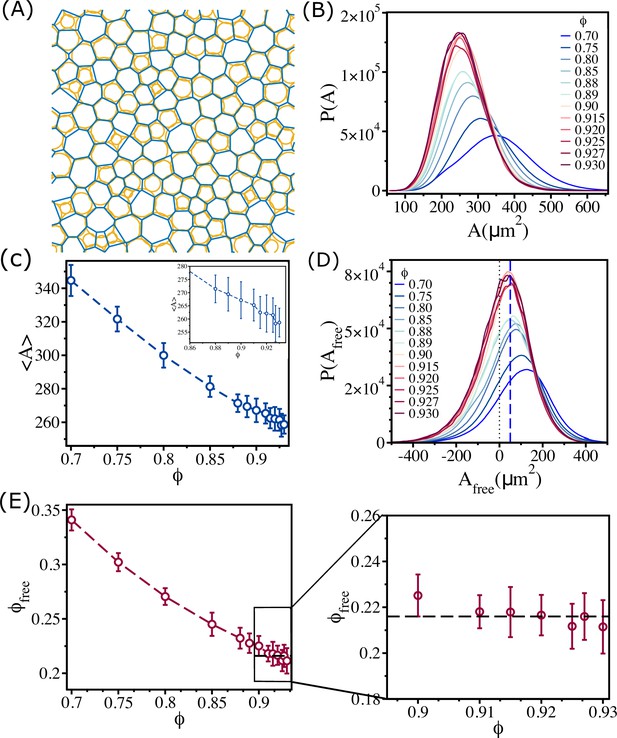
Changes in free area fraction with .
(A) Voronoi tessellation of cells for for a single realization. The orange circles represent actual cell sizes. The blue polygons show the Voronoi cell size. (B) Distribution of Voronoi cell size as a function of . (C) Mean Voronoi cell size as a function of . A zoomed-in view for is shown in the inset. (D) Distribution of free area for all . The vertical blue dashed line shows that the maximum in the distribution is at . (E) Free area fraction as a function of . Note that saturates beyond . An expanded view of the saturated region is shown in the right panel of (E). The error bars in (C) and (D) are the standard deviation in and , respectively, for 24 independent simulations.

Relaxation in the polydisperse cell system is independent of the waiting time.
(A) for at different waiting times (. Regardless of the value of , all the curves collapse onto a master curve. (B) Relaxation time, , as a function of . Over a three orders of magnitude change in , the variation in relaxation times is less than the sample-to-sample fluctuations, as shown by the error bar. The error bars in (B) are the standard deviation in for 24 independent simulations.
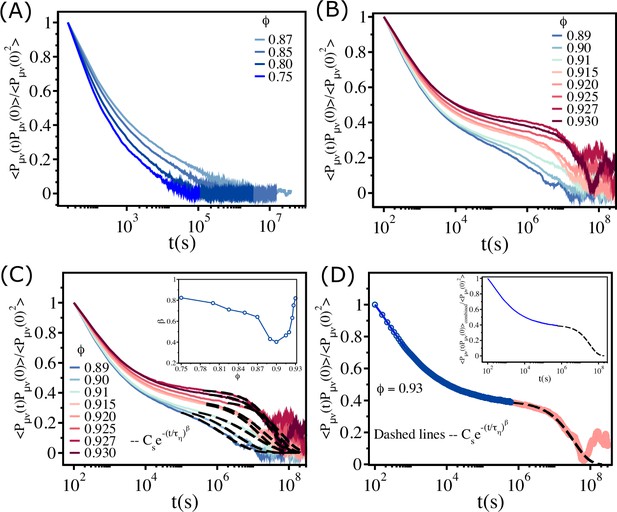
Fit of the stress–stress correlation functions to stretched exponential functions.
(A) The stress–stress correlation function divided by the value at as a function of for . (B) Similar plot for . (C) The long time decay of is fit to , as shown by the dashed lines. The inset shows the dependence of on . (D) The data that is fit using the stretched exponential function (black dashed line) is combined with the short time data (blue solid line), which is fit using the cubic spline function. The resulting fits produces a smooth curve , as shown in the inset.
