The scheduling of adolescence with Netrin-1 and UNC5C
Figures
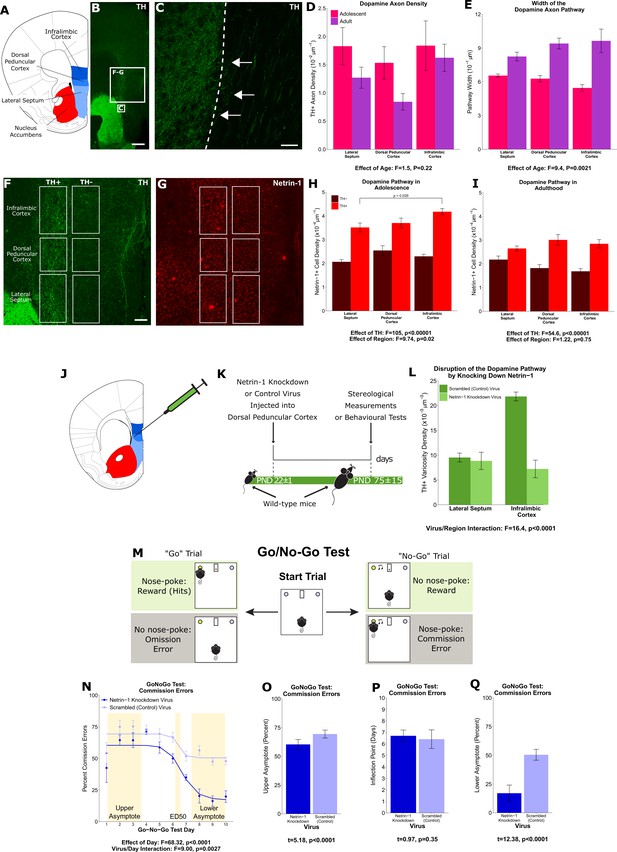
A ‘pathway’ of Netrin-1-expressing cells ‘paves the way’ for dopamine axons growing from the nucleus accumbens to the medial prefrontal cortex during adolescence.
(A) The brain regions containing the dopamine fibres passing to the medial prefrontal cortex are highlighted in a line drawing of a coronal mouse brain section derived from Paxinos and Franklin, 2013. (B) An image of a coronal section through the forebrain of an adult mouse at low magnification (×4). Green fluorescence indicates immunostaining for tyrosine hydroxylase (TH), used here as a marker for dopamine. The smaller and larger white squares indicate the regions enlarged in panel C and panels F and G, respectively. Scale bar = 500 μm. (C) The nucleus accumbens (left of the dotted line) is densely packed with TH+ axons (in green). Some of these TH+ axons can be observed extending from the nucleus accumbens medially toward TH+ fibres oriented dorsally toward the medial prefrontal cortex (white arrows). Scale bar = 10 μm. (D) Modified stereological quantification revealed no significant difference in TH+ axon density between adolescence (21 days of age) and adulthood (75 days of age). Mixed-effects analysis of variance (ANOVA), effect of age: F=1.53, p=0.22; region by age interaction: F=1.44, p=0.49. Sample sizes: 11 adolescent, 9 adult (E) The average width of the area that dopamine axons occupy increased significantly from adolescence to adulthood, revealing that there is an increase in the total number of fibres passing to the medial prefrontal cortex during this period. Mixed-effects ANOVA, effect of age: F=9.45, p=0.0021; region by age interaction: F=5.74, p=0.057. Sample sizes: 11 adolescent, 9 adult (F) In order to quantify the Netrin-1-positive cells along the TH+ fibre pathway, the pathway was contoured in each region, and a contour of equal area was placed medial to the dopamine pathway as a negative control. Scale bar = 200 μm. (G) Using quantitative stereology, Netrin-1-positive cell density was determined along and adjacent to the pathway for each region. Red fluorescence indicates immunostaining for Netrin-1. (H) In adolescent mice there are more Netrin-1-positive cells along the fibres expressing TH (‘TH+’) than medial to them (‘TH-’). This is what we refer to as the ‘Netrin-1 pathway’. Along the pathway, there is a significant increase in Netrin-1-positive cell density in regions closer to the medial prefrontal cortex, the innervation target. Mixed-effects ANOVA, effect of TH: F=105, p<0.0001. Effect of region: F=9.74, p=0.021. A post hoc Tukey test revealed a difference (p=0.029) between the densities of the lateral septum and infralimbic