Metformin regulates bone marrow stromal cells to accelerate bone healing in diabetic mice
Figures
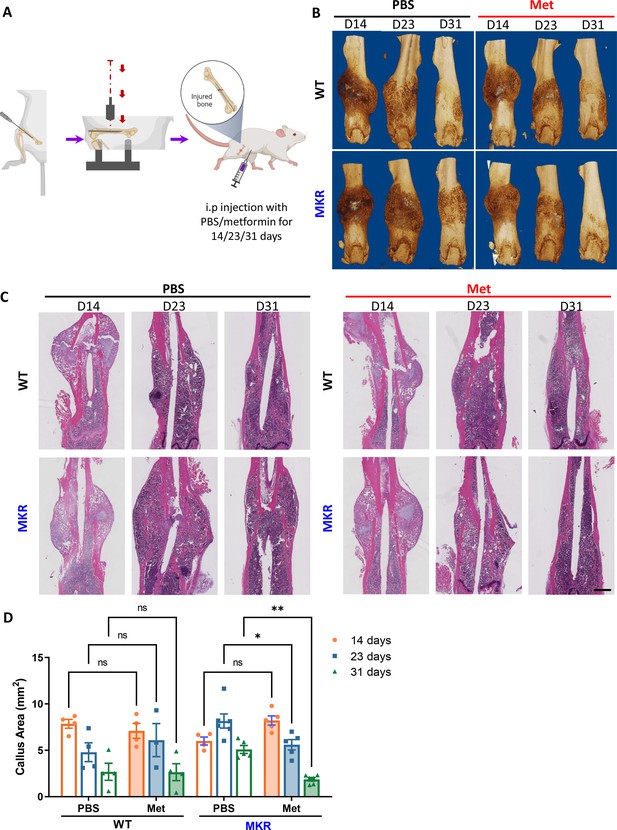
Improved healing in closed transverse fracture.
(A) Schematic representation of the experimental design (created with BioRender.com). (B) Representative µCT images of femurs from each treatment group. (C) H&E staining of longitudinal femur sections (scale bar, 1 mm). (D) Histomorphometry analysis was performed on those H&E slides to evaluate the callus area at the fracture site from each treatment group (ANOVA, followed by Tukey’s post hoc test), Bars show mean ± SEM. N = 3–6, *p<0.05, **p<0.005.
-
Figure 1—source data 1
Source data for Figure 1D.
- https://cdn.elifesciences.org/articles/88310/elife-88310-fig1-data1-v2.xlsx
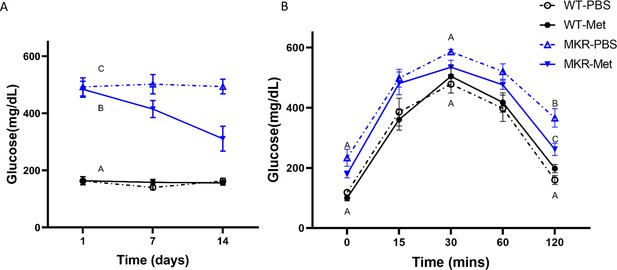
Metformin’s effect on glucose levels in MKR mice.
(A) Glucose levels in WT and MKR mice throughout the 14-day PBS or metformin treatment. (B) Glucose tolerance test (GTT) after fasting post 14-day PBS or metformin treatment (N = 6, SD). If different letters are shown at a time point, they are statistically different from one another (ANOVA, p<0.05 by post hoc Tukey’s).
-
Figure 1—figure supplement 1—source data 1
Source data for Figure 1—figure supplement 1A and B.
- https://cdn.elifesciences.org/articles/88310/elife-88310-fig1-figsupp1-data1-v2.xlsx
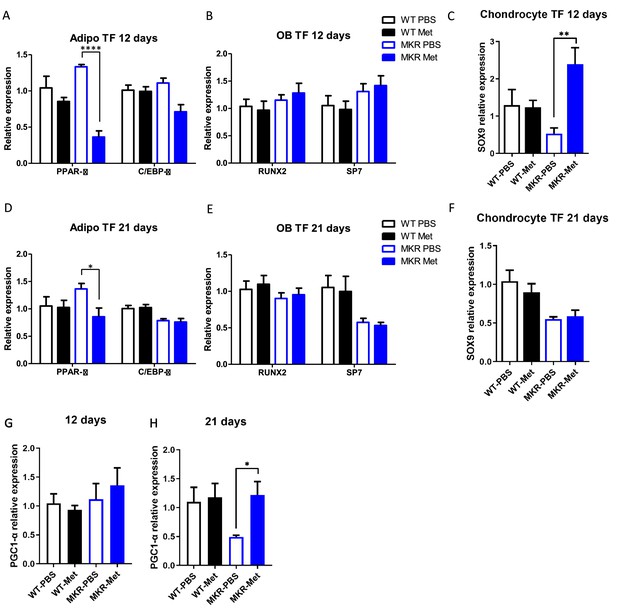
Transcript factor expressions within the femoral fracture callus tissue.
