Thalamic regulation of ocular dominance plasticity in adult visual cortex
Figures
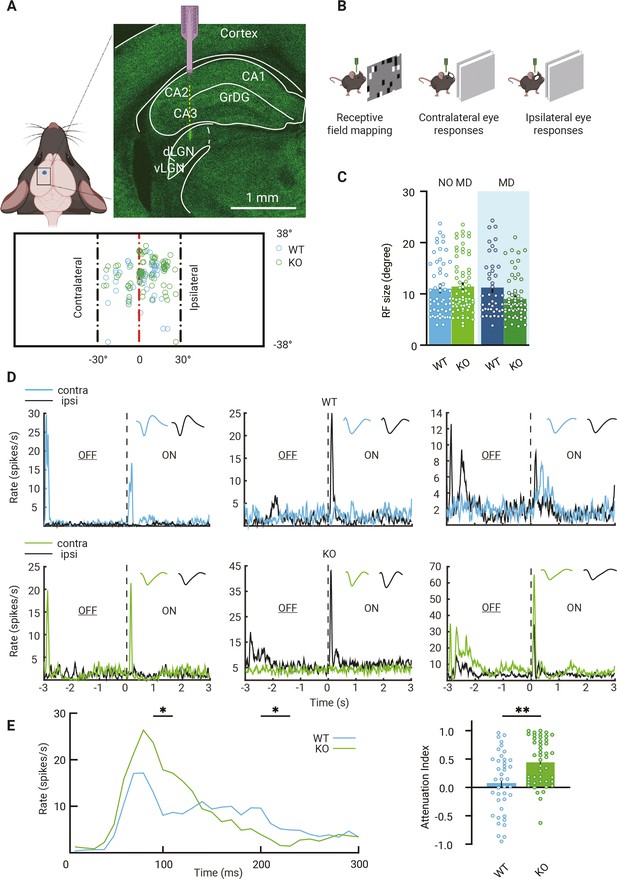
Visual responses of dorsolateral geniculate nucleus (dLGN) neurons in mice lacking thalamic Gabra1.
(A) Recording electrodes were placed in the ipsilateral projection zone of dLGN (see green fluorescent trace of actual electrode penetration in dLGN). All receptive field (RF) centers of multiunits recorded in wild-type (WT, blue) and Gabra1 cKO (KO, green) mice (n = 61 units from 13 non-deprived or monocularly deprived [MD] mice and n = 80 units from 18 NO MD or MD mice). Nose position is at 0° horizontally and vertically. The black dashed lines indicate –30° and +30° horizontal angles. (B) Experimental setup to measure RF and single-eye responses. (C) RF sizes of multiunits in NO MD and MD (shaded area) Gabra1 cKO and WT mice do not differ (two-way ANOVA, interaction of genotype with MD: p=0.07; Tukey’s post hoc test; WT NO MD vs. Gabra1 cKO NO MD: p=0.19; WT MD vs. Gabra1 cKO MD: p=0.11). (D) Examples of dLGN neuron responses to full-screen OFF-ON flash stimuli in WT and Gabra1 cKO mice. Colored and black lines indicate responses of contra- and ipsilateral eyes, respectively. Waveforms of each unit responding to the contra- or ipsilateral eye are shown in the upper-right corner. WT: left panel is a monocular unit, right panels are binocular units. Gabra1 cKO: left two panels are monocular units, right panel is a binocular unit (ZETA test). (E) Left, average responses of contralateral eye in WT and Gabra1 cKO mice. Gabra1 cKO mice show higher peak (90–110 ms) and lower prolonged responses (200–230ms ). (Repeated-measure two-way ANOVA, interaction of genotype with time, p=0.0001; post hoc, Fisher’s LSD test.) Right, attenuation index of visual responses in WT and Gabra1 cKO mice. * p<0.05, ** p<0.01 (C, E) Error bars indicate standard error of the mean.