cortex, but only within the dopamine pathway. Sample size: 8 (I) In adult mice the Netrin-1 pathway is maintained, however there is no longer an increasing density of Netrin-1-positive cells toward the medial prefrontal cortex. Mixed-effects ANOVA, effect of TH: F=54.56, p<0.0001. Effect of region: F=1.22, p=0.75. Sample size: 8 (J) The virus injection location within the mouse brain. A Netrin-1 knockdown virus, or a control virus, was injected into the dopamine pathway at the level of the dorsal peduncular cortex. (K) Our experimental timeline: at the onset of adolescence a Netrin-1 knockdown virus, or a control virus, was injected in wild-type mice. In adulthood the mice were sacrificed and stereological measurements taken. (L) TH+ varicosity density was quantified in the region below the injection site, the lateral septum, and in the region above the injection site, the infralimbic cortex. There was a significant decrease in TH+ varicosity density only in the infralimbic cortex. Mixed-effects ANOVA, virus by region interaction: F=16.41, p<0.0001. Sample sizes: knockdown 11, control 8 (M) The experimental set-up of the final (test) stage of the Go/No-Go experiment. A mouse that has previously learned to nose-poke for a reward in response to a visual cue (illuminated nose-poke hole) must now inhibit this behaviour when the visual cue is paired with an auditory cue (acoustic tone). (N) Mice injected with the Netrin-1 knockdown virus show improved action impulsivity compared to controls; they incur significantly fewer commission errors across the Go/No-Go task. Mixed-effects ANOVA, effect of day: F=68.32, p<0.0001. Day by virus interaction: F=9.00, p=0.0027. A sigmoidal curve is fit to each group of mice to determine how the two groups differ. Points indicate group means and error bars show standard error means. Sample sizes: knockdown 10, control 10 (O) During the first days of Go/No-Go testing, both groups incur commission errors with high frequency, but the Netrin-1 knockdown group has fewer errors than the control group (t-test, t=5.18, p<0.0001). (P) The ED50 – the inflection point in each sigmoidal curve – does not differ between groups, indicating that all mice improve their ability to inhibit their behaviour at around the same time (t-test, t=0.97, p=0.35). (Q) Mice microinfused with the Netrin-1 knockdown virus incur substantially fewer commission errors in the last days of the Go/No-Go task compared to mice injected with the control virus (t-test, t=12.38, p<0.0001). For all barplots, bars indicate group means and error bars show standard error means.
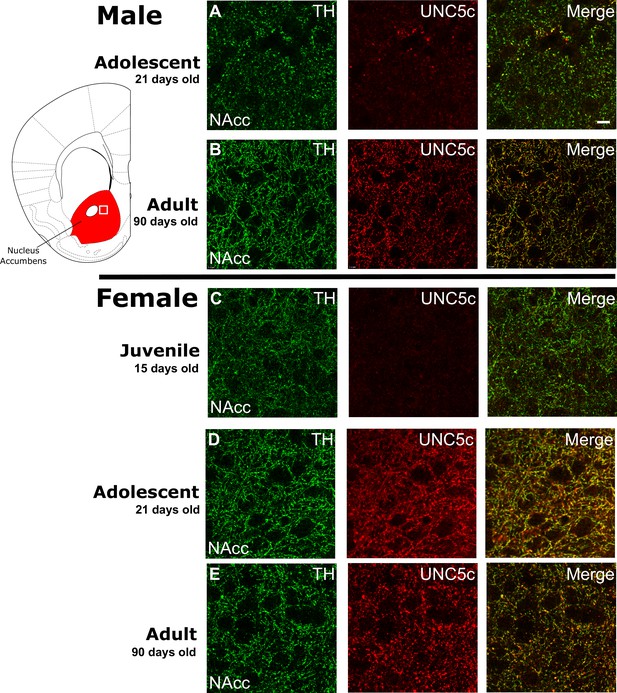
The age of onset of UNC5C expression by dopamine axons in the nucleus accumbens of mice is sexually dimorphic.
