An optogenetic cell therapy to restore control of target muscles in an aggressive mouse model of amyotrophic lateral sclerosis
Figures
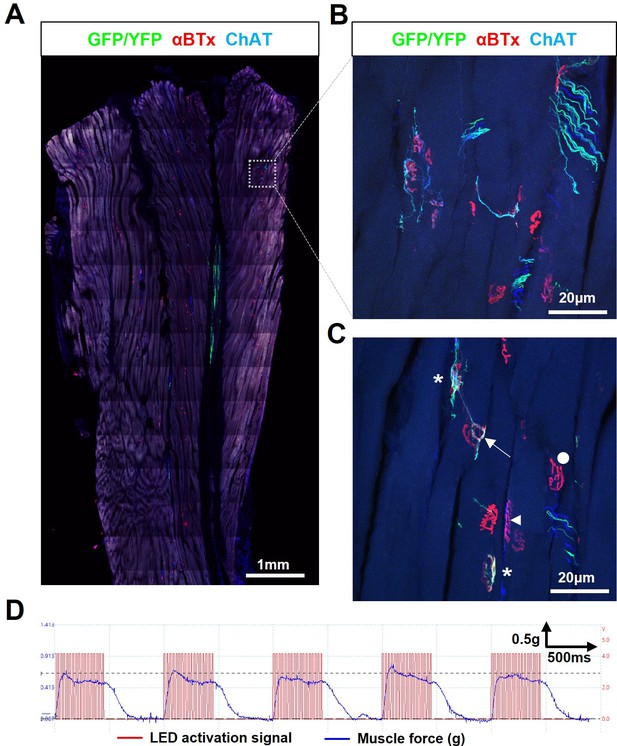
Engrafted allogeneic ChR2+ motor neuron can survive and robustly reinnervate target muscles in SOD1G93A mice but occurs rarely.
(A) Confocal tile-scan of a longitudinal section of the triceps surae muscle from a 135d SOD1G93A mouse, 45d post-engraftment, showing a rare example of graft survival, in the absence of immunosuppression, with robust intramuscular axon projection. (B) Maximum intensity projection (MIP) images of a confocal z-stack through region of interest (dashed box in (A)) and (C), showing NMJs fully (asterisks) and partially (arrow) innervated by ChR2+ motor neuron axons, as well as innervated by endogenous (GFP/YFP-negative, ChAT-positive) motor axons (arrowhead) and fully denervated endplates (circle). (D) Representative recording (n=1/3 positive responders) showing characteristically weak contractile responses to repetitive 20 Hz optical stimulation.
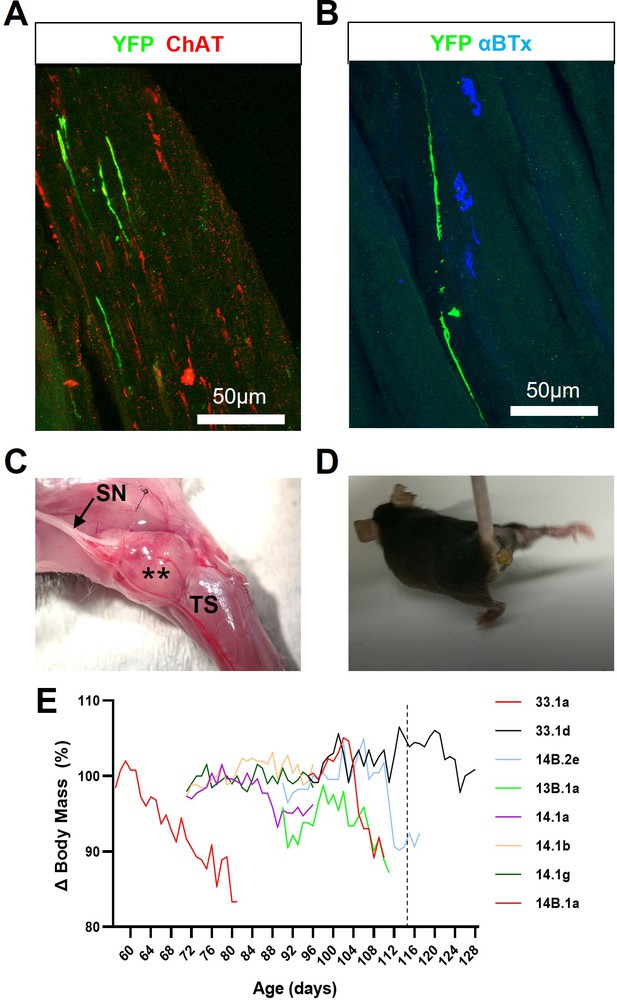
FK506 enables long-term survival of engrafted ChR2+ motor neurons but inhibits muscle reinnervation and exacerbates disease progression in SOD1G93A mice.
(A) Representative confocal image showing that GFP/YFP+ axons are able to project within intramuscular branches, following intraneural engraftment of ChR2+ motor neurons and daily immunosuppression with FK506; obtained from a 112d SOD1G93A mouse at 27d post-engraftment. (B) GFP/YFP+ axons fail to reinnervate NMJs despite the proximity of ChR2+ motor axon terminals to denervated endplates. (C) FK506-mediated immunosuppression permits exuberant growth of carry-over pluripotent stem cells that form intraneural tumours (**) within engrafted sciatic nerve (SN) branches; (D) these tumours cause severe movement impairment of the affected hindlimb. (E) FK506 caused body mass loss in a subset (8/18) of SOD1G93A mice, treated at different ages, that precedes onset of normal decline in this model (indicated by vertical dashed line at 115d).