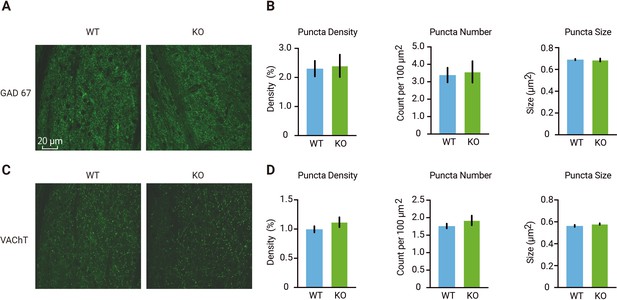
No differences in GAD67 or VACht puncta in dLGN of wild-type mice and mice lacking thalamic Gabra1.
(A) Examples of immunohistochemical staining for GAD67 in dorsolateral geniculate nucleus (dLGN) of adult wild-type (WT, blue) and Gabra1 cKO (KO, green) mice. (B) Quantification of the density, number, and sizes of GAD67 puncta (putative inhibitory boutons). No differences were detected between WT and Gabra1 cKO mice (t-test; puncta density, p=0.85; puncta number, p=0.82; puncta size, p=0.80. n = 3 mice per group, three slices were imaged per mouse). (C) Examples of immunohistochemical staining for VAChT puncta (putative cholinergic boutons) in dLGN of adult WT and Gabra1 cKO mice. Same scale was used as in panel A. (D) Quantification of the density, number, and sizes of VAChT. No differences were detected between WT and Gabra1 cKO mice (t-test; puncta density, p=0.29; puncta number, p=0.35; puncta size, p=0.22. n = 3 mice per group, three slices were imaged per mouse). (B, D) Error bars represent stand error of the mean.
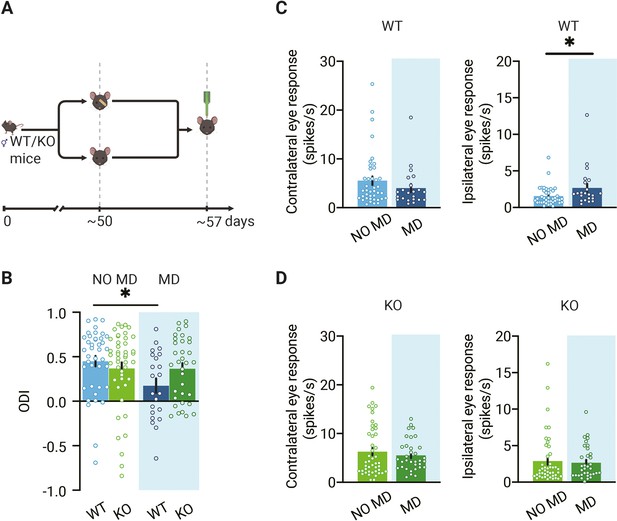
Loss of ocular dominance (OD) plasticity in dorsolateral geniculate nucleus (dLGN) of mice lacking thalamic synaptic inhibition.
(A) Illustration of the experiment design. In experiments, four groups of animals were used: deprived (MD) or non-deprived (NO MD) wild-type (WT, blue) and Gabra1 cKO (KO green) mice. Mice in the MD group had the eyelids of the eye contralateral to the recording side sutured for 7 d. (B) Seven days of MD reduces the ODI in WT mice but not in Gabra1 cKO animals (interaction of genotype with MD: two-way ANOVA, p=0.046, Tukey’s post hoc test; WT NO MD vs. WT MD, p=0.040; WT NO MD, n = 40 units, seven mice; WT MD, n = 22 units, six mice; Gabra1 cKO NO MD, n = 45 units, nine mice; Gabra1 cKO MD, n = 34 units, nine mice). (C) In WT mice, responses to the ipsilateral eye are significantly increased after 7-day MD. Responses to the contralateral eye are unchanged (Mann–Whitney; contralateral, NO MD vs. MD, p=0.29; ipsilateral, NO MD vs. MD, p=0.032). (D) In Gabra1 cKO mice, MD causes no significant changes in responses to either the contralateral or the ipsilateral eye (Mann–Whitney; contralateral, NO MD vs. MD, p=0.73; ipsilateral, NO MD vs. MD, p=0.59). (B-D) Error bars represent standard error of the mean. * p<0.05.
