Long-term hematopoietic transfer of the anti-cancer and lifespan-extending capabilities of a genetically engineered blood system by transplantation of bone marrow mononuclear cells
Figures
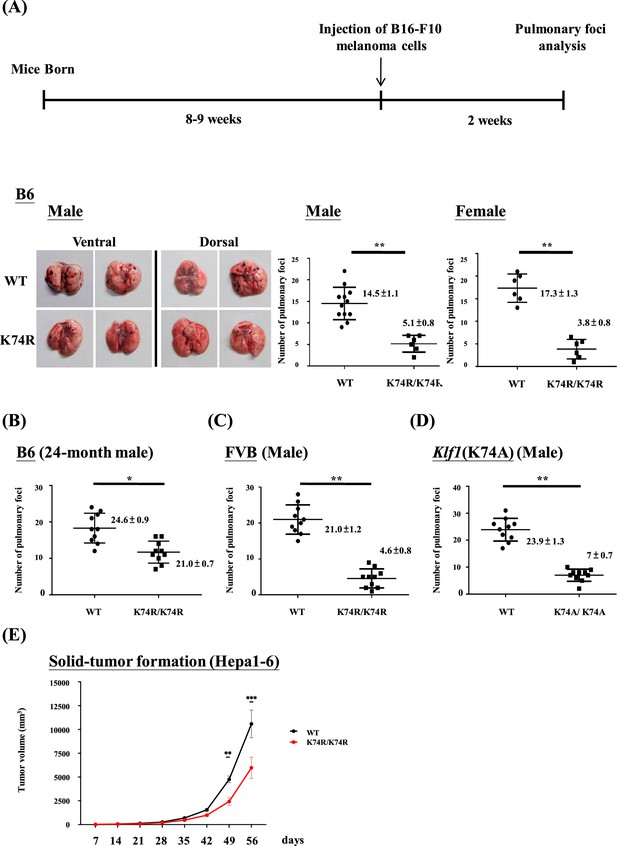
Anti-cancer capability of Klf1(K74R) mice as analyzed by the experimental melanoma metastasis assay.
(A) Flow chart illustrating the strategy of the pulmonary tumor foci assay. Left panels: representative photographs of pulmonary metastatic foci on the lungs of wild-type (WT) and Klf1(K74R) male mice in the B6 background 2 wk after intravenous injection of B16-F10 cells (105 cells/ mouse). Statistical comparison of the numbers of pulmonary foci is shown in the two histograms on the right. N = 10 (male) and N = 7 (female), **p<0.01. Note that only the number of large pulmonary foci (>1 mm diameter) was scored. N > 6, **p<0.01. (B) Pulmonary tumor foci assay of 24-month-old WT and Klf1(K74R) male mice. Statistical comparison is shown in the two histograms. N = 10 (male), *p<0.05. (C) Pulmonary tumor foci assay of male mice in the FVB background. Statistical comparison is shown in the histograph on the right. N = 10, **p<0.01. (D) Pulmonary tumor foci assay of Klf1(K74A) male mice. Statistical comparison of the 3-month-old WT and Klf1(K74A) mice numbers of pulmonary foci is shown in the two histograms. N = 10 (male), **p<0.01. (E) The Klf1(K74R) mice and WT mice were subcutaneously injected with Hepa1-6 cells to form tumors. The tumor volumes were measured once a week using the formula: length × weight × height × π/6. The curves of tumor growth started to show difference between the Klf1(K74R) and WT mice at 49 d after Hepa1-6 cell injection. N > 6, *p<0.05, **p<0.01, and ***p<0.001.