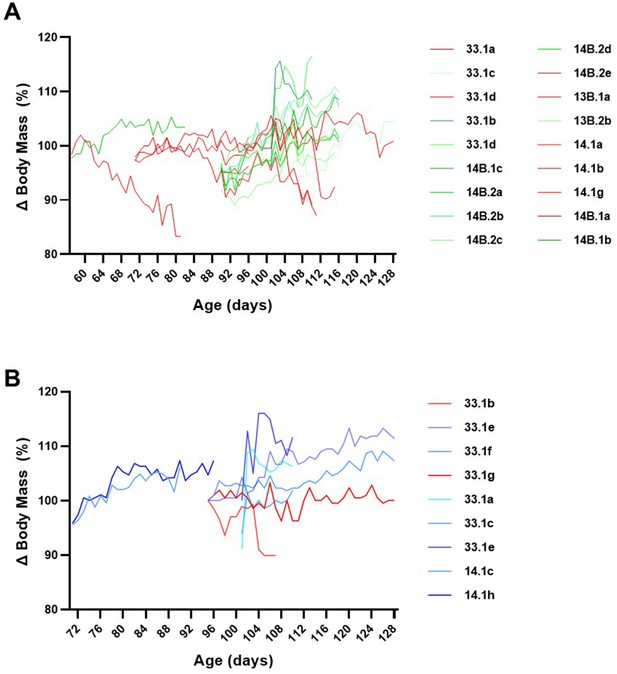
FK506 severely affects body mass in most SOD1G93A but not wild-type mice.
(A) Longitudinal body mass analysis of individual SOD1G93A mice (n = 18) treated with FK506 (5mg/kg/d), spanning different age ranges, reveals that FK506 prevents body mass increase in the majority of SOD1G93A mice and causes body mass decline in a subset of SOD1G93A mice (44.4%; 8/18 animals, highlighted in red) that precedes body mass decline associated with disease phenotype that normally commences at 115d. (B) Body mass of most (77.8%; 7/9) wild-type mice treated with FK506 continued to increase throughout the study period.
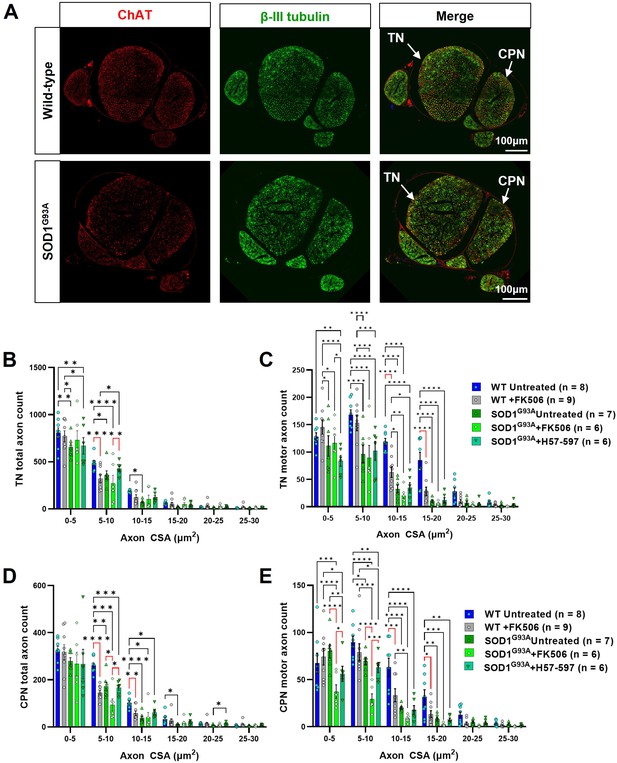
FK506 causes peripheral nerve axonopathy in SOD1G93A and wild-type mice.
(A) Representative examples of wild-type (top) and SOD1G93A (bottom) sciatic nerve transverse sections, showing common peroneal nerve (CPN) and tibial nerve (TN), labeled for total axons (βIII tubulin; green) and motor axons (choline acetyl transferase; ChAT; red); automated axon size distribution analysis of (B) total and (C) motor axon in the tibial nerve (TN); (D) total and (E) motor axons in the common peroneal nerve (CPN) in both wild-type and SOD1G93A mice. Data shown as mean; error bars = SEM; two-way ANOVA analysis: *denotes p ≤ 0.05; ** denotes p ≤ 0.0002; *** denotes p ≤0.002; **** denotes p ≤ 0.00002; significance bars displayed in red highlight changes directly attributable to FK506, independent of genotype.
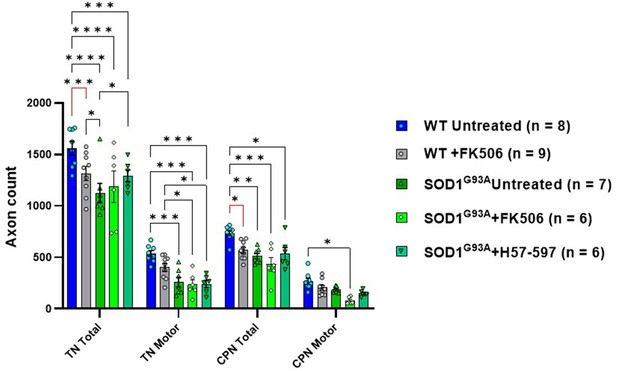
FK506 moderately reduces total sciatic nerve axon counts in wild-type mice but loss of total and motor axons is not observed in SOD1G93A mice when all axon calibers are grouped.
Automated analysis of total (i.e. sensory and motor) axon numbers reveals that daily treatment with FK506 causes a significant reduction of total axon counts in wild-type tibial nerve (TN) and common peroneal nerve (CPN) nerve branches; when all axonal calibers are grouped, the deleterious effect of FK506 on motor axon loss is not observed, suggesting that specific calibers may be preferentially vulnerable and the expected axonal loss in SOD1G93A mice does not appear to be exacerbated by FK506 treatment in terms of overall numbers. Data shown as mean; error bars = SEM; two-way ANOVA; *denotes p = ≤0.05; ** denotes p = ≤0.0002; *** denotes p = ≤0.002; **** denotes p = ≤0.00002; significance bars displayed in red highlight changes directly attributable to FK506, independent of genotype.