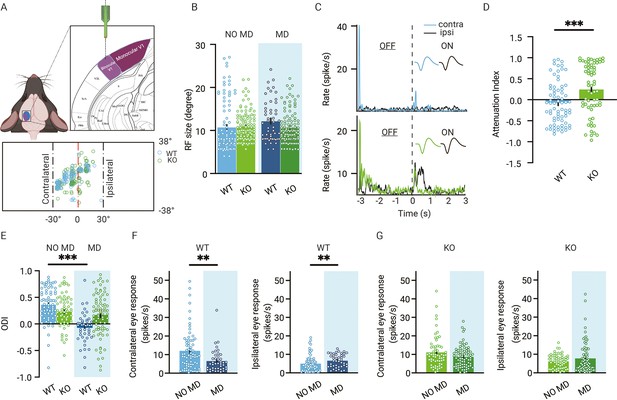
Reduced ocular dominance (OD) plasticity in adult V1 lacking thalamic OD plasticity.
(A) Recording electrodes are located in binocular V1. All receptive field (RF) centers of multiunits recorded in wild-type (WT, blue) and Gabra1 cKO (KO, green) mice (n = 112 units from 13 NO MD or MD mice and n = 138 units from 18 NO MD or MD mice). Nose position is at 0° horizontally and vertically. The black dashed lines indicate –30° and +30° horizontal angles. (B) RF sizes of units in WT and Gabra1 cKO mice do not differ (interaction of genotype with MD: two-way ANOVA, p=0.07). (C) Two examples of single-unit responses in V1 of a WT and Gabra1 cKO mouse to the contra- and ipsilateral eyes to ON and OFF visual stimuli. Each stimulus lasted 3 s. Colored and black lines indicate contra- and ipsilateral eye responses, respectively. (D) Attenuation index of contralateral eye responses in V1 of WT and Gabra1 cKO mice. (E) Seven days of MD reduces the OD index (ODI) in WT but not Gabra1 cKO mice (interaction of genotype with MD: two-way ANOVA, p<0.001, Tukey’s post hoc test; WT NO MD vs. WT MD, p<0.001; WT NO MD, n = 71 units, seven mice; WT MD, n = 42, six mice; Gabra1 cKO NO MD, n = 63 units, nine mice; Gabra1 cKO MD, n = 78 units, nine mice). (F) In WT mice, responses to the contralateral eye are significantly reduced after 7-day MD, while those to the ipsilateral eye are significant increased (Mann–Whitney; contralateral, NO MD vs. MD, p=0.0043; ipsilateral, NO MD vs. MD, p=0.0062). (G) In Gabra1 cKO mice, MD causes no significant changes in responses to either the contralateral or the ipsilateral eye (Mann–Whitney; contralateral, NO MD vs. MD, p=0.17; ipsilateral, NO MD vs. MD, p=0.66). (B, D, E-G) Error bars represent standard error of the mean. ** p<0.01, *** p<0.001.