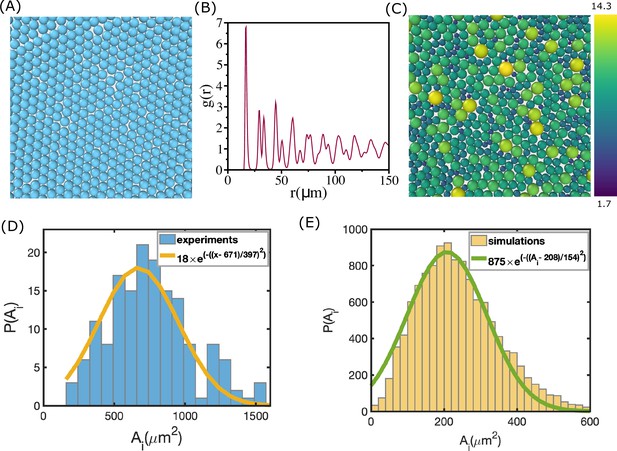
Area distribution of the cells.
(A) Simulation snapshot for monodisperse cell system. The number of cells in the two-dimensional periodic box is . (B) Pair correlation function, , as a function of . There is clear evidence of order, as reflected in the sharp peaks at regular intervals, which reflects the packing in (A). (C) A schematic picture of polydisperse cell system from the simulations. Color bar on the right shows the scale of radii in µm. There is no discernible order. (D) Distribution of cell area extracted from experiment during morphogenesis of zebrafish blastoderm (extracted from Fig. S2(A)) (Petridou et al., 2021). (E) Same as (D) except, , used in a typical simulation. Cell radii vary from 2 µm to 15 µm.
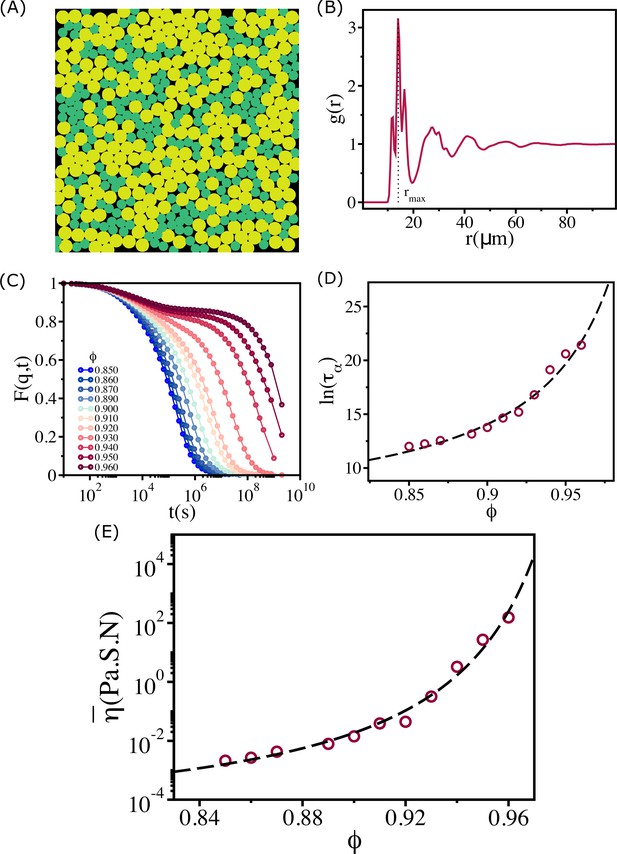
Structure and relaxation behavior for a binary mixture of cells.
(A) A typical simulation snapshot for binary mixture of cells at . (B) The corresponding pair correlation function, , between all the cells. The vertical dashed line is at the first peak position (). (C) , with , where is the location of the first peak in the , as a function of time at various values. (D) The logarithm of the relaxation time, , as a function of . Over the entire range of , the increase in is well fit by the Vogel–Fulcher–Tammann (VFT) (VFT) relation. Most importantly, the relaxation time does not saturate, which means the evolving tissue cannot be modeled using a 50:50 binary mixture. (E) Effective shear viscosity as a function of reflects the behavior of as a function of in (D).