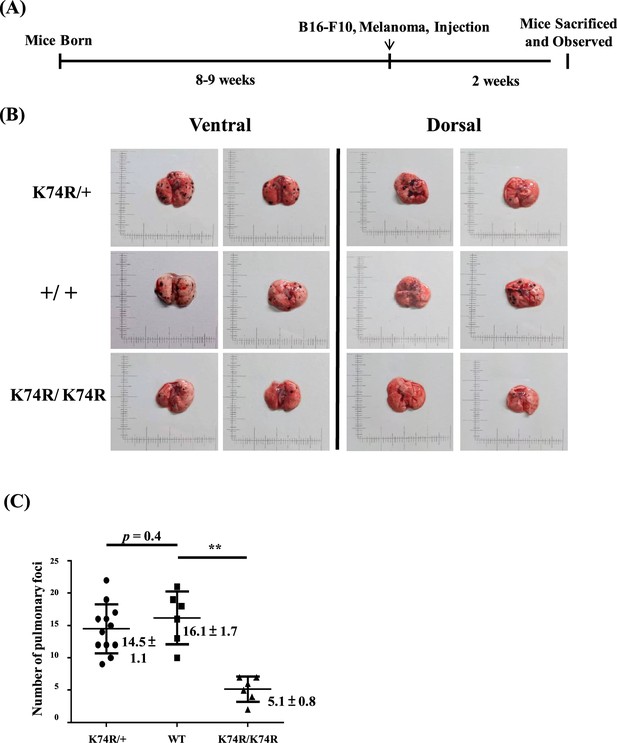
Cancer resistance of Klf1(K74R) homozygous and heterozygous mice.
(A) Flow chart illustrating the strategy of the pulmonary tumor foci assay. (B) Representative photographs of pulmonary metastatic foci on the lungs of wild-type (WT), heterozygous Klf1(K74R), and homozygous Klf1(K74R) male mice in the B6 background 2 wk after intravenous injection of B16-F10 melanoma cells (105 cells/ mouse). (C) Statistical comparison of the numbers of pulmonary foci is shown in the three histograms. N = 12 (heterozygous), N = 7 (WT), and N = 6 (homozygous), **p<0.01. Note that only the numbers of large pulmonary foci (>1 mm diameter) were scored.
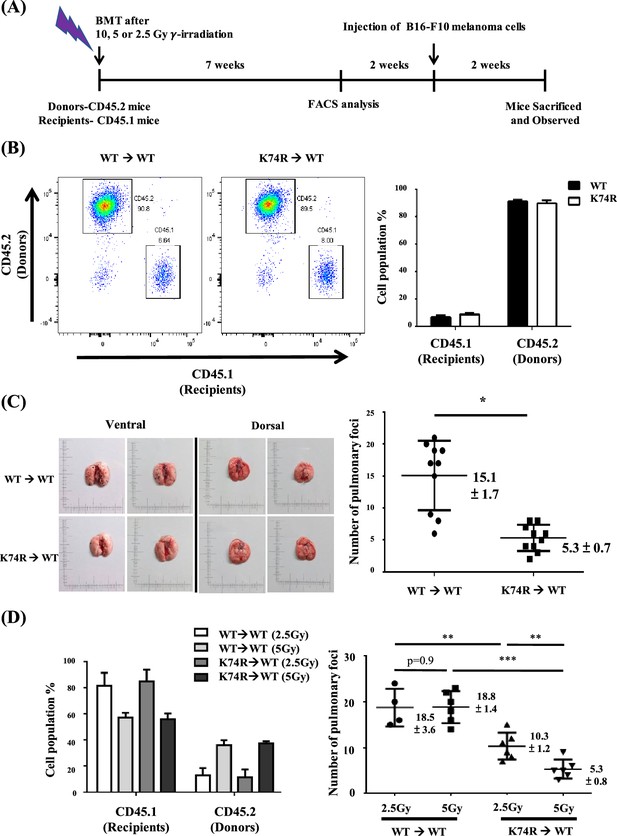
Transfer of cancer resistance of Klf1(K74R) mice to wild-type (WT) mice by bone marrow transplantation (BMT).
(A) Flow chart illustrating the experimental strategy. (B) Fluorescence-activated cell sorting (FACS) analysis of the efficiency of BMT by using 10 Gy γ-irradiation. The percentages of CD45.1/CD45.2 cells in the peripheral blood (PB) of the recipient male mice were analyzed by flow cytometry, with the representative FACS charts shown on the left and the statistical histobar diagram on the right. (C) Transfer of the anti-metastasis capability of 8-week-old Klf1(K74R) male mice to age-equivalent WT male mice by BMT by using 10 Gy γ-irradiation. Left panels: representative photographs of lungs with pulmonary metastatic foci in the recipient WT (CD45.1) mice after BMT from WT (CD45.2) or Klf1(K74R) (CD45.2) donor mice and challenged with B16-F10 cells. Statistical analysis of the number of pulmonary B16-F10 metastatic foci on the lungs is shown in the right histogram. n = 10, *p<0.05. (D) Transplantation of 8-week-old male WT (CD45.1) mice with BMMNC from age-equivalent WT (CD45.2) male mice or from Klf1(K74R) (CD45.2) male mice by using the γ-irradiation dosage 2.5 Gy or 5 Gy. The histobar diagram comparing the percentages of CD45.1 and CD45.2 PB cells of the recipient WT mice after BMT is shown on the left. The statistical analysis of the average number of pulmonary foci on the lungs of recipient WT mice after BMT and injected with the B16-F10 cells is shown in the right histogram, N = 6. **p<0.01, ***p<0.001.