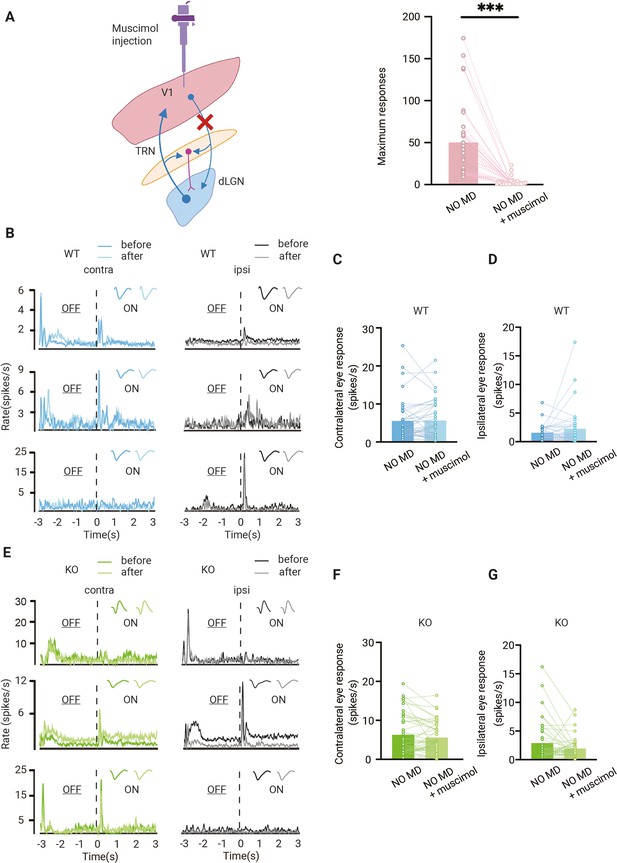
Effect of feedback from V1 to dorsolateral geniculate nucleus (dLGN) responses.
(A) Left, illustration of corticothalamic-thalamocortical feedback network. dLGN is innervated by V1 and receives glutamatergic feedback. All these projections send excitatory collaterals to the thalamic reticular nucleus (TRN) which sends inhibitory inputs to dLGN. By muscimol injection in V1, corticothalamic projections are silenced. Right, V1 is effectively silenced by muscimol injection (Wilcoxon signed rank, p<0.001, n = 31 mice). (B) Examples of dLGN responses before and after muscimol injection in V1 of non-deprived wild-type (WT, blue) mice. Waveforms of each unit are shown in the upper-right corner. Left and right panels correspond to contralateral and ipsilateral eye responses, respectively. Dark and light lines represent responses before and after muscimol injection, respectively. (C, D) Silencing V1 feedback has no significant effect on contralateral (C) or ipsilateral (D) responses in WT mice (Wilcoxon signed rank; contralateral, WT NO MD vs. WT NO MD with muscimol, p=0.62; ipsilateral, WT NO MD vs. WT NO MD with muscimol, p=0.94, n = 40 units, seven mice). (E) Examples of dLGN responses before and after muscimol injection in V1 of non-deprived Gabra1 cKO (KO, green) mice. Waveforms of each unit are shown in the upper-right corner. Left and right panels correspond to contralateral and ipsilateral eye responses, respectively. Dark and light lines represent responses before and after muscimol injection, respectively. (F, G) There is no significant effect of V1 silencing on contralateral (F) or ipsilateral (G) eye responses in Gabra1 cKO mice, but a trend towards decreased ipsilateral eye responses is present (Wilcoxon signed rank; contralateral, Gabra1 cKO NO MD vs. Gabra1 cKO NO MD with muscimol, p=0.19; ipsilateral, Gabra1 cKO NO MD vs. Gabra1 cKO NO MD with muscimol, p=0.059, n = 45 units, nine mice).