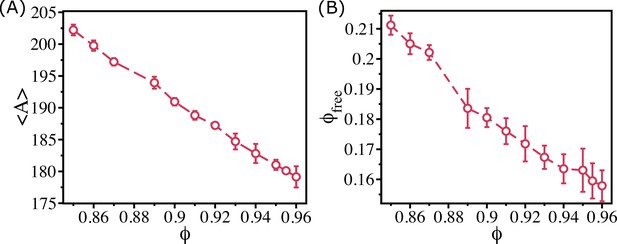
Free area decreases monotonically for the binary mixture of cells.
(A) Mean Voronoi cell size, , as a function of for the 50:50 binary system. (B) The free area fraction, , as a function of shows that decreases monotonically as increases. The error bars in (B) are the standard deviation in $\phi_{free}$ for 24 independent simulations.
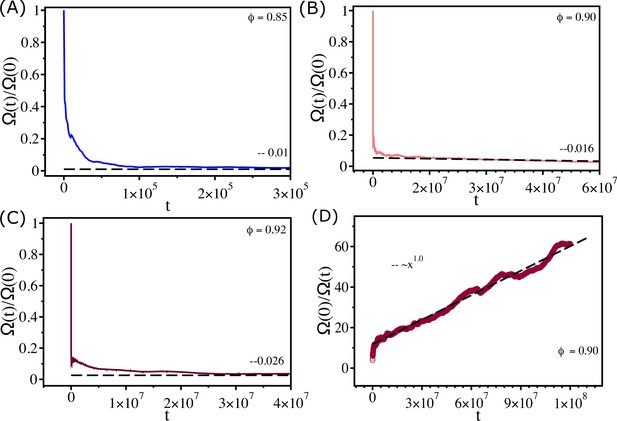
Measure of ergodicity.
(A) Ergodic measure scaled by the value at () as a function of for . (B, C) Similar plots for and , respectively. At long time, reach , , and for , , and , respectively. (D) as a function of for . The dashed line shows a linear fit. The time t is in second.
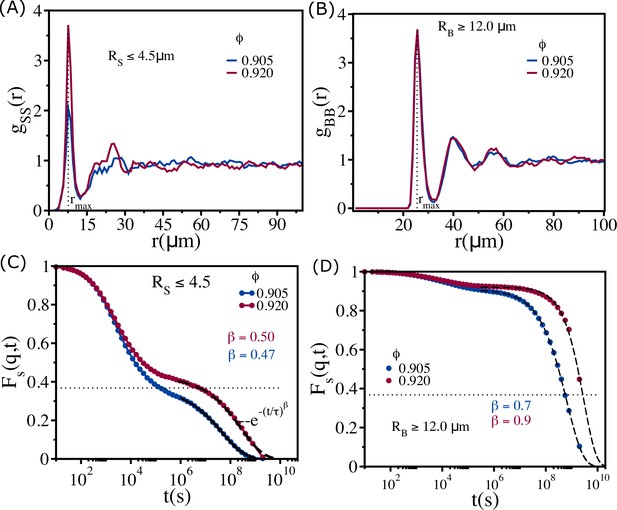
Cell size-dependent structures and dynamics.
(A) Radial distribution function between small-sized cells () at (blue) and (red). These values are greater than . (B) Same as (A) except the results are for between large cells (). (C) for cells with at and . Note that even at these dense packings, the mobility of the smaller-sized cells is substantial, which is reflected in the time dependence of . (D) for cells with at and . The black dashed lines are fits to stretched exponential functions, , where is the relaxation time and is the stretching exponent. The dotted lines correspond to the value .