Images are representative of observed immunofluorescence patterns in the nucleus accumbens (approx. location highlighted as a white square in the coronal mouse brain section plate 19, modified from Paxinos and Franklin, 2013). No qualitative differences were noted between the shell and core of the nucleus accumbens. For each row, six individuals were sampled. In males (A–B), UNC5C expression on dopamine fibres (here identified by immunofluorescent staining for tyrosine hydroxylase [TH]) in the nucleus accumbens appears during adolescence. (A) At the onset of adolescence (21 days of age) dopamine fibres do not express UNC5C. Scale bar = 10 μm. (B) By adulthood (90 days of age), dopamine fibres express UNC5C. In females (C–E), UNC5C expression on dopamine fibres in the nucleus accumbens appears prior to adolescence. (C) In juvenile (15 days of age) mice, there is no UNC5C expression on dopamine fibres. (D) By adolescence, dopamine fibres express UNC5C. (E) In adulthood, dopamine fibres continue to express UNC5C.
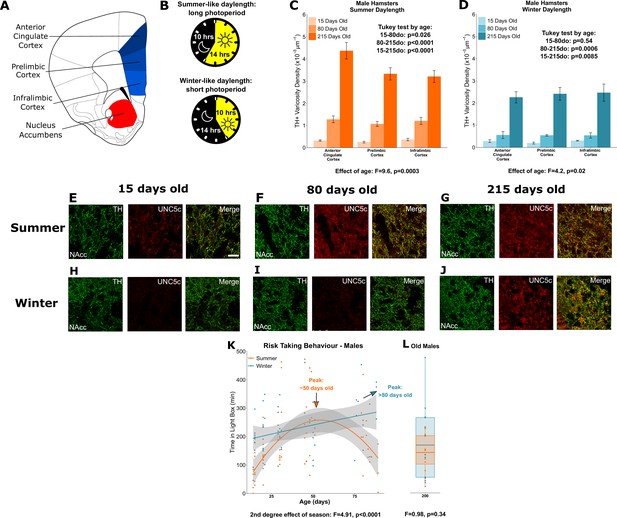
Plasticity of adolescent development in male Siberian hamsters according to seasonal phenotype.
All results illustrated in this figure refer to results in male hamsters. (A) Dopamine innervation was quantified in three subregions of the medial prefrontal cortex, highlighted in blue. UNC5C expression was examined in the nucleus accumbens, highlighted in red. Line drawing of a coronal section of the mouse brain was derived from plate 19 of Paxinos and Franklin, 2013. (B) Hamsters were housed under either summer-mimicking long days and short nights (‘summer hamsters’) or winter-mimicking short days and long nights (‘winter hamsters’). (C) In male hamsters housed under a summer-mimicking daylength there is an increase in dopamine varicosity density in the medial prefrontal cortex between 15 and 80 days of age. Mixed-effects analysis of variance (ANOVA), effect of age: F=9.6, p=0.000255. Tukey test, 15–80 days old (do): p=0.026; 80–215do: p<0.0001; 15–215do: p<0.0001. Sample sizes: 15-days-old 8, 80-days-old 8, 215-days-old 10 (D) In male hamsters housed under a winter-mimicking daylength there is no increase in dopamine varicosity density until hamsters have reached 215 days of age. Mixed-effects ANOVA, effect of age: F=4.17, p=0.0205. Tukey test, 15–80do: p=0.54; 80–215do: p=0.0006; 15–215do: p=0.0085. Sample sizes: 15-days-old 4, 80-days-old 8, 215-days-old 8 (E) At 15 days of age, dopamine axons (here identified by immunofluorescent staining for tyrosine hydroxylase [TH]) in the nucleus accumbens of male summer daylength hamsters largely do not express UNC5C. Scale bar = 20 µm (bottom right). (F–G) At 80 (F) and 215 (G) days of age, dopamine axons in the nucleus accumbens express UNC5C. (H–I) At 15 (H) and 80 (I) days of age, dopamine axons in the nucleus accumbens of male winter hamsters largely do not express