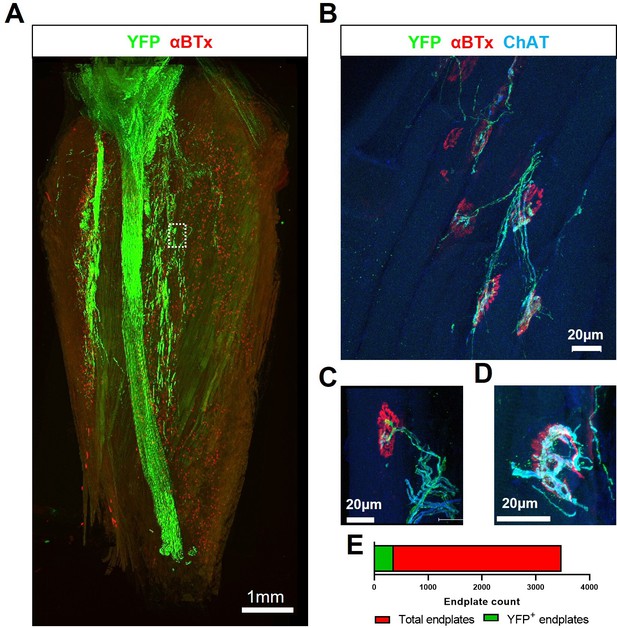
Transient H57-597 mAb treatment confers complete ChR2+ motor neuron allograft survival and, importantly, allows robust triceps surae muscle reinnervation up until late-stage disease in SOD1G93A mice.
(A) 3D reconstruction of 67 individual tile-scans, acquired from serial sections from an entire triceps surae muscle, from a 135d SOD1G93A mouse following engraftment of ChR2+ motor neurons at 95d and H57-597 treatment, showing the full extent of axonal projection throughout the whole muscle; see also Video 1. (B) A high-resolution maximum intensity projection (MIP) image of a confocal z-stack, revealing multiple NMJs innervated to varying extents (α-bungarotoxin; α-BTx; red) by YFP + engrafted motor neuron axons (green) labeled for choline-acetyl transferase (ChAT; blue – note; overexposure of blue channel enables visualization of muscle fibres). (C) MIP image of a confocal z-stack showing an example of a partially innervated NMJ. (D) MIP image of a confocal z-stack showing an example of a fully innervated NMJ; note, poly-innervation, shown in (C), and terminal sprouting, shown in (D) which are signs of immaturity. (E) Automated quantification of total endplate number (count = 3482; labeled with α-BTx) and manual counts of endplates (count = 364) that showed YFP colocalization, indicating innervation, from the same muscle.
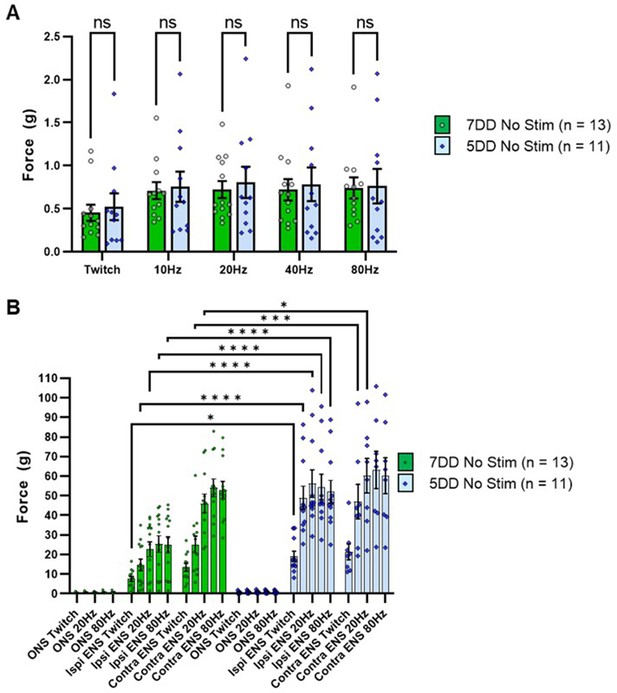
Subtype identity of engrafted ChR2+ motor neurons does not affect the maximum contractile response of the targeted muscle to acute optical stimulation in SOD1G93A mice.
(A) Fast-firing motor neurons (produced using a 7-day differentiation protocol thus labeled as ‘7DD’) or slow-firing ChR2+ motor neurons (produced using a 5-day differentiation protocol thus labeled as ‘5DD’) were engrafted in age matched SOD1G93A mice and the maximum contractile response elicited by acute optical stimulation (at 133.9 ±7.2 days versus 133 ±6.9 days, respectively) was determined using isometric muscle tension analysis; optical nerve stimulation (ONS) was delivered at the indicated frequencies to fully interrogate the muscle response. (B) Electrical nerve stimulation (ENS) of the ipsilateral and contralateral triceps surae muscles were also determined for reference, using stimulation frequencies designed to mirror the ONS patterns; ONS responses included for scale. Data shown as mean; error bars = SEM; multiple unpaired t tests with Tukey post hoc correction (A) or two-way ANOVA (B) *denotes p=≤0.05; ** denotes p=≤0.0002; *** denotes p=≤0.002; **** denotes p=≤0.00002.
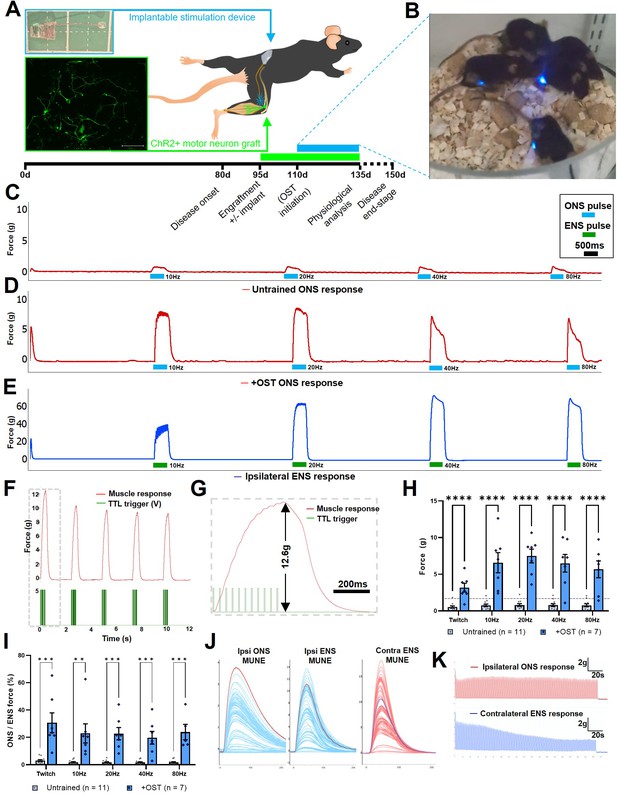
Daily optical stimulation training (OST) of post-symptom onset SOD1G93A mice engrafted with ChR2+ motor neurons, significantly enhances contractile response to optical stimulation.
