Associative memory neurons of encoding multi-modal signals are recruited by neuroligin-3-mediated new synapse formation
Figures
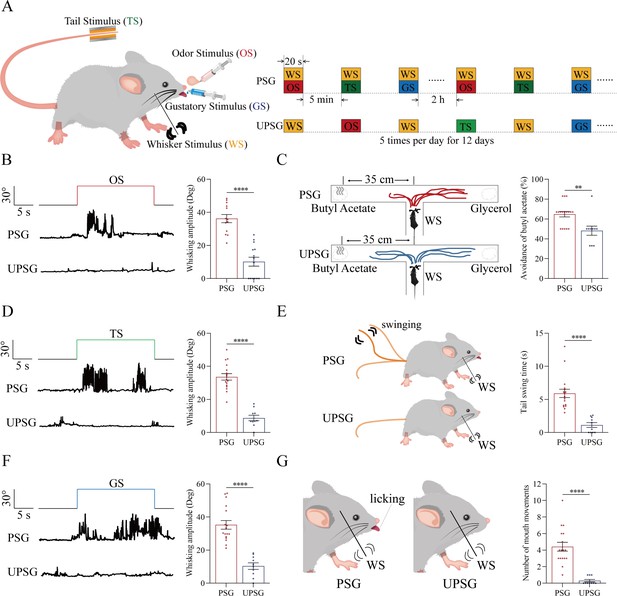
The pair-stimulations of the whisker stimulus (WS) with the odor stimulus (OS), the tail-heating stimulus (TS), and the gustatory stimulus (GS) sequentially lead to odorant-induced whisker motion versus whisking-induced olfactory response, tail-heating-induced whisker motion versus whisking-induced tail swing, and gustation-induced whisker motion versus whisking-induced taste response.
(A) The associative learning in C57BL/6JThy1-YFP mice was conducted by the pair-stimulations of the whisker tactile signal with the olfactory butyl acetate signal, the whisker tactile signal with the sucrose taste signal, and the whisker tactile signal with the tail-heating signals sequentially in mice, which were assigned in paired-stimulus group (PSG), compared to mice in unpaired-stimulus group (UPSG), for 12 days. (B, D, F) The OS, the TS, and the GS appear to induce whisker motions in a fluctuation pattern in PSG mice, respectively, but not in UPSG mice. Calibration bars are 30°of whisker deflection and 5 s. The statistical analyses in right panels show whisking amplitudes in response to the OS, TS, and GS in PSG mice (red bar) and in UPSG mice (blue bar). (C) As indicated by the moving trace, PSG mice prefer to move away from the butyl acetate side as the olfactory response to the whisker stimulus compared with the UPSG mice (left panel). The percentage of avoidance of butyl acetate in PSG mice (red bar, right panel) and in UPSG mice (blue bar). (E) The WS appears to induce tail swing after training in PSG, but not in UPSG mice (left panel). The statistical analyses about tail swing time in response to the WS in PSG mice (red bar, right panel) and in UPSG mice (blue bar). (G) The WS appears to induce mouth movements after training in PSG mice, but not in UPSG mice (left panel). The statistical analyses about number of mouth movements in response to the WS in PSG mice (red bar, right panel) and in UPSG mice (blue bar).
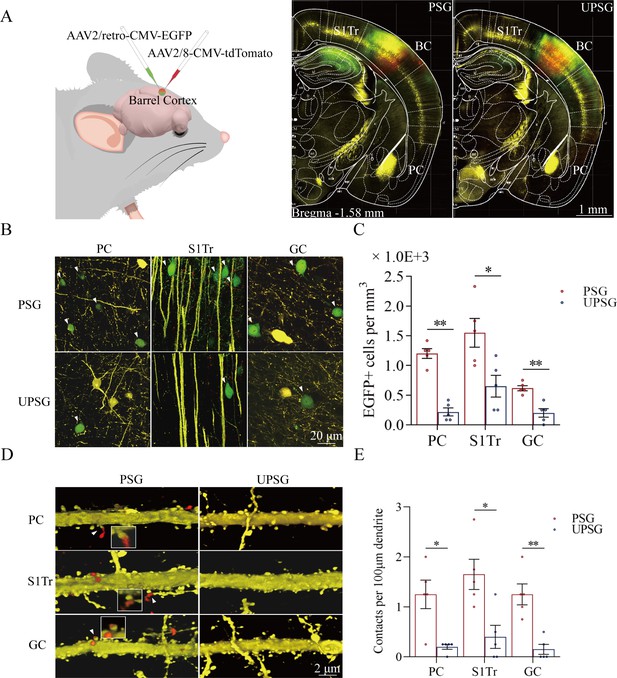
Associative learning by pairing whisker stimulus (WS) with the odor stimulus (OS), WS with tail-heating stimulus (TS), and WS with gustatory stimulus (GS) induces synapse innervations from PC, S1-Tr, and GC to BC, as well as from BC to PC, S1-Tr, and GC.