UNC5C. (J) By 215 days of age there is UNC5C expression in dopamine axons in the nucleus accumbens of male winter hamsters. (E–J) Representative images of the nucleus accumbens shell, six individuals were examined per group. (K) Male hamsters house under a summer-mimicking daylength show an adolescent peak in risk taking in the light/dark box apparatus. Those raised under a winter-mimicking photoperiod show a steady increase in risk taking over the same age range. Arrows indicate the ages at which risk-taking peaks in summer (orange) and winter (blue) hamsters. Polynomial regression, effect of season: F=3.551, p=0.00056. Curves show polynomial functions, shaded areas show uncertainty in the functions. Sample sizes: summer 66, winter 57 (L) In male hamsters, at 215 days of age, there is no difference in risk taking between hamsters raised under summer and winter photoperiods. t-Test, effect of season: t=0.975, p=0.341. The central line through each box indicates the group mean, the upper and lower bounds of each box indicate the third and first quartiles respectively, and the whiskers indicate the maximum and minimum values. Sample sizes: 12 summer, 12 winter. For all barplots, bars indicate group means and error bars show standard error means.
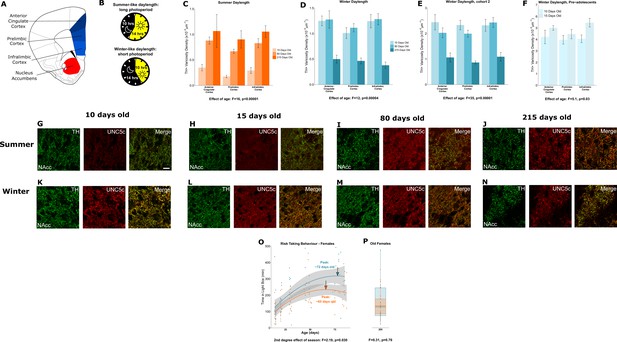
Plasticity of adolescent development in female Siberian hamsters according to seasonal phenotype.
All results illustrated in this figure refer to results in female hamsters. (A) Dopamine innervation was quantified in three subregions of the medial prefrontal cortex, highlighted here in blue. UNC5C expression was examined in the nucleus accumbens, highlighted in red. Line drawing of a coronal section of the mouse brain was derived from Paxinos and Franklin, 2013. (B) Hamsters were housed under either a summer-mimicking or winter-mimicking daylength. (C) In female hamsters housed under a summer daylength dopamine varicosity density in the medial prefrontal cortex increases between 15 and 80 days of age. Mixed-effects analysis of variance (ANOVA), effect of age: F=16.72, p<0.0001. Sample sizes: 15-days-old 6, 80-days-old 8, 215-days-old 4 (D) In female hamsters housed under a winter daylength there is no increase in dopamine varicosity density post-adolescence. Instead, there is a steep decline in density between 80 and 215 days of age. Mixed-effects ANOVA, effect of age: F=12.33, p=0.000043. Sample sizes: 15-days-old 8, 80-days-old 8, 215-days-old 8 (E) As our results in panel D were unexpected, we replicated them with a second cohort of hamsters and found qualitatively identical results. Mixed-effects ANOVA, effect of age: F=34.871, p<0.0001. 