(A) Schematic indicating intraneural engraftment site in distal tibial nerve and reinnervated triceps surae (TS) muscle, along with stimulation device (top inset) implantation site and subcutaneous LED position;+4 div MMC-treated ChR2+ motor neurons express YFP (green; lower inset box); experimental timescale is shown below. (B) Still frame (taken from Video 5) showing daily OST. Representative isometric muscle tension physiology recordings from the TS muscle in response to specified pulses of ONS in untrained (C) and + OST (D) late-stage SOD1G93A mice, along with ENS response from the same muscle (E). (F) Delivery of an optimized pulse pattern elicits maximal response to ONS that can be used to finely control repetitive contractions; (G) Dashed box is shown at higher temporal resolution to indicate square-wave TTL pulse pattern (that drives LED stimulator) and an individual tetanic contraction. (H) Quantification of maximum contractile responses to indicated pulse patterns of acute ONS shows a highly significant improvement in force generation in late-stage SOD1G93A mice that underwent OST versus untrained controls (dashed horizontal line indicates maximum value from our previous study in nerve ligated WT mice). (I) The proportion of total muscle capacity (determined by supramaximal ENS minus ONS value) elicited by acute ONS is also significantly higher following OST; data represent mean ± SEM. (J) Motor unit number estimate (MUNE) traces obtained from a representative late-stage SOD1G93A mouse, following OST, in response to ipsilateral ONS and ENS, along with contralateral ENS (note, different scales). (K) Fatigue trace recordings comparing ipsilateral ONS (top) and contralateral ENS (bottom) in the same late-stage SOD1G93A mouse, in response to 250ms 20 Hz pulses, repeated every 1 s for 180 s.
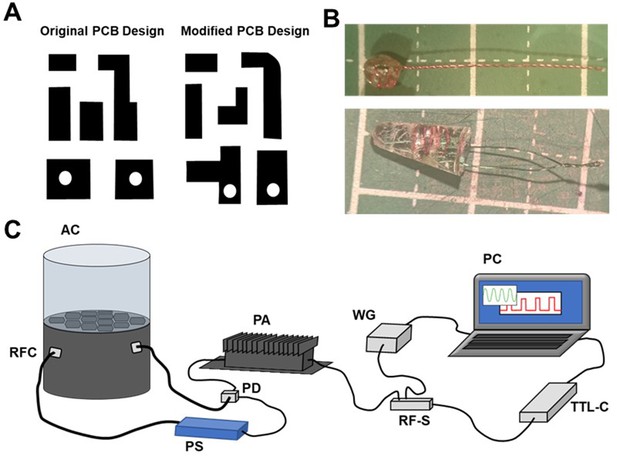
An existing implantable device underwent minor modifications to improve suitability for optical stimulation training experiments.
(A) Modifications to the original PCB design and (B) encapsulation method, conferred long-term functionality of the wireless stimulation following in vivo implantation. (C) Incorporation of a radio frequency (RF) switch and TTL control device to deliver empirically-determined RF pulse patterns to activate the implanted wireless LED devices in vivo: a PC controlled wave-form generator (WG) emits a continuous 1dB 1493GHz signal that is interrupted by a RF-switch (RF-S), modulated by a PC operated TTL-control device (TTL-C); the RF signal is relayed to a power amplifier (PA) before passing through a power divider (PD); the orientation of one output is rotated by 90° by a phase-shifter (PS) before being relayed to the resonance frequency cavity (RFC), the other output is fed directly to the RFC; during optical stimulation sessions, mice are placed in the animal chamber (AC).
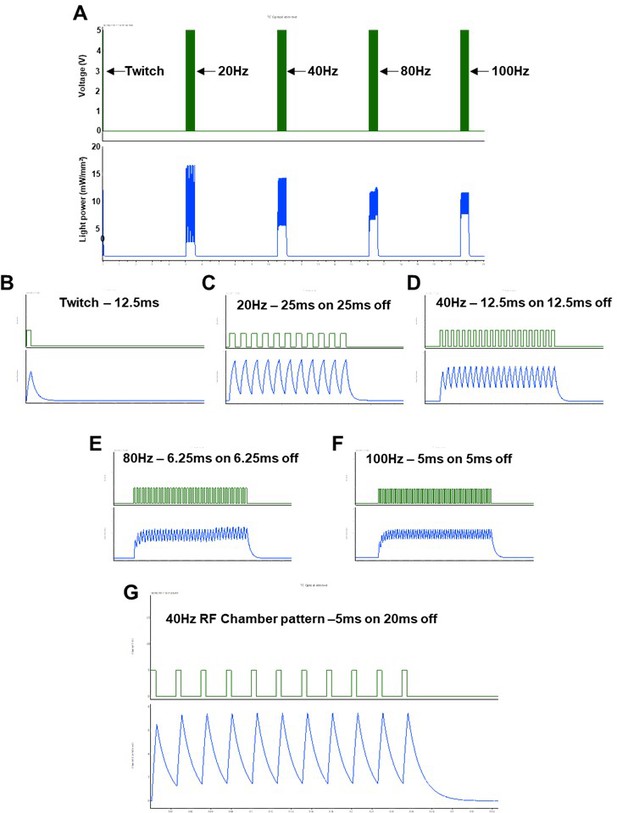
Light power recording and stimulation pattern recordings used to elicit acute optical nerve stimulation (ONS) throughout study.
(A) Low temporal resolution recording show electrical TTL trigger pulses (green; top), used to activate an LED stimulator, and light power recordings measured using a digital light meter; note: LED liquid light guide was positioned an equivalent distance (1cm) from the light meter, compared to the distance from the exposed sciatic nerve for ONS studies. (B–F) Higher temporal resolution images of the same pulse patterns; (G) recording of the optimized pulse pattern that induced maximal tetanic contraction and was subsequently used for long-term optical stimulation training.