(A) The AAV2/retro-CMV-EGFP and AAV2/8-CMV-tdTomato were microinjected into the barrel cortex. (B) EGFP-labeled neurons in the PC, S1-Tr, and GC in paired-stimulus group (PSG) mice and unpaired-stimulus group (UPSG) mice. (C) The densities of EGFP-labeled neurons in the PC, S1-Tr, and GC in PSG mice (red bar) and UPSG mice (blue bar). (D) Td-Tomato-labeled axonal boutons from BC and their contacts on the dendritic spine of PC, S1-Tr, and GC neurons in PSG mice and UPSG mice. (E) The densities of synapse contacts consisting of tdTomato-labeled boutons and yellow fluorescent protein (YFP)-labeled spines in the PC, S1-Tr, and GC in PSG mice (red bar) and UPSG mice (blue bar).
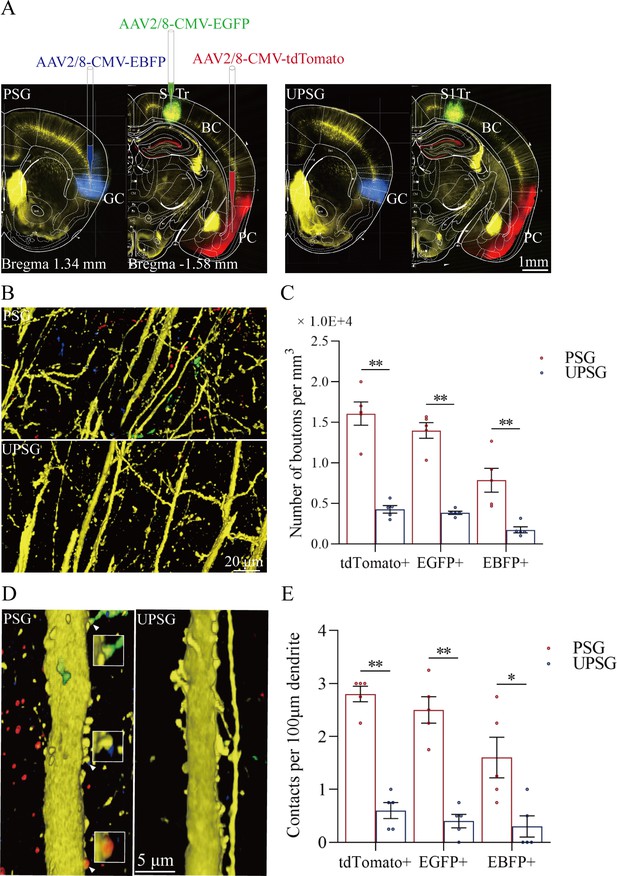
The convergent synapse innervations of neurons from the piriform cortex, gustatory cortex, and S1-Tr cortex onto barrel cortical neurons.