15-days-old 8, 80-days-old 8, 215-days-old 7 (F) To try and determine when dopamine varicosities innervate the medial prefrontal cortex, we examined a cohort of 10- and 15-day-old hamsters. We found that varicosity density increases in the medial prefrontal cortex during this time, indicating that dopamine innervation to the medial prefrontal cortex is accelerated in female winter hamsters. Mixed-effects ANOVA, effect of age: F=5.05, p=0.03. Sample sizes: 10-days-old 10, 15-days-old 8 (G–H) In 10- and 15-day-old female summer hamsters there is little UNC5C expression in nucleus accumbens dopamine axons (here identified by immunofluorescent staining for tyrosine hydroxylase [TH]). Sample size: 4 (panel G) or 6 (panel H). (I–J) By 80 days of age (panel I), and continuing at 215 days of age (panel J), dopamine axons in the nucleus accumbens express UNC5C in female summer hamsters. Sample sizes: 6. Scale bar = 20 µm (panel G bottom right). (K–N) At all ages which winter female hamsters were examined, dopamine axons in the nucleus accumbens express UNC5C in winter female hamsters. Sample sizes: 4 (panel K) or 6 (panels L–N). (O) In female hamsters, those raised under summer and winter daylengths both show an increase in risk taking over time. The winter hamsters peak later compared to the summer daylength hamsters. Arrows indicate the ages at which risk taking peaks in summer (orange) and winter (blue) hamsters. Polynomial regression, effect of season: F=3.305, p=0.00126. Curves show polynomial functions, shaded areas show uncertainty in the functions. Sample sizes: summer 66, winter 61 (P) In female hamsters, at 215 days of age, there is no difference in risk taking between hamsters raised under summer and winter photoperiods. t-Test, effect of season: t=0.309, p=0.76. The central line through each box indicates the group mean, the upper and lower bounds of each box indicate the third and first quartiles respectively, and the whiskers indicate the maximum and minimum values. Sample sizes: 15 summer, 12 winter. For all barplots, bars indicate group means and error bars show standard error means.
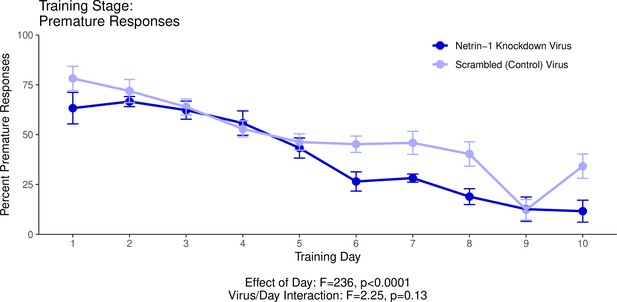
This graph illustrates the ability of adult (60+ days of age) mice to perform a learned response to a stimulus in order to receive a food reward as part of a learning phase leading up to the Go/No-Go behavioural experiment.
’Percent Premature Responses’ quantifies the percentage of trials in which the mice performed the learned response before the onset of the cue, and therefore ended the trial without receiving a reward. The statistical results presented below the graph are from the analysis of variance (ANOVA) presented below. The data presented in this graph and analyzed below correspond to the second training stage of the Go/No-Go behavioural experiment, referred to as Reaction Time. See our Methods subsection ‘Behaviour – Go/No-Go’ for details.
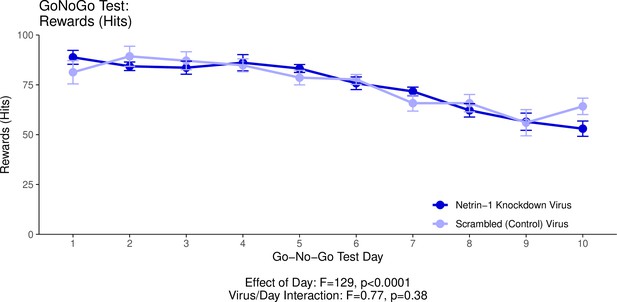
This graph illustrates the ability of adult (60+ days of age) mice to perform a learned response to a stimulus in order to receive a food reward as part of the Go/No-Go behavioural experiment.