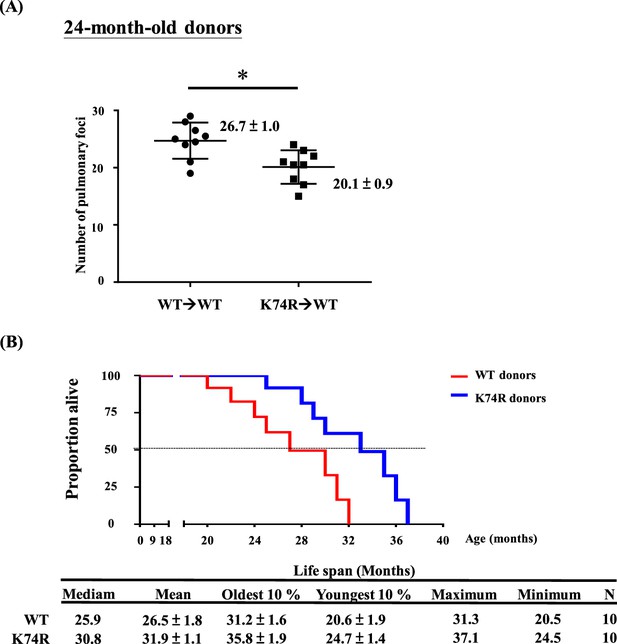
Transfer of cancer resistance of aging Klf1(K74R) mice to young wild-type (WT) mice by bone marrow transplantation (BMT), and lifespan prolonging by young mice bone marrow transplantation.
(A) Transfer of the anti-metastasis capability of 24-month-old male Klf1(K74R) mice to 2-month-old male wild-type (WT) mice by bone marrow transplantation (BMT) by using 10 Gy γ-irradiation. Statistical comparison of the number of pulmonary foci is shown in the two histograms on the right. N = 10 (male), *p<0.05. Note that only the number of large pulmonary foci (>1 mm diameter) were scored. (B) Survival curves of 2-month-old WT mice receiving BMT from 2-month-old Klf1(K74R) or WT donor mice. N = 10, p<0.05.
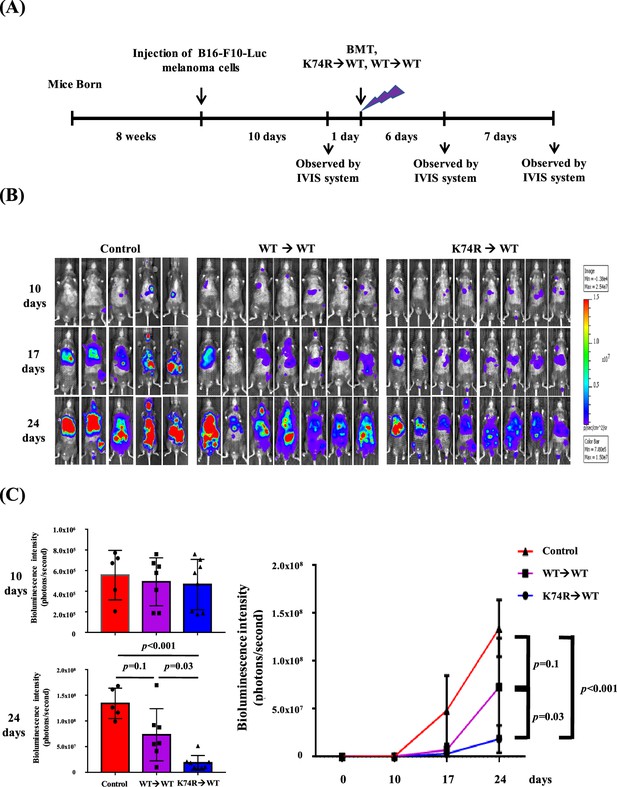
Inhibition of tumor growth in wild-type (WT) mice by bone marrow transplantation (BMT) from Klf1(K74R) mice.