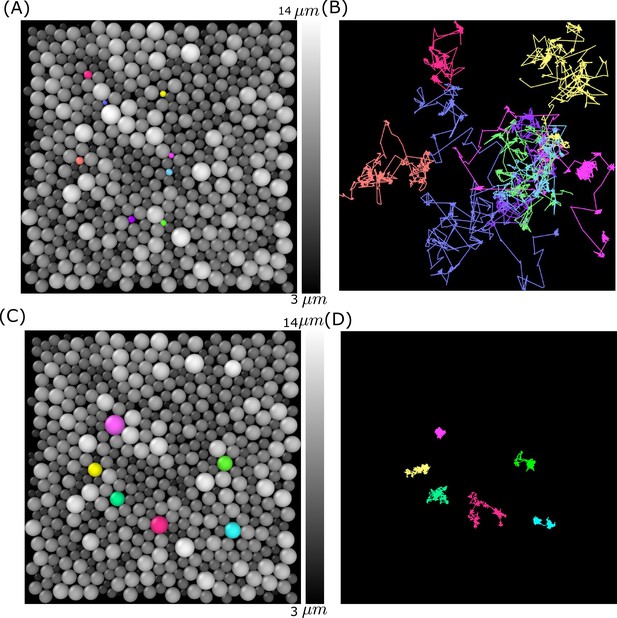
Simulation snapshot and trajectories for a few smaller- and bigger-sized cells.
(A) Cells () are colored according to their sizes (gray colors). A few small-sized cells are shown in different colors (pink, blue, orange, purple, cyan, light purple, and yellow). (B) The corresponding trajectories are shown over the entire simulation time. (C) Similar plot as (A) but for a few bigger-sized cells shown in purple, yellow, light green, red, cyan, and green colors. (D) Same as (B) except the trajectories of the large-sized cells are highlighted. Clearly, the large cells are jammed.
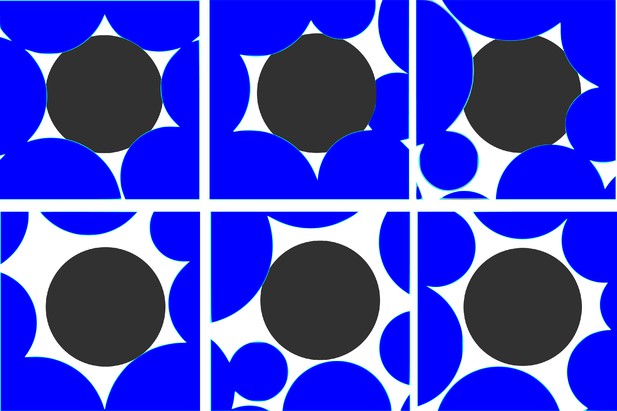
Dynamical rearrangement of jammed cells.
The changing local environment of a randomly selected cell (black) over time. Top panels: from left to right, , and . The black-colored cell is completely jammed by other cells. Bottom panels: from left to right, , and . Dynamical facilitation, resulting in collective rearrangement of the cells surrounding the black cell, enables it to move in the dynamically created free volume.
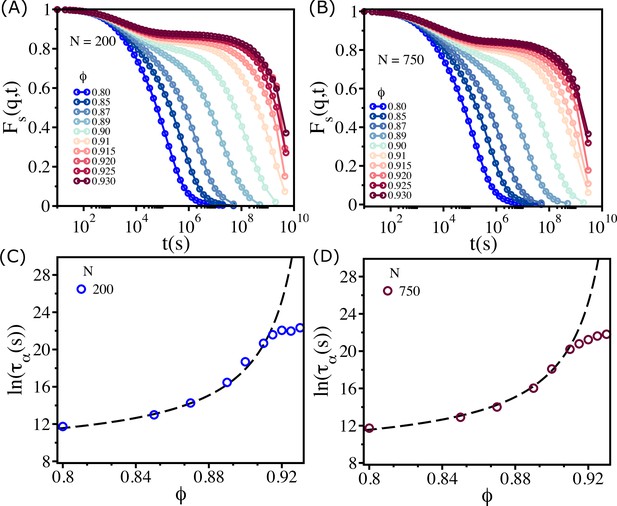
Finite size effects.
for (A) and (B). Logarithm of as a function of for (C) and for (D). The dashed lines are the Vogel–Fulcher–Tammann (VFT) fits.