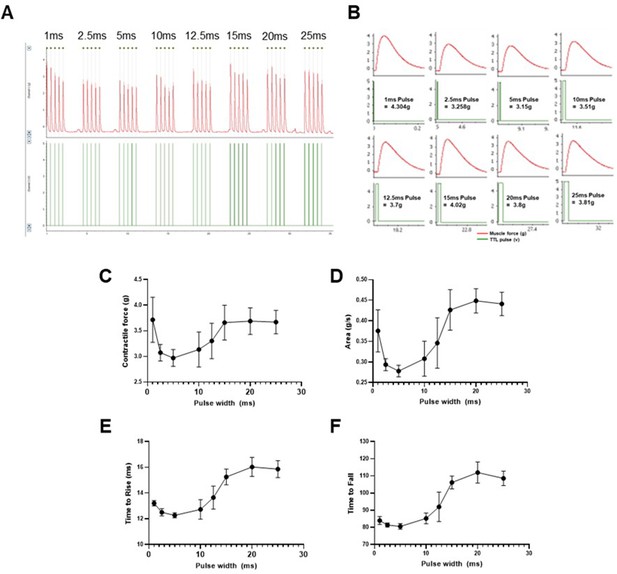
Optimization of optical nerve stimulation (ONS) pulse width to evoke maximum twitch contractile force.
Isometric muscle tension force was recorded in response to 5 repeated ONS pulses, with varying pulse width from 1ms – 25ms (A); higher temporal resolution images of ONS evoked muscle twitch contractions (B) show electrical TTL trigger pulses, which activate the LED stimulator, in relation to twitch contractions. Automated analysis of ONS evoked contractile responses shows the relationship between pulse duration and contractile force (C), area under the curve (D), time take for force to rise from baseline to 5% of maximum (E) and time taken for force to fall back to below 10% of maximum response (F). Note: the shortest 1ms pulse induces maximal contractile force, with a rapid rise and fall time, whereas longer pulses (>15ms) can induce similar contractile force but at the cost of delayed muscle relaxation. Data shown as mean; error bars = st dev.
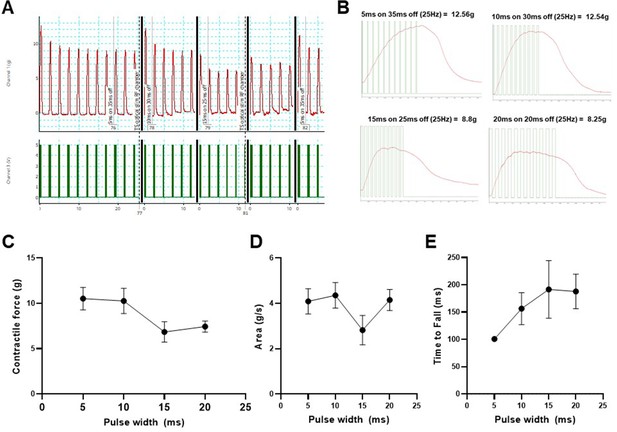
Optimization of optical nerve stimulation (ONS) pulse pattern to evoke maximum tetanic contractile force.
Repetitive tetanic contractions following delivery of custom designed 25Hz ONS pulse patterns, in a ChR2+ motor neuron engrafted SOD1G93A mouse; pulse width varied from 5 to 20ms and pulse interval varied from 35 to 20ms (A); individual tetanic contractions for each of the four tested pulse patterns are shown at higher resolution, with overlayed TTL pulses to indicate when LED is activated (B). Automated quantification of 5 tetanic contractions per pulse pattern revealed important contractile characteristics of maximal contractile force (C), area under curve (D) and time for contractile force to fall to 80% of peak value (E). Note: electrical TTL trigger pulses <5ms frequently failed to activate the LED stimulator, therefore 5ms was the shortest pulse duration tested and incorporated into the optical stimulation training (OST) program. Data shown as mean; error bars represent st dev.
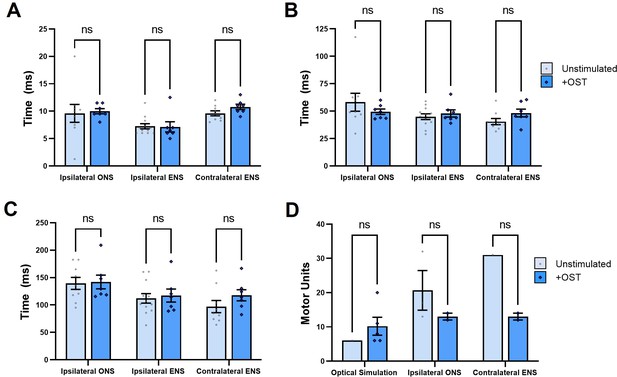
Daily optical stimulation training (OST) in SOD1G93A mice does not affect muscle contractile characteristics in response to acute optical nerve stimulation (ONS).
Automated peak analysis of brief twitch contractions elicited by acute optical stimulation at the experimental end-point revealed that OST did not significantly alter any of the following muscle characteristics compared to untrained SOD1G93A mice: (A) time taken for muscle contractile force to rise above 5% of baseline value from onset of ONS pulse (Time to Rise); (B) time between initial contraction and peak force generation (Time to Peak); or (C) time taken to relax to 50% of peak force (1/2 Relaxation Time). Motor unit number estimates (MUNE) were difficult to obtain in untrained SOD1G93A mice in response to ONS, but greater contractile force of twitch contractions in mice that underwent OST enabled MUNE values to be determined (D). Data shown as mean; error bars = SEM; multiple unpaired t tests with Bonferroni-Dunn post hoc correction; ns = not significant.