(A) A flow chart of the experiments. Luciferase-positive B16-F10 cells were injected into the tail vein of 8-week-old WT male mice (day 0). The mice were then transplanted with bone marrow mononuclear cells (BMMNCs) from WT or Klf1(K74R) male mice on day 11 after the luciferase-positive B16-F10 cell injection. In vivo imaging system (IVIS) was used to follow the tumor growth in mice on days 0, 10, 17, and 24, respectively. (B) Representative images of bioluminescence reflecting the luciferase activity from melanoma cancer cells in mice. The color bar indicates the scale of the bioluminescence intensity. (C) Statistical analysis of the intensities of bioluminescence in the cancer-bearing mice (WT→WT, purple, N = 7; Klf1(K74R)→WT, blue, N = 8; control [no BMT], red, N = 3).
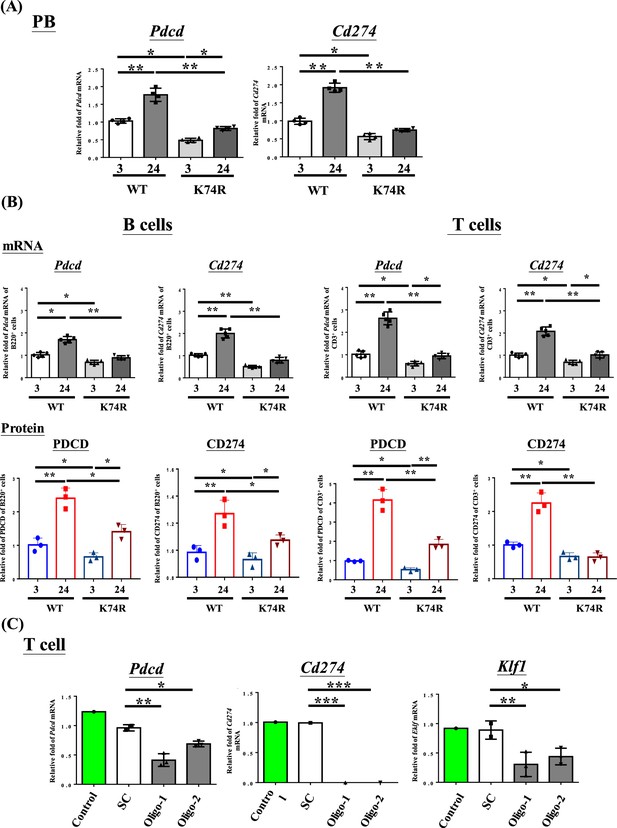
Decrease in Pdcd and Cd274 expression in blood cells of Klf1(K74R) mice.
(A) Levels of Pdcd and Cd274 mRNAs in the peripheral blood (PB) of wild-type (WT) and Klf1(K74R) male mice at the ages of 3 mo and 24 mo, respectively, as analyzed by RT-qPCR. Note the relatively low levels of Pdcd and Cd274 mRNAs in the Klf1(K74R) mice at both ages in comparison to the WT mice. (B) Upper panels: comparison of the mRNA levels of Pdcd and Cd274 of CD3+ T cells and B220+ B cells isolated from the PB of 8-week-old WT and Klf1(K74R) male mice. N = 5. *p<0.05; **p<0.01. Lower panels: comparison of the protein levels of PDCD and CD274, as analyzed by flow cytometry, of CD3+ T cells and B220+ B cells from 8-week-old WT and Klf1(K74R) male mice. N = 3. *p<0.05; **p<0.01. (C) Comparison of the levels of Pdcd, Cd274, and Klf1 mRNAs, as analyzed by RT-qPCR, in CD3+ T cells, which were isolated from splenocytes, without or with RNAi knockdown of Klf1 mRNA. Two oligos (oligo-1 and oligo-2) were used to knock down Klf1 mRNA by ~60–70%, which resulted in the reduction of Pdcd mRNA level by 30–60% and nearly complete depletion of Cd274 mRNA. Control, T cells transfected with GFP-plasmid; SC, T cells transfected with scrambled oligos. N > 3. *p<0.05; **p<0.01; ***p<0.001.