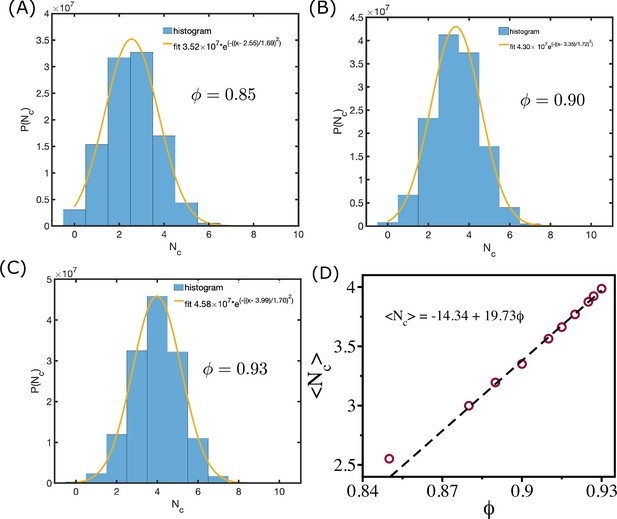
Mean coordination number and cell area fraction.
(A––C) shows the distribution of coordination number for = 0.85, and , respectively. The orange lines are Gaussian fits to the histograms. (D) shows mean as a function of . The dashed line shows the linear relationship between them.
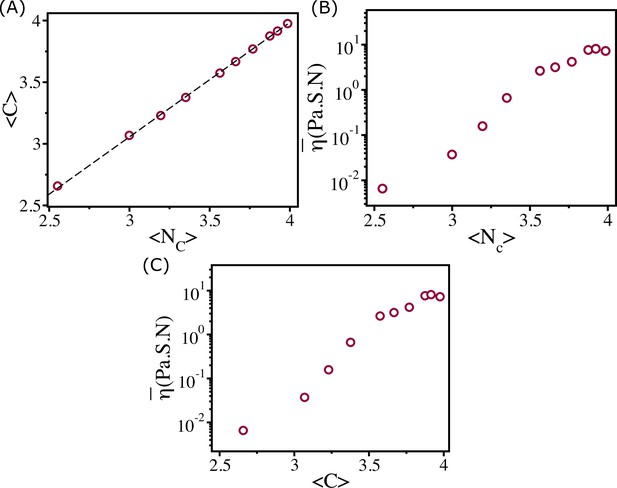
Viscosity and coordination number.
(A) shows as a function of . Clearly they are linearly related as shown by the dashed line. Viscosity as a function of (B) and (C).

Connectivity profile.
Connectivity maps for , and are shown in (A), (B), (C), (D), (E), and (F), respectively. For , there is a path that connects the cells in the entire sample. The percolation transition occurs over a very narrow range of (roughly at ; orange map), which also coincides with the sharp increase in , thus linking equilibrium transition to geometric connectivity.
Videos
Shows multiple rearrangements of smaller sized cells (blue and green cells) causes the big cells (yellow cells) to move in a highly jammed environment ().
Bright colors show the cell-cell overlap. Note that the overlap values are higher than those in lower area fractions. Free spaces (black background) are changing dynamically around a cell.
Shows how a big cell (yellow) moves in the crowded environment .
Note that the smaller-sized cells (colored as deep blue) always move faster. Again, the multiple rearrangement causes the bigger cell to move substantially. The amount of overlap is smaller than that at .
Shows the movements of cells at a low area fraction ().
Note that the smaller and bigger-sized cells are almost equally faster at lower area fractions () because of the huge available free areas.
Tables
Parameters used in the simulation.
| Parameters | Values | References |
|---|---|---|
| Timestep () | This paper | |
| Self-propulsion (µ) | This paper | |
| Friction coefficient () | This paper | |
| Mean cell elastic modulus ( | Galle et al., 2005; Malmi-Kakkada et al., 2018 | |
| Mean cell Poisson ratio () | 0.5 | Schaller and Meyer-Hermann, 2005; Malmi-Kakkada et al., 2018 |