(A) Adipocyte, (B) osteoblast differentiation, and (C) chondrogenesis transcript factors expression in the callus tissue at 12 d post-fracture. (D) adipocyte, (E) osteoblast differentiation, and (F) chondrogenesis transcript factors expression in the callus tissue at 21 d post-fracture. PGC1α expression in the callus tissue at (G) 12 d and (H) 21 d post-fracture.
-
Figure 1—figure supplement 2—source data 1
Source data for Figure 1—figure supplement 2A–H.
- https://cdn.elifesciences.org/articles/88310/elife-88310-fig1-figsupp2-data1-v2.xlsx
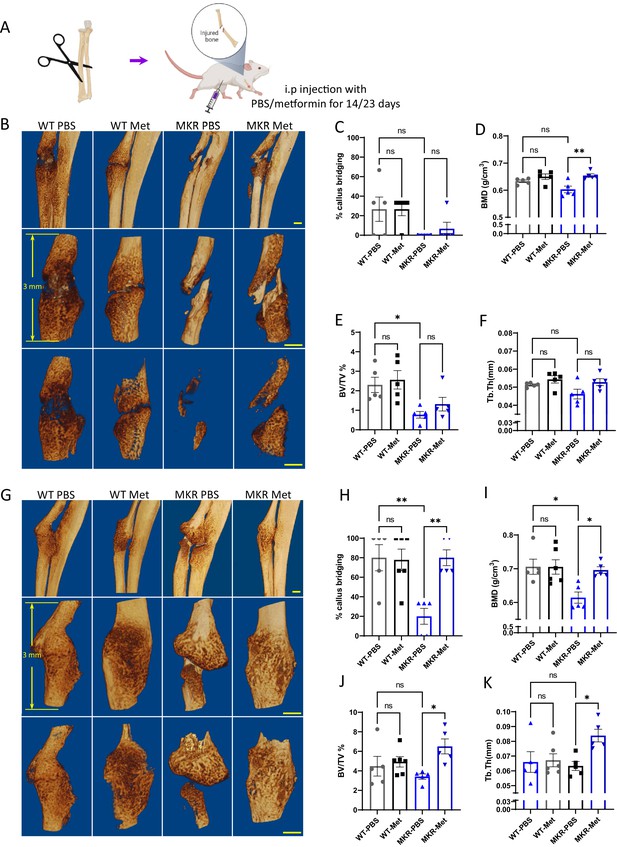
Improved healing in non-fixed radial fracture at two different time points (14 d and 23 d post-fracture).
(A) Schematic representation of the experimental design (created with BioRender.com). (B) Representative µCT images of mouse radiuses (top) and fracture sites (bottom) 14 d post-fracture (scale bar, 500 µm). Measured parameters (C–F) by µCT at 14 d post-fracture. (C) Percentage of callus bridging (%). (D) Bone mineral density (BMD; g/cm3). (E) Bone volume/tissue volume (BV/TV; %). (F) Trabecular thickness (Tb.Th; mm). (G) Representative µCT images of mouse radiuses (top) and fracture sites (bottom) 23 d post-fracture (scale bar, 500 µm). Measured parameters (H–K) by µCT at 23 d post-fracture. (H) Percentage of callus bridging (%). (I) BMD (g/cm3). (J) BV/TV (%). (K) Tb.Th (mm). Results of quantitative µCT data analysis (ANOVA, followed by Tukey’s post hoc test). Bars show mean ± SEM; N = 5–6.
-
Figure 2—source data 1
Source data for Figure 2C–F and H–K.