’Hits’ quantifies the percentage of trials in which the mice performed correctly in response to a visual cue and in the absence of an auditory cue (a ‘hit’), and received a food reward as a result. The statistical results presented below the graph are from the analysis of variance (ANOVA) presented below. The data presented in this graph and analyzed below correspond to the test stage of the Go/No-Go behavioural experiment, referred to as the Go/No-Go task. See our Methods subsection ‘Behaviour – Go/No-Go’ for details.
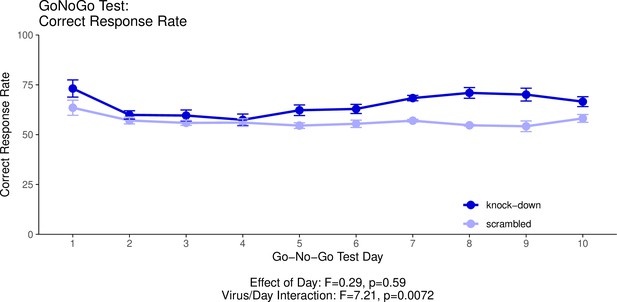
This graph illustrates the ability of adult (60+ day old) mice to perform both ‘Go’ trials and ‘No-Go’ trials correctly.
A ‘Go’ trial requires a behavioural response to a visual cue. A ‘No-Go’ trial requires the inhibition of the behavioural response to the visual cue when it is presented with a second, auditory cue. The ‘Correct Response Rate’ quantifies the trials where the mice respond correctly whether the trial is a ‘Go’ trial or a ‘No-Go’ trial. The statistical results presented below the graph are from the analysis of variance (ANOVA) presented below. The data presented in this graph and analysed below correspond to the test stage of the Go/No-Go behavioural experiment, referred to as the Go/No-Go task. See our Methods subsection ‘Behavior – Go/No-Go’ for details.
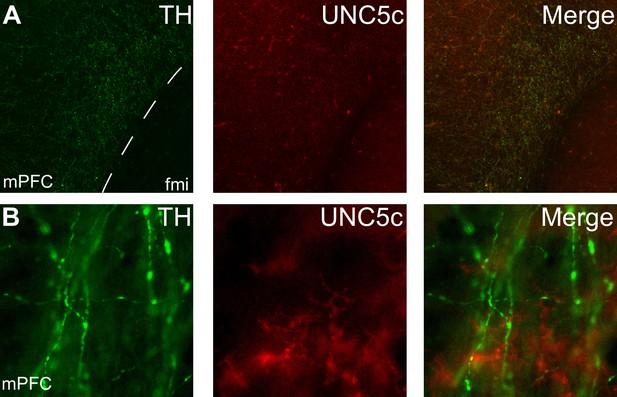
Expression of UNC5c protein in the medial prefrontal cortex of an adult male mouse.
Low (A) and high (B) magnification images demonstrate that there is little UNC5c expression in dopamine axons in the medial prefrontal cortex. Here, we identify dopamine axons by immunofluorescent staining for tyrosine hydroxylase (TH). See our Methods subsection ‘Immunohistochemistry’ for details. Abbreviations: fmi: forceps minor of the corpus callosum, mPFC: medial prefrontal cortex.
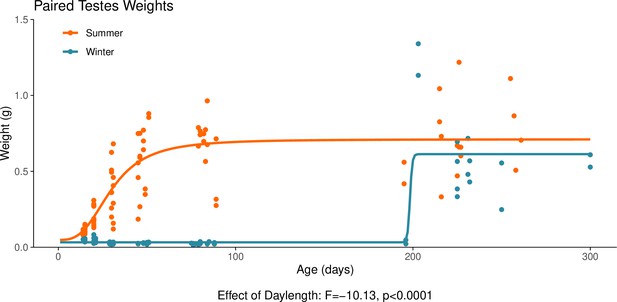
This graph illustrates the effect of daylength on the reproductive development of male hamsters.