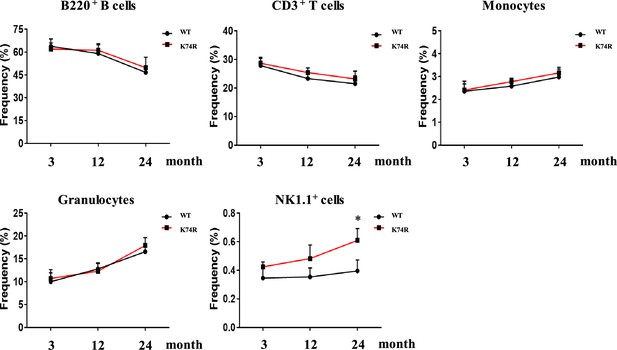
Comparison of hematopoietic cell populations between wild-type (WT) and Klf1(K74R) mice.
Line graph for comparison of the percentages of peripheral CD3+ T cells, B220+ B cells, monocytes, granulocytes, and natural killer (NK) cells, respectively, in the peripheral blood of 3-month-old, 12-month-old, and 24-month-old WT vs. Klf1(K74R) male mice. N > 5. *p<0.05.
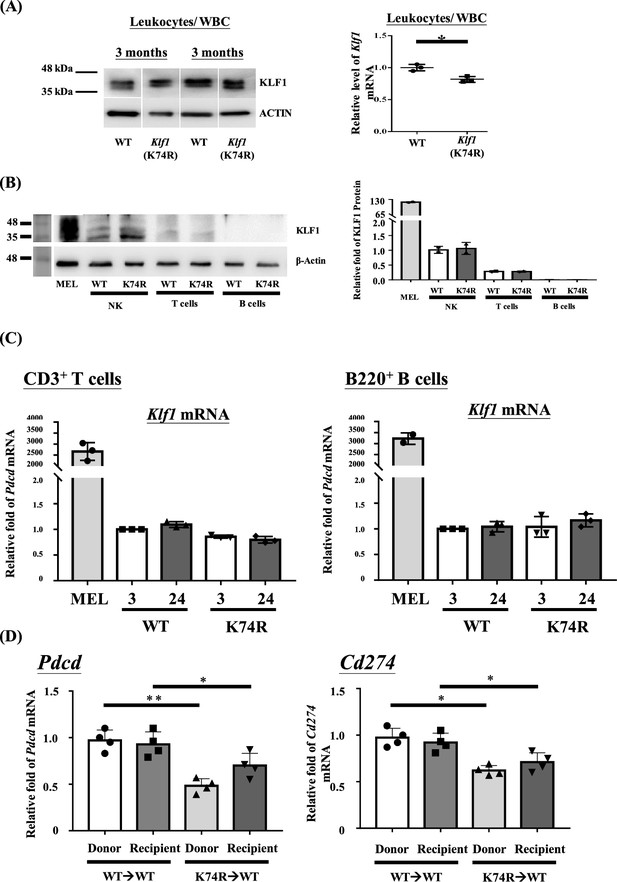
Comparative analysis of Klf1 gene expression in hematopoietic cells of wild-type (WT) and Klf1(K74R) mice.
(A) Comparative western blotting (WB) and RT-qPCR analysis of Klf1 expression in leukocytes isolated from peripheral blood (PB) of 3-month-old WT and Klf1(K74R) mice. The representative WB gel patterns of leukocytes/WBC are shown on the left panels, and the statistical analysis of the RT-qPCR data of Klf1 mRNA in the leukocytes/white blood cells (WBCs) is shown on the right panels. N = 3. (B) KLF1 protein expression in natural killer (NK) cells, CD3+ T cells, and B220+ B cells isolated from spleen were compared by WB. Extract from mouse erythroleukemia cell line (MEL) was used as a positive control. The extracts from different types of cells were first standardized by analysis by using anti-actin antibody (lower panel on the left). They were then analyzed by anti-KLF1. Note that the amount/lane of extracts from NK, T, and B cells were all 12-fold higher than the MEL cell extract loaded considering the relatively much higher level of KLF1 in MEL cells. N > 4. (C) Comparison of the Klf1 mRNA levels in CD3+ T cells and B220+ B cells isolated from the PB of 3- and 24-month-old WT and Klf1(K74R) male mice. N = 3. Klf1 mRNA of the MEL cells was also analyzed as the positive control. (D) RT-qPCR was used to compare the levels of Pdcd and Cd274 mRNAs in the PB of donor WT and Klf1(K74R) mice before bone marrow transplantation (BMT) and the PB of recipient WT mice after BMT. N = 4. *p<0.05; **p<0.01.