- https://cdn.elifesciences.org/articles/88310/elife-88310-fig2-data1-v2.xlsx
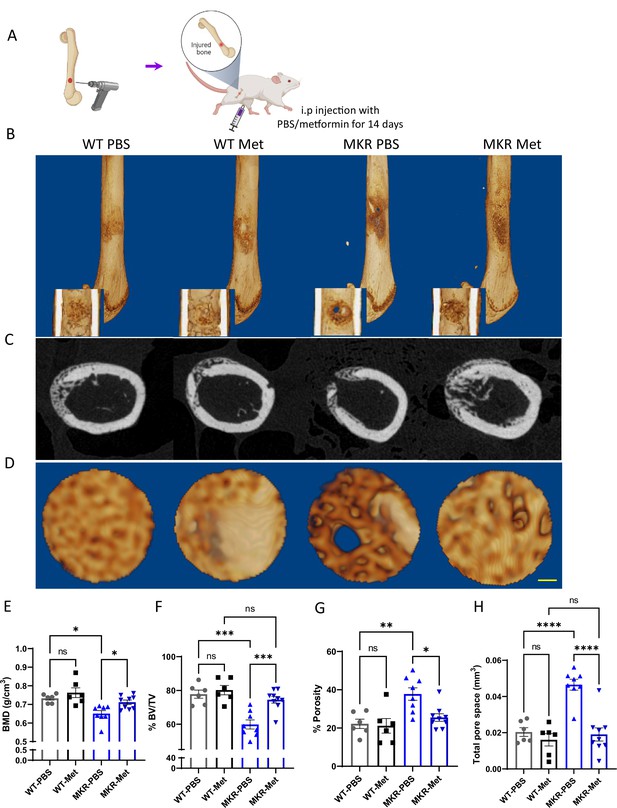
Improved healing in drill-hole bone repair model.
(A) Schematic representation of the experimental design (created with BioRender.com). (B) Representative 3D images of mouse femurs with both exterior and interior general view at 14 d post-surgery. (C) Cross-plane images at the center of the drill site. (D) Representative 3D images within the drill site (scale bar, 100 µm). and (E, H) Results of quantitative µCT data analysis (ANOVA, followed by Tukey’s post hoc test; bars show mean ± SEM; n = 6–9, *p<0.05, **p<0.01, ***p<0.005, **** p<0.0001). (E) Bone mineral density (BMD; g/cm3). (F) Bone volume/tissue volume ratio (BV/TV; %). (G) Porosity (%). (H) Total pore space (mm3).
-
Figure 3—source data 1
Source data for Figure 3E–H.
- https://cdn.elifesciences.org/articles/88310/elife-88310-fig3-data1-v2.xlsx
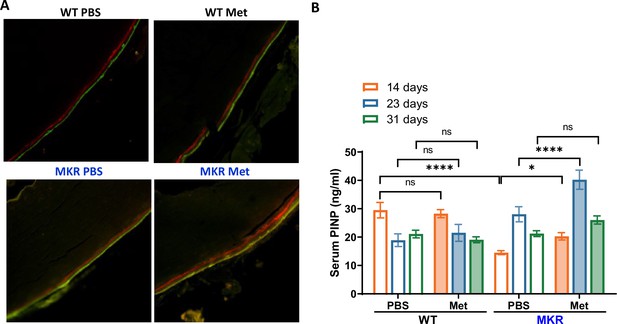
Metformin promotes bone formation.
(A) Representative images of calcein double labeling in cortical periosteum of the femur mid-shaft. (B) Serum P1NP level (ng/ml) from each treatment group at 14, 23, and 31 d post- femoral fracture (ANOVA, followed by Sidak’s post hoc test). Bars show mean ± SEM; N = 5–8, *p<0.05, ****p<0.0001.
-
Figure 4—source data 1
Source data for Figure 4B.
- https://cdn.elifesciences.org/articles/88310/elife-88310-fig4-data1-v2.xlsx
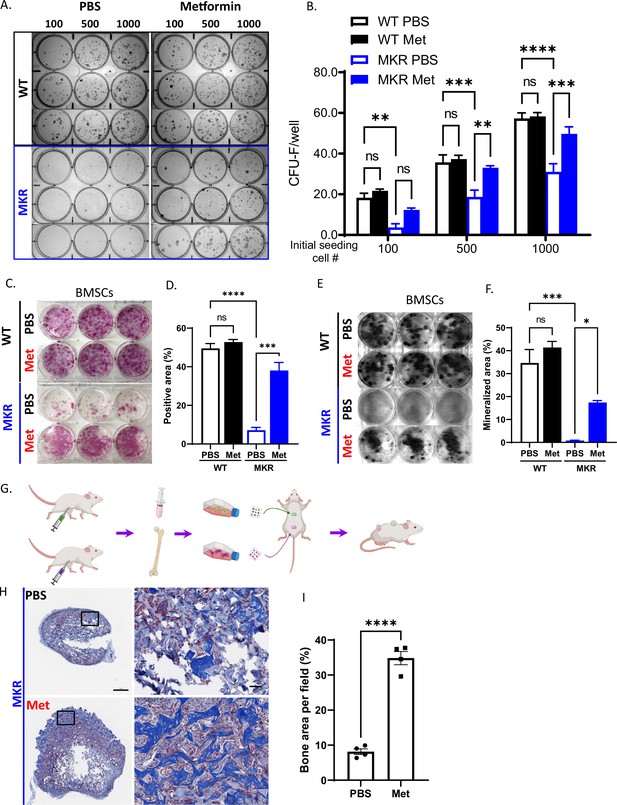
Improved osteogenesis of bone marrow stromal cell (BMSC) from metformin-treated MKR mice.