(A) Neuronal tracing was done by injecting AAV2/8-CMV-EBFP into the GC, AAV2/8-CMV-tdTomato into the PC, and AAV2/8-CMV-EGFP into the S1-Tr, and by detecting their presence in the BC. (B) The axon boutons labeled by EBFP, EGFP, and tdTomato are detected in the BC of paired-stimulus group (PSG) mice (top panel), compared with those in unpaired-stimulus group (UPSG) mice (bottom panel). (C) The densities of EBFP-labeled, EGFP-labeled, and tdTomato-labeled boutons in the BC in PSG mice (red bar) and in UPSG mice (blue bar). (D) Synapse contacts between the spines of yellow fluorescent protein (YFP)-labeled glutamatergic neurons and the axon boutons labeled by EBFP, EGFP, or tdTomato are detected in the BC of PSG mice (left panel), compared with those in UPSG (right panel). (E) The densities of synapse contacts between the spines of YFP-labeled neurons and the axon boutons labeled by EBFP, EGFP, or tdTomato in PSG mice (red bar) and in UPSG mice (blue bar).
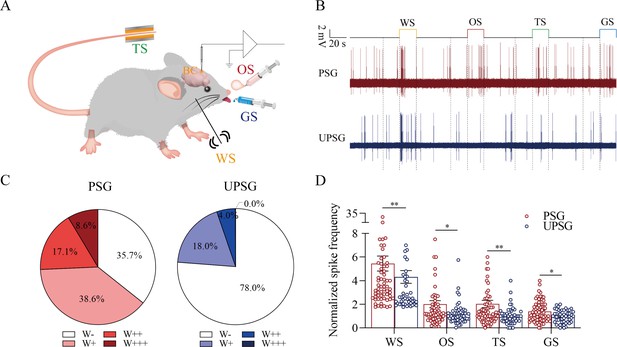
The barrel cortical neurons were responding to odor, tail-heating, and gustatory sucrose signals alongside the whisker tactile signal in paired-stimulus group (PSG) mice.
(A) A diagram illustrates a recording of local field potentials (LFPs) in the barrel cortex. (B) An example of barrel cortical neuron in response to butyl acetate, tail-heating, sucrose, and whisker tactile signals from PSG mouse (red trace) and unpaired-stimulus group (UPSG) mouse (blue trace). The calibration bars are 2 mV and 20 s. (C) The percentages of associative neurons in PSG mice (left panel) and in UPSG mice (right panel). Neurons that respond only to whisker stimulus (WS) are labeled as W-, and those that respond to WS and one, two, or all three other signals are labeled as W+, W++, and W+++, respectively. (D) The normalized spike frequencies in response to whisker tactile signals, butyl acetate, tail-heating signal, and sucrose signal in PSG (red bar) and in UPSG (blue bar), respectively.
The interconnections of the barrel cortex with the piriform, S1-Tr, and gustatory cortices enable this core of the barrel cortex constitute a linkage among piriform, S1-Tr, and gustatory cortices for the piriform cortex, the S1-Tr cortex, and the gustatory cortex to be indirectly interconnected.
(A) The barrel cortex can become the core station for the first-order and secondary-order associative memory. (B) The tail-heating or sucrose appears to induce the olfactory responses in paired-stimulus group (PSG) mice, in which the tail-heating or the sucrose induces mice away from the butyl acetate due to their smelling this odorant (examples in top and bottom ‘T’ mazes, respectively), but not in unpaired-stimulus group (UPSG) mice. (C) Percentages away from the butyl acetate block by the tail-heating and the sucrose in PSG mice (red bar) and in UPSG mice (blue bar). (D) The butyl acetate or the sucrose appears to induce the tail swing besides the whisker fluctuation in PSG mice, but not in UPSG mice. (E) The durations of tail swing by the butyl acetate and the sucrose in PSG mice (red bar) and in UPSG mice (blue bar). (F) The butyl acetate or the tail-heating appears to induce the gustatory responses besides the whisker fluctuation in PSG mice, but not in UPSG mice. (G) The times of tongue-out licking by the butyl acetate and the tail-heating in PSG mice (red bar) and in UPSG mice (blue bar).
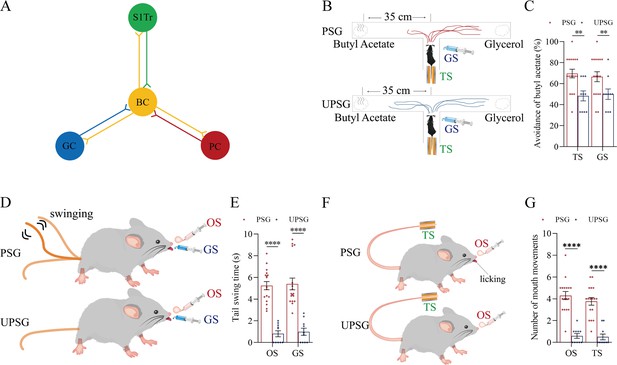
Neuroligin-3 knockdown significantly downregulates the joint storage and the reciprocal retrieval of the associated signals.