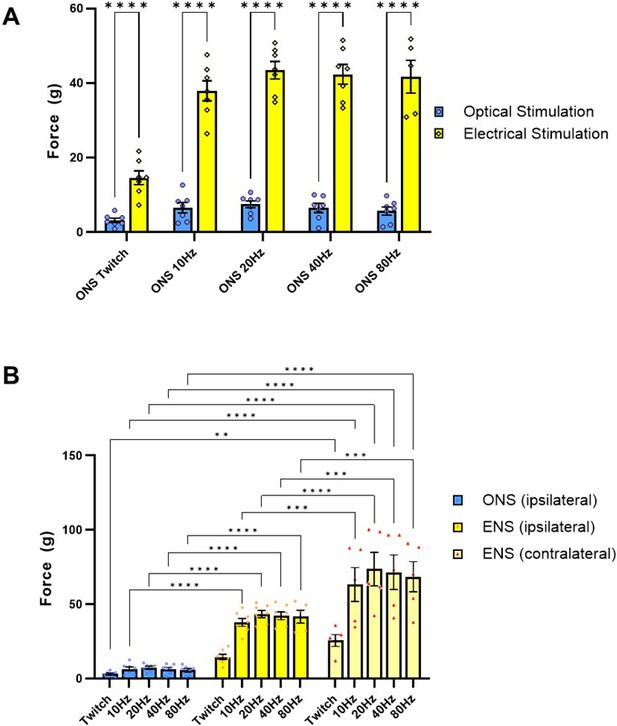
Comparison of optical nerve stimulation (ONS) versus electrical nerve stimulation (ENS) in late-stage SOD1G93A mice shows that supramaximal ENS still induces stronger contractile force, even after optical stimulation training (OST).
(A) Comparison of maximal twitch and tetanic force values, acquired from the same TS muscle in each animal in response to either supramaximal ONS or ENS stimuli delivered at specified pulse patterns. (B) Maximal twitch and tetanic recordings from the ipsilateral and contralateral TS muscle in response to ONS and ENS stimuli. Data shown as mean; error bars = SEM; multiple unpaired t tests with Tukey post hoc correction (A) or two way ANOVA (B) *denotes p = ≤ 0.05; ** denotes p = ≤0.0002; *** denotes p = ≤0.002; **** denotes p = ≤0.00002.
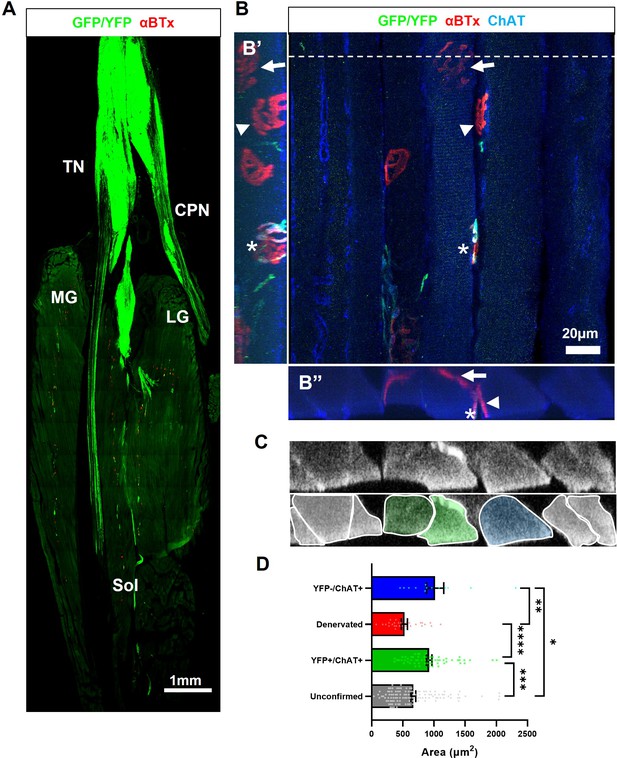
Optical stimulation training prevents atrophy of muscle fibres that have been reinnervated by ChR2+ motor neurons in late-stage SOD1G93A mice.
(A) Confocal tile-scan showing a single longitudinal section through the triceps surae muscle of 135d SOD1G93A mouse (35d post-engraftment), following daily OST; endplates (labeled with αBTx) innervated by GFP/YFP+ engrafted motor neurons are evident throughout the whole muscle group, including fast-twitch medial gastrocnemius (MG) and lateral gastrocnemius (LG) muscles and the slow-twitch soleus (Sol) muscle. (B) Representative top-down maximum intensity projection (MIP) view of a confocal z-stack through a 30μ m longitudinal section obtained from the same mouse; including side-on (B’) and end-to-end (B”) MIP views of the same z-stack; a de novo NMJ, innervated by a ChR2+ motor neuron (asterisk), is indicated on a muscle fibre that also has a denervated endplate (arrow), along with another muscle fibre still innervated by an endogenous choline-acetyltransferase (ChAT+) positive, GFP-negative motor neuron (arrowhead). (C) A digital slice (top) through the 3D z-stack obtained at the y-axis plane indicated by dashed line in (B) and colorized masks delineating individual muscle fibres (bottom); see Video 6. (D) Average cross-sectional area of individual muscle fibres with an innervation status that was unconfirmed (117 fibres), innervated by GFP +motor neurons (62 fibres), denervated (28 fibres), or innervated by endogenous motor axons (13 fibres); n=3 late-stage engrafted SOD1G93A mice that had undergone OST; Data shown as mean; error bars = SEM; one-way ANOVA with Tukey’s post hoc correction: *denotes p ≤0.05; ** denotes p ≤0.0002; *** denotes p ≤0.002; **** denotes p ≤0.00002.
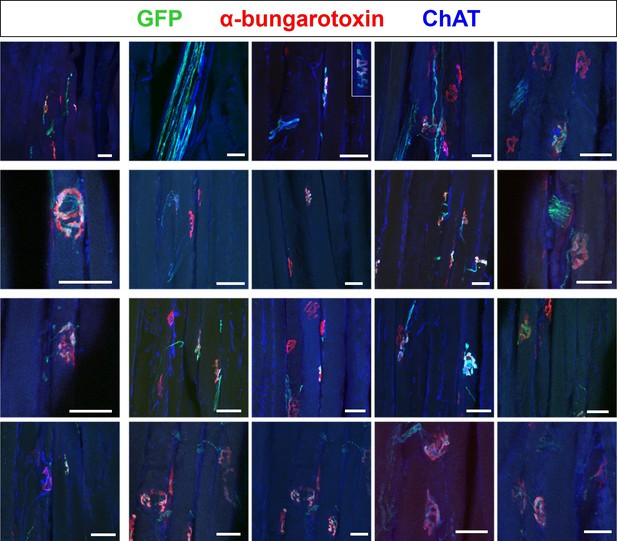
Daily optical stimulation training (OST) appears to enhance the extent of innervation of end-plates by engrafted ChR2+ motor neurons.