Testicular weight is a commonly used proxy for the timing of puberty in male hamsters. The increase in paired testes weight, signalling puberty, is delayed when the hamsters are housed under a winter-mimicking short daylength, compared to a summer-mimicking long daylength. The statistical result below the graph is from the linear model presented below. For details on the hamster treatment protocol, see our Methods subsection ‘Animals’.
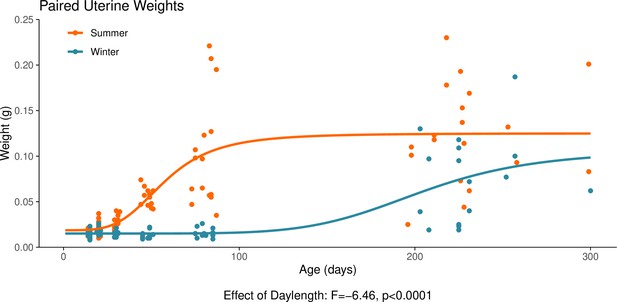
This graph illustrates the effect of daylength on the reproductive development of female hamsters.
Uterine weight is a commonly used proxy for the timing of puberty in female hamsters. The increase in uterine weight, signalling puberty, is delayed in hamsters that are housed under a winter-mimicking short daylength, compared to a summer-mimicking long daylength. The statistical result below the graph is from the linear model presented below. For details on the hamster treatment protocol, see our Methods subsection ‘Animals’.
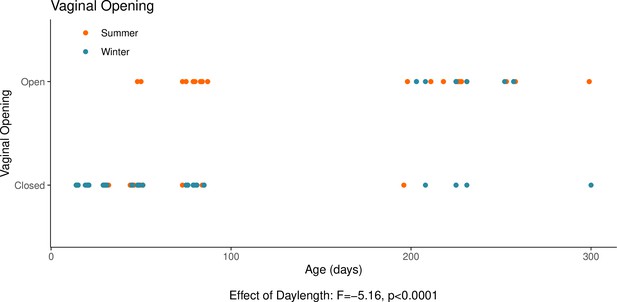
This graph illustrates the effects of daylength on the reproductive development of female hamsters.
The timing of the opening of the vagina is, in females, a commonly used proxy for the timing of puberty alongside uterine weight. The opening of the vagina, signalling puberty, is delayed when housed under a winter-mimicking short daylength, compared to a summer-mimicking long daylength. The statistical result below the graph is from the model presented below. For details on the hamster treatment protocol, see our Methods subsection ‘Animals’.
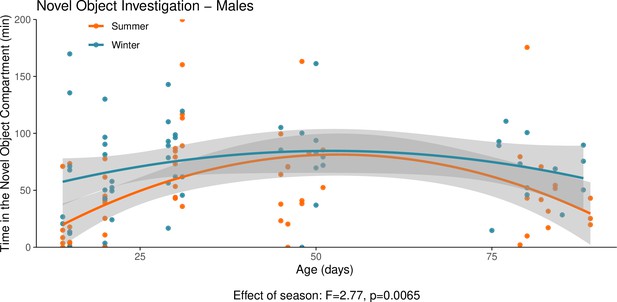
This graph illustrates the results of a novel object behavioural experiment with male summer and winter hamsters between the ages of 15 and 90 days.
The protocol used for this experiment was identical to that described in the Methods subsection ‘Behaviour – light/dark box’, the only difference being that for this experiment a novel object (a small wire-framed rodent cage) was placed in the light compartment of the box. The time spent in the compartment of the box that contained the novel object was quantified as our measure of novel object investigation. Summer hamsters were housed under a summer-mimicking long photoperiod, while winter hamsters were housed under a winter-mimicking short photoperiod. For more details on our hamster housing protocol, see our Methods subsection ‘Animals’. The statistical result below the graph is from the polynomial model shown below.