(A) The downregulation of neuroligin-3 through short-hairpin RNA (shRNA) is expected to suppress the innervation of new synapses as well as the recruitment of associative memory neurons in the BC. (B) shRNA specific for neurolingin-3 and its scramble control RNA were microinjected into the barrel cortices in shRNA group and shRNA scramble subgroups. (C) The amplitudes of whisker motion induced by the odor stimulus (OS), tail-heating stimulus (TS), and gustatory stimulus (GS) in scramble group (red bar) and shNlgn3 group (blue bar). (D) The left panel shows the rates of avoidance to the butyl acetate in whisking-induced olfactory response in neuroligin-3 knockdown mice (blue bar) and in shRNA scramble control mice (red bar). The middle panel shows the durations of whisking-induced tail swing in neuroligin-3 knockdown mice and in shRNA scramble control mice. The right panel shows the times of whisking-induced lip lickings in neuroligin-3 knockdown mice and in shRNA scramble control mice. (E) Percentages away from the butyl acetate block by the tail-heating and the sucrose in shRNA scramble control mice (red bar) and in neuroligin-3 knockdown mice (blue bar). (F) The durations of tail swing by the butyl acetate and the sucrose in shRNA scramble control mice (red bar) and in neuroligin-3 knockdown mice (blue bar). (G) The times of tongue-out licking by the butyl acetate and the tail-heating in shRNA scramble control mice (red bar) and in neuroligin-3 knockdown mice (blue bar).
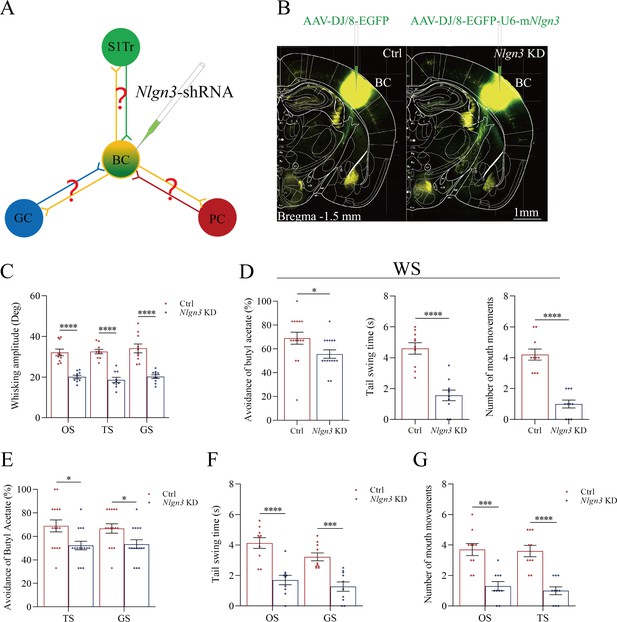
The suppression of convergent synapse innervations on barrel cortical neurons by neuroligin-3 knockdown.
(A, D, G) AAV-DJ/8-EGFP was injected into the BC in scramble control mice and AAV-DJ/8-EGFP-U6-mNlgn3 was injected into the BC in neuroligin-3 short-hairpin RNA (shRNA) mice before training. AAV2/8-CMV-tdTomato was microinjected into the S1-Tr cortex, AAV2/8-CMV-EBFP was into the piriform cortex (A). AAV2/8-CMV-tdTomato was microinjected into the S1-Tr cortex, AAV2/8-CMV-EBFP was into the gustatory cortex (D). AAV2/8-CMV-tdTomato was microinjected into the piriform cortex, AAV2/8-CMV-EBFP was into the gustatory cortex (G). (B, E, H) Neuroligin-3 knockdown appears to prevent the learning-induced formation of the synapse contacts on barrel cortical neurons in neuroligin-3 shRNA mice, compared to those in scramble control mice. (C, F, I) The densities of synapse contacts (contacts per 100 μm dendrite) between the spines of yellow fluorescent protein (YFP)-labeled neurons and the axon boutons labeled by EBFP or tdTomato in scramble control group (red bar) compared to those in neuroligin-3 knockdown group (blue bar).