-
Figure 4—figure supplement 2—source data 1
Original file for the western blot analysis in Figure 4—figure supplement 2A (anti-KLF1, anti-β-actin).
- https://cdn.elifesciences.org/articles/88275/elife-88275-fig4-figsupp2-data1-v1.pdf
-
Figure 4—figure supplement 2—source data 2
Original file for the western blot analysis in Figure 4—figure supplement 2B (anti-KLF1, anti-β-actin).
- https://cdn.elifesciences.org/articles/88275/elife-88275-fig4-figsupp2-data2-v1.pdf
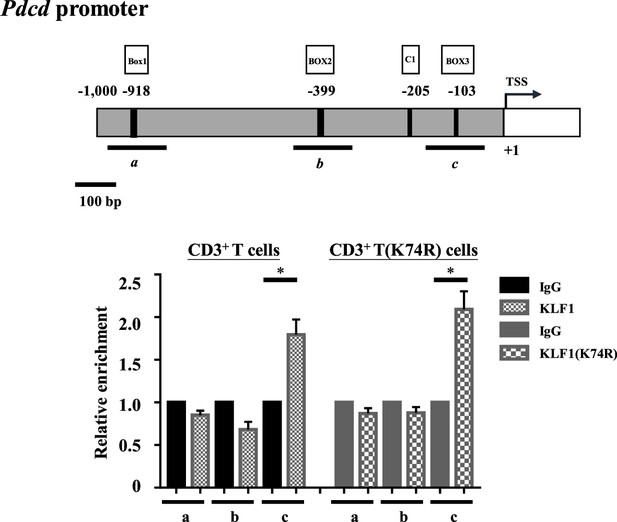
ChIP-qPCR analysis of KLF1-binding on the Pdcd promoter of CD3+ T cells from wild-type (WT) and Klf1(K74R) mice.
The map of the Pdcd promoter region is shown on the top, with the CACCC boxes (Box1, Box2, Box3), CCAAT boxes (C1), and the transcription start site (TSS,+1) indicated. The histogram below shows the relative signals from ChIP-qPCR analysis of the regions a, b, and c centered around Box1, Box2, and Box3, respectively. Note that KLF1 binds to Box3 of Pdcd promoter in CD3+ T cells from either WT or Klf1(K74R) mice. ChIP-qPCR analysis of KLF1-binding in the βmaj globin gene promoter in MEL cells was carried out as a positive control (data not shown). N = 2, *p<0.05.
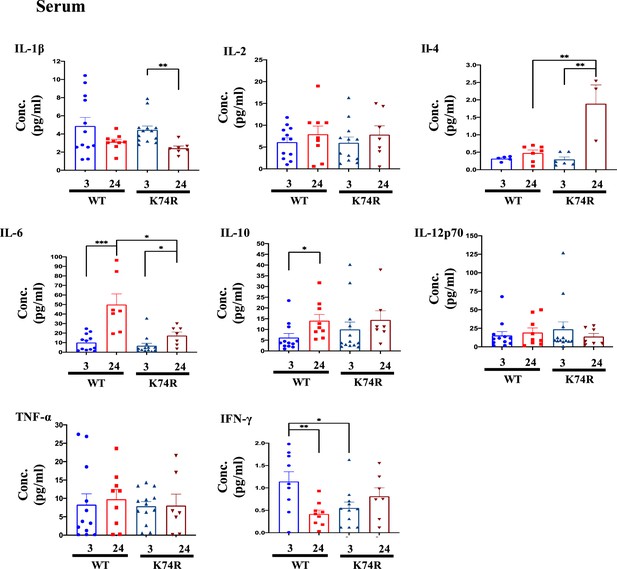
Comparison of the serum levels of different cytokines in wild-type (WT) and Klf1(K74R) mice.