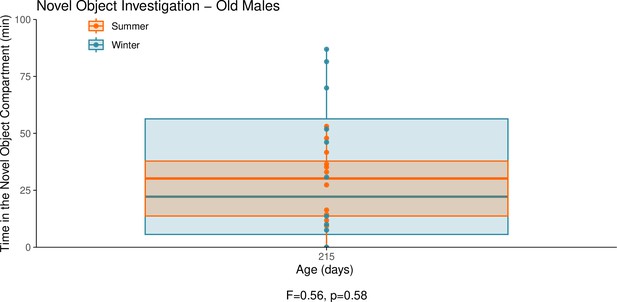
This graph illustrates the results of a novel object behavioural experiment with 215-day-old male summer and winter hamsters.
The protocol used for this experiment was identical to that described in the Methods subsection ‘Behaviour – light/dark box’, the only difference being that for this experiment a novel object (a small wire-framed rodent cage) was placed in the light compartment of the box. The time spent in the compartment of the box that contained the novel object was quantified as our measure of novel object investigation. Summer hamsters were housed under a summer-mimicking long photoperiod, while winter hamsters were housed under a winter-mimicking short photoperiod. For more details on our hamster housing protocol, see our Methods subsection ‘Animals’. The statistical result below the graph is from the linear model shown below.
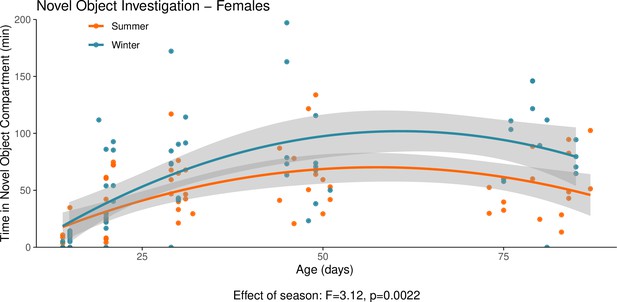
This graph illustrates the results of a novel object behavioural experiment with female summer and winter hamsters between the ages of 15 and 90 days.
The protocol used for this experiment was identical to that described in the Methods subsection ‘Behaviour – light/dark box”, the only difference being that for this experiment a novel object (a small wire-framed rodent cage) was placed in the light compartment of the box. The time spent in the compartment of the box that contained the novel object was quantified as our measure of novel object investigation. Summer hamsters were housed under a summer-mimicking long photoperiod, while winter hamsters were housed under a winter-mimicking short photoperiod. For more details on our hamster housing protocol, see our Methods subsection ‘Animals’. The statistical result below the graph is from the polynomial model shown below.
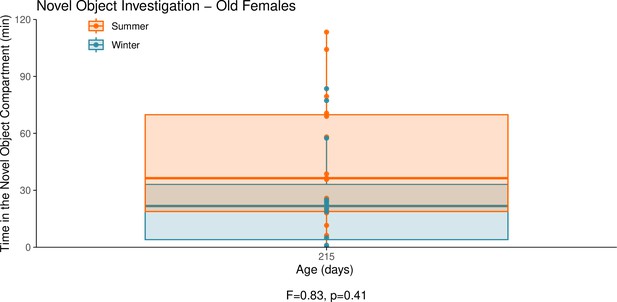
This graph illustrates the results of a novel object behavioural experiment with 215-day-old female summer and winter hamsters.
The protocol used for this experiment was identical to that described in the Methods subsection ‘Behaviour – light/dark box’, the only difference being that for this experiment a novel object (a small wire-framed rodent cage) was placed in the light compartment of the box. The time spent in the compartment of the box that contained the novel object was quantified as our measure of novel object investigation. Summer hamsters were housed under a summer-mimicking long photoperiod, while winter hamsters were housed under a winter-mimicking short photoperiod. For more details on our hamster housing protocol, see our Methods subsection ‘Animals’. The statistical result below the graph is from the linear model shown below.