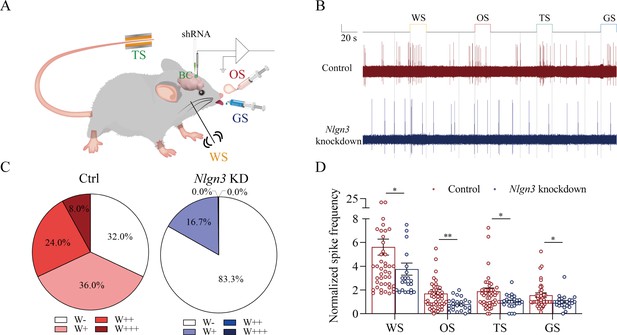
The response of barrel cortical neurons to odor, tail-heating, and gustatory sucrose signals alongside the whisker tactile signal were downregulated in shNlgn3 mice.
(A) A diagram illustrates microinjection of adeno-associated viruses (AAVs) and a recording of local field potentials (LFPs) in the barrel cortex. (B) An example of barrel cortical neuron in response to butyl acetate, tail-heating, sucrose, and whisker tactile signals from scramble mouse (red trace) and shNlgn3 mouse (blue trace). The calibration bars are 2 mV and 20 s. (C) The percentages of associative neurons in scramble mice (left panel) and in shNlgn3 mice (right panel). Neurons that respond only to whisker stimulus (WS) are labeled as W-, and those that respond to WS and one, two, or all three other signals are labeled as W+, W++, and W+++, respectively. (D) The normalized spike frequencies in response to whisker tactile signals, butyl acetate, tail-heating signal, and sucrose signal in scramble mice (red bar) and in shNlgn3 mice (blue bar), respectively.
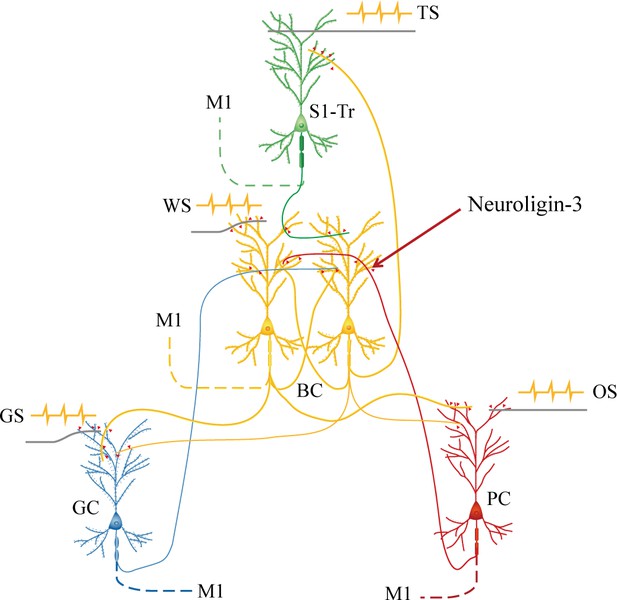
Barrel cortical neurons are recruited to be associative memory neurons in the hub-like core station, based on the synapse interconnections with piriform, S1-Tr, and gustatory cortices, in addition to their intramodal interconnection.
Molecular substrates for these cellular changes are based on the activity-dependent epigenetic processes and neuroligin-3-mediated synapse linkage. The associative learning by pairing the whisker stimulus (WS) with the odor stimulus (OS), the WS with the tail-heating stimulus (TS), and the WS with gustatory stimulus (GS) induces mutual synapse innervations from PC (red), S1-Tr (green), and GC (blue) to BC (yellow), as well as from BC to PC, S1-Tr, and GC, which drives the recruitment of barrel cortical neurons to be associative memory cells. The newly formed neural circuits constitute the foundation of multiple cross-modal memories.