(A) Primary BMSCs were isolated from animals that were treated with PBS or metformin in vivo for 14 d and were seeded at indicated density for CFU-F culture. (B) Results from quantitative analysis of colony counts measured using ImageJ. (C) Primary BMSCs from mice received 14-day treatment of PBS or metformin were plated in 6-well plates and cultured using differentiation medium and were tested for ALP activity, (D) Total ALP-positive area per well was measured using ImageJ. (E) von Kossa staining to examine mineralization. (F) Calculation of mineralized area. (G) Schematic representation of the experimental design. (H) Masson’s Trichrome staining on the ossicle sections. (I) Percentage of bone area (blue) per field was measured using ImageJ. Bars show mean ± SEM. n = 6–9, *p<0.05, **p<0.01, ***p<0.005, ****p<0.0001.
-
Figure 5—source data 1
Source for Figure 5B, D, F and I.
- https://cdn.elifesciences.org/articles/88310/elife-88310-fig5-data1-v2.xlsx
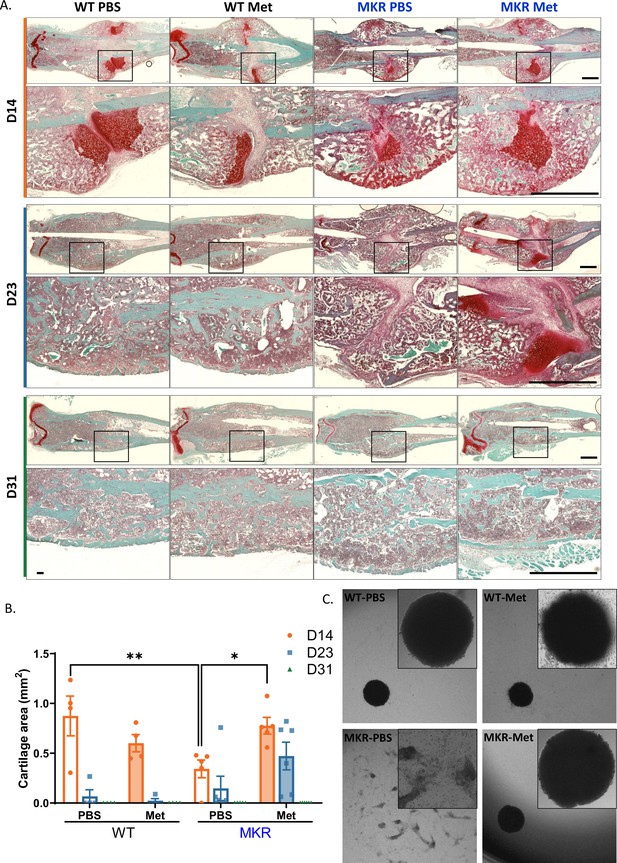
Improved chondrogenesis of bone marrow stromal cell (BMSC) from the metformin-treated MKR mice.
(A) Safranin O staining of longitudinal femur sections from femoral fractures at different time points (scale bar, 1 mm). (B) Cartilage area at fracture site was measured using ImageJ. (C) Chondrocyte pellet culture of BMSCs from the PBS- or metformin-treated animals. Micromass culture were generated by seeding 5 µl of primary BMSCs (1.6 × 107 cells/ml) in the center of 48-well plate and cultured under chondrogenic condition for 3 d (ANOVA, followed by Sidak’s post hoc test). Bars show mean ± SEM. n = 4–6, *p<0.05, **p<0.01.
-
Figure 6—source data 1
Source data for Figure 6B.
- https://cdn.elifesciences.org/articles/88310/elife-88310-fig6-data1-v2.xlsx