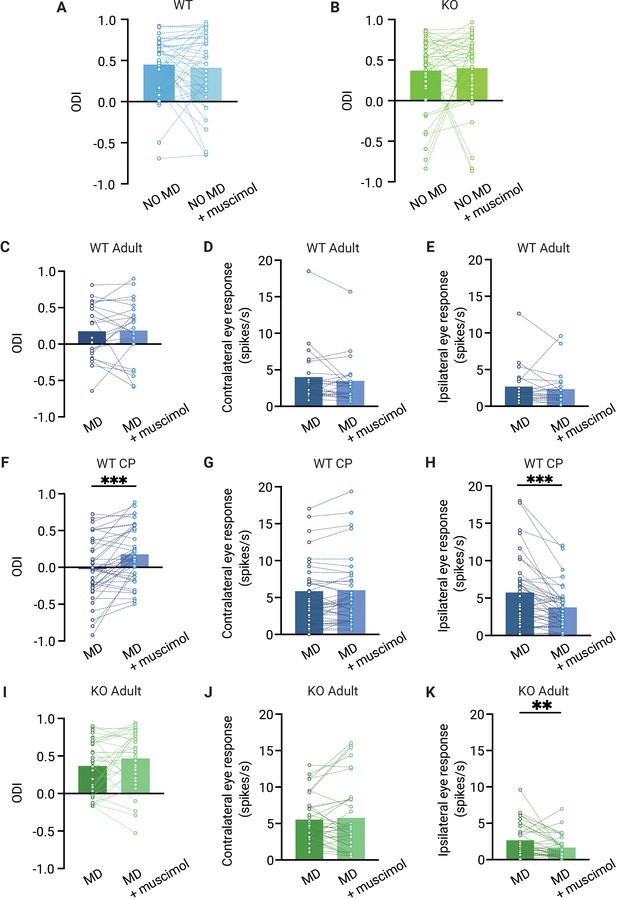
The ocular dominance (OD) shift in dorsolateral geniculate nucleus (dLGN) is independent from V1 feedback in adult mice but not in critical period mice.
(A, B) Muscimol injection in V1 has no effect on the OD index (ODI) in dLGN of adult non-deprived wild-type (WT, blue) (A) and Gabra1 cKO (KO, green) (B) mice (Wilcoxon signed rank; WT NO MD vs. WT NO MD with muscimol, p=0.86, n = 40 units, seven mice; Gabra1 cKO NO MD vs. Gabra1 cKO NO MD with muscimol, p=0.45, 45 units, nine mice). (C–E) Muscimol injection in V1 has no significant effect on the ODI (C), or contralateral (D) or ipsilateral (E) eye responses in dLGN of monocularly deprived WT mice (Wilcoxon signed rank; ODI, WT MD vs. WT MD with muscimol, p=0.89; contralateral, WT MD vs. WT MD with muscimol, p=0.21; ipsilateral, WT MD vs. WT MD with muscimol, p=0.10, n = 22 units, six mice). (F–H) During the critical period, V1 silencing has a significant influence on the ODI (F) and ipsilateral eye responses (H) in dLGN of monocularly deprived WT mice, but not on contralateral eye responses in these mice (G) (Wilcoxon signed rank; ODI, WT MD vs. WT MD with muscimol, p<0.001; contralateral, WT MD vs. WT MD with muscimol, p=0.46; ipsilateral, WT MD vs. WT MD with muscimol, p=0.003, n = 41 units, 10 mice). (I–K) Muscimol injection in V1 has no significant influence on the ODI and contralateral eye responses in Gabra1 cKO MD mice, but significantly modulates dLGN ipsilateral eye responses (Wilcoxon signed rank; ODI, Gabra1 cKO MD vs. Gabra1 cKO MD with muscimol, p=0.13; contralateral, Gabra1 cKO MD vs. Gabra1 cKO MD with muscimol, p=0.97; ipsilateral, Gabra1 cKO MD vs. Gabra1 cKO MD with muscimol, p=0.004, n = 34 units, nine mice). ** p< 0.01, *** p<0.001.

Ocular dominance (OD) plasticity and the effect of cortical silencing on OD during the critical period.
(A) Monocular deprivation (MD) induces ocular dominance plasticity in dorsolateral geniculate nucleus (dLGN) of wild-type (WT) mice during the critical period (Mann–Whitney; WT NO MD vs. WT MD, p=0.0015; WT NO MD, n = 36, 10 mice; WT MD, n = 41, 10 mice). (B) During the critical period, V1 feedback has no effect on ocular dominance (OD) in non-deprived WT mice (Wilcoxon signed rank; ODI, WT NO MD vs. WT MD with muscimol, p=0.20). *** p<0.001.