Confocal images showing examples of intramuscular nerves and end-plates, within the triceps surae muscle, innervated by engrafted ChR2+ motor neurons in late-stage SOD1G93A mice (n = 3) that had undergone OST. This sampling of NMJs provides an indication of the high-level of occupancy of innervated end-plates; many of these images were extracted from confocal z-stacks of regions of interest (ROIs) used for digital cross-sectional area Analysis of Longitudinal Muscle Sections (dCALMS) analysis (see Figure 6). Scale bars = 20 μm.
Videos
3D reconstruction of innervated endplates from SOD1G93A mice in the absence of immunosuppression.
FK506 facilitates graft survival but allows intraneural tumour formation that causes severe motor dysfunction.
3D reconstruction of an entire triceps surae muscle group from a late-stage SOD1G93A mouse, after ChR2+ motor neuron engraftment showing extent of reinnervation.
3D reconstruction of individual endplates (red) reinnervated by engrafted ChR2+ motor neuron (green) in a 135d SOD1G93A mouse (35d post-engraftment) in combination with transient H57-597 mAb treatment.
Daily optical stimulation training significantly enhances elicited muscle force in SOD1G93A mice.
3D visualization of longitudinal muscle section from an engrafted SOD1G93A mouse along with ‘dCALMS’ muscle fiber analysis technique.
Tables
Single Nucleotide Polymorphism (SNP) analysis of Clone #C9G and control mESC lines to confirm genetic background strain.
SNP analysis confirmed that mESC Clone #C9G, used in this study, originated from a different genetic background compared to host mice (C57Bl/6J background strain). C57BL/6J mESCs and HBG3 mESCs were included as controls.
| Conformity of Sample to Reference Strain Allelic Profile | |||||
|---|---|---|---|---|---|
| Sample ID-Code | Reference | # Called | Call Rate | Percent Match | Percent Het |
| 001-Clone C9G mESCs | 129S1SvImJ | 382 | 99.5% | 97.5% | 0.3% |
| 002-C57BL/6 J mESCs | B6J | 383 | 99.7% | 99.7% | 0.0% |
| 003-Clone HBG3 mESCs | B6J | 377 | 98.2% | 62.5% | 10.9% |
Summary of data from mice treated with FK506, including graft outcome (where applicable).
List of experimental mice, including genotype, sex, age at start and end of experiment, initial and final body mass and % change (values that decreased or failed to increase are highlighted in red). Engrafted ChR2+ motor neurons were histologically determined to be present in all animals. Age at which ipsilateral hindlimb motor deficits were initially observed in vivo and post-mortem observation of intraneural tumour formation: *, **, and *** denotes small, medium and large tumour size, respectively (see Figure 2E). †Denotes animals that died during the course of treatment.
| ID | Genotype | Sex | Age (g) | Body mass (g) | δ BM (%) | Onset (days) | Tumor | ||
|---|---|---|---|---|---|---|---|---|---|
| Start | End | Initial | Final | ||||||
| 14.1 c | Wild-Type | M | 71 | 93 | 24.8 | 26.3 | 106.0 | - | * |
| 14.1 h | Wild-Type | F | 71 | 98 | 19.1 | 20.5 | 107.3 | 82 | - |
| 14.1 a | SOD1G93A | M | 71 | 100 | 26.5 | 25.5 | 96.2 | 82 | ** |
| 14.1b | SOD1G93A | M | 71 | 101 | 25.6 | 26 | 101.6 | 82 | ** |
| 14.1 g | SOD1G93A | F | 71 | 98 | 19.4 | 19.1 | 98.5 | 80 | * |
| 14B.1a | SOD1G93A | M | 57 | 83 | 25.2 | 21 | 83.3 | 69 | *** |
| 14B.1b | SOD1G93A | M | 57 | 101 | 26.3 | 27.2 | 103.4 | 70 | ** |
| 14B.1c | SOD1G93A | M | 92 | 121 | 28.2 | 28.4 | 100.7 | 101 | not recorded |
| 14B.2a | SOD1G93A | F | 90 | 124 | 21.1 | 22.9 | 108.5 | 104 | not recorded |
| 14B.2b | SOD1G93A | F | 90 | 124 | 20.8 | 22.3 | 107.2 | 104 | not recorded |
| 14B.2c | SOD1G93A | F | 90 | 127 | 20.2 | 22.2 | 109.9 | 104 | not recorded |
| 14B.2d | SOD1G93A | F | 90 | 120 | 21.9 | 22.2 | 101.4 | 104 | not recorded |
| 14B.2e | SOD1G93A | F | 90 | 117 | 22.4 | 20.7 | 92.4 | 104 | *** |
| 13B.1a | SOD1G93A | M | 90 | 113 | 24.2 | 21.1 | 87.2 | 101 | ** |
| 13B.2b | SOD1G93A | M | 85 | 112 | 23.8 | 23.5 | 98.7 | 101 | ** |
| Animals listed below underwent FK506 treatment in the absence of intraneural engraftment | |||||||||
| 20.1d | Wild-Type | F | 101 | 131 | 19.2 | 20.95 | 109.1 | Not applicable | |
| 20.1 f | Wild-Type | F | 101 | 131 | 21.3 | 23.3 | 109.4 | ||
| 20.1 h | Wild-Type | F | 101 | 131 | 18.1 | 21.2 | 117.1 | ||
| 33.1e | Wild-Type | F | 95 | 128 | 21.1 | 22.5 | 106.6 | ||
| 33.1 f | Wild-Type | F | 95 | 128 | 22 | 22.5 | 102.3 | ||
| 33.1 g | Wild-Type | F | 95 | 128 | 21.3 | 21 | 98.6 | ||
| 33.1b | Wild-Type | M | 95 | 107† | 26.7 | 24 | 89.9 | ||
| 20.1e | SOD1G93A | F | 101 | 131 | 17.5 | 19.8 | 113.1 | ||
| 20.1 g | SOD1G93A | F | 101 | 131 | 17 | 21.2 | 124.7 | ||
| 33.1 a | SOD1G93A | M | 95 | 111† | 25.8 | 23 | 89.1 | ||
| 33.1 c | SOD1G93A | M | 95 | 128 | 24.7 | 25.8 | 104.5 | ||
| 33.1d | SOD1G93A | M | 95 | 128 | 23.2 | 23.2 | 100.0 | ||
Summary of data following in vivo engraftment of ChR2+ motor neurons in SOD1G93A mice.