Bead-based multiplex analysis was used to measure the serum levels of IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12p70, TNF-α, and IFN-γ in the sera of the male mice of the ages of 3 mo and 24 mo. N > 3. *p<0.05; **p<0.01; ***p<0.001.
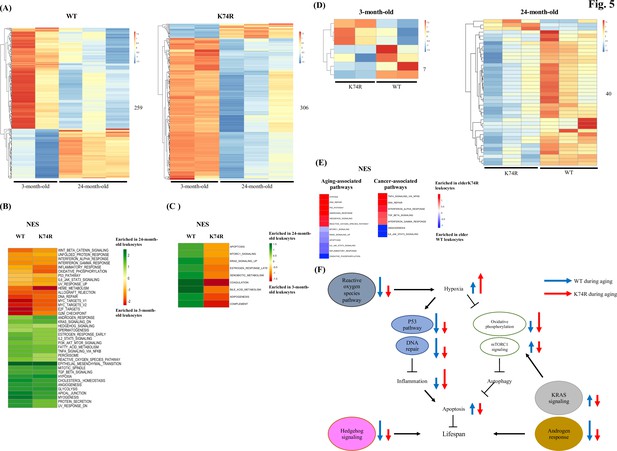
Proteomics analysis of mouse leukocytes.
(A) Heatmap plots representing age-dependent differentially expressed proteins (DEPs) in the leukocytes from the wild-type (WT) male mice (left) and Klf1(K74R) male mice (right). Differential test threshold: expression fold change >1.5 and p-value <0.01. (B, C) Pathway analysis of the age-dependent DEPs changed in the concordant (B) and reverse (C) directions, respectively, in the WT and Klf1(K74R) mice. NES, normalized enrichment score. (D) Heatmap plots representing strain-dependent DEPs in leukocytes of 3-month-old (left) and 24-month-old (right) mice. (E) Pathway analyses of the strain-dependent DEPs in elder WT leukocytes (enrichment indicated by blue color) and elder Klf1(K74R) leukocytes (enrichment indicated by red color). The aging- and cancer-associated pathways are presented in the left and right diagrams, respectively. (F) A model of the regulation of the mouse lifespan by different cellular pathways in the leukocytes. The inter-relationship of 11 aging-associated cellular pathways differentially expressed in the leukocytes of Klf1(K74R) male mice in comparison to the WT male mice, among themselves and with respect to the regulation of lifespan (for references, see text), is depicted here. The directions and lengths of the arrows indicate changes in the individual pathways, upregulation (up arrows) or repression (down arrows), in the leukocytes during aging of the WT male mice (blue arrows) and Klf1(K74R) male mice (red arrows), respectively.
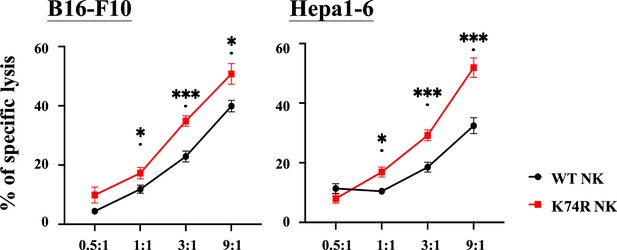
Higher in vitro cancer cell cytotoxicity of NK(K74R) cells than wild-type (WT) natural killer (NK) cells.
B16-F10 and Hepa1-6 cells were labeled with Calcein-AM (BioLegend) and co-cultured with NK cells in different E:T ratio for 4 hr. Killing rates of B16-F10 and Hepa 1–6 cancer cells by NK cells were determined by the intensities of fluorescence in the medium in comparison to the controls of spontaneous release and maximal release.
Additional files
-
Supplementary file 1
Cell surface markers of different hematopoietic blood cells.
(a) Cell surface markers of different hematopoietic blood cells. (b) DNA primers used in ChIP-qPCR experiments.
- https://cdn.elifesciences.org/articles/88275/elife-88275-supp1-v1.zip
-
MDAR checklist
- https://cdn.elifesciences.org/articles/88275/elife-88275-mdarchecklist1-v1.docx