Table shows a full list of all experimental animals reported in the optical stimulation section of this study, including animal ID, sex, age at start and end of study period, duration of graft, body mass at the start and end of the study period and change in body mass. The table shows the three main cohorts reported in this study, based on type of motor neurons that were engrafted and presence/absence of optical stimulation training. All animals exhibited a positive graft survival, determined by histology and/or acute optical nerve stimulation (ONS) at the experimental end-point; data is shown for 20Hz ONS, which elicited maximal tetanic contractile force.
| 2*ID | 2*Sex | Age (g) | 2*Duration (d) | BM (g) | 2*Δ BM (%) | 2*Max Force (g) | ||
|---|---|---|---|---|---|---|---|---|
| Start | End | Start | End | |||||
| Fast-firing Motor neurons (derived from 7DD dissociated EBs pretreated with MMC) | ||||||||
| 21.1 a | M | 103 | 139 | 36 | 25.2 | 23 | 91.3 | 0.39 |
| 21.1e | M | 103 | 140 | 37 | 23.3 | 21.7 | 93.1 | 0.33 |
| 25A1b | M | 118 | 137 | 19 | 28.3 | 23.2 | 82.0 | 0.55 |
| 25 A.1d | M | 118 | 137 | 19 | 27 | 23.2 | 85.9 | 0.54 |
| 24.2 a | M | 117 | 137 | 20 | 27.2 | 23.6 | 86.8 | 0.57 |
| 25B.1b | M | 109 | 135 | 26 | 24.2 | 21 | 86.8 | 0.47 |
| 25 C.1a | M | 101 | 137 | 36 | 26.3 | 24.5 | 93.2 | 0.88 |
| 21.1 f | F | 103 | 140 | 37 | 20.8 | 19 | 91.3 | 0.43 |
| 22.1d | F | 100 | 120 | 20 | 20.5 | 19.5 | 95.1 | 1.10 |
| 22.1e | F | 100 | 121 | 21 | 23.4 | 20.4 | 87.2 | - |
| 24.2d | F | 114 | 137 | 23 | 19.5 | 18.2 | 93.3 | 0.50 |
| 25B.1c | F | 109 | 135 | 26 | 19.8 | 19.2 | 97.0 | 1.04 |
| 25 C.1d | F | 100 | 122 | 22 | 18.5 | 18.6 | 100.5 | 1.11 |
| 25 C.1f | F | 100 | 138 | 38 | 20.2 | 18.5 | 91.6 | 1.48 |
| Average: | 106.8±7.2 | 133.9±7.2 | 27.1±7.8 | 23.2±0.7 | 21.0±0.9 | 91.1±1.6 | 0.72±0.1 | |
| Slow-firing Motor neurons (derived from 5DD dissociated EBs pretreated with MMC) - untrained | ||||||||
| 28.2d | F | 97 | 142 | 45 | 20.3 | 18.4 | 90.6 | 0.57 |
| 28.2 f | F | 97 | 142 | 45 | 20.2 | 17.8 | 88.1 | 0.63 |
| 28.1b | F | 104 | 136 | 32 | 22 | 19.3 | 87.7 | 0.33 |
| 26.1 a | M | 104 | 143 | 39 | 24.8 | 22.3 | 89.9 | 0.24 |
| 28.2b | M | 97 | 128 | 31 | 26.2 | 23.1 | 88.2 | 1.26 |
| 31.1 c | M | 95 | 137 | 42 | 28 | 23.9 | 85.4 | 0.64 |
| 1.1 a | M | 90 | 125 | 35 | 25.9 | 26 | 100.4 | 1.31 |
| 1.1b | M | 90 | 126 | 36 | 28.9 | 30.1 | 104.2 | 2.24 |
| 35–1 c | M | 93 | 124 | 31 | 26.4 | 25.9 | 98.1 | 0.22 |
| 35-1b | M | 93 | 130 | 37 | 29 | 28.3 | 97.6 | 0.97 |
| 35-1e | M | 93 | 130 | 37 | 23.7 | 24 | 101.3 | 0.46 |
| 40–1 c | M | 95 | 133 | 38 | 24 | 23 | 95.8 | - |
| Average: | 95.7±4.6 | 133±6.9 | 37.3±4.8 | 25.0±0.7 | 23.5±0.9 | 93.9±1.7 | 0.81±0.18 | |
| Slow-firing Motor neurons (derived from 5DD dissociated EBs pretreated with MMC)+optical stimulation training | ||||||||
| 28.2 c | M | 97 | 128 | 31 | 25.6 | 24.4 | 95.3 | 8.96 |
| 1.1 c | M | 90 | 121 | 31 | 23.4 | 22.6 | 96.6 | 6.81 |
| 35-1d | M | 93 | 131 | 38 | 27.4 | 28 | 102.2 | 8.69 |
| 40–1 a | M | 95 | 132 | 37 | 25.1 | 24.2 | 96.4 | 3.60 |
| 38–1 c | F | 93 | 136 | 43 | 20.2 | 19.8 | 98.0 | 10.67 |
| 38.1e | F | 95 | 142 | 47 | 22.4 | 19 | 84.8 | 8.73 |
| 45.1 c | F | 96 | 137 | 41 | 21.6 | 21.4 | 99.1 | 4.99 |
| Average: | 94.1±2.3 | 132.4±6.8 | 38.3±6.0 | 23.7±1.0 | 22.8±1.2 | 96.1±2.2 | 7.49±0.94 | |





