Ryanodine receptor 2 inhibition reduces dispersion of cardiac repolarization, improves contractile function, and prevents sudden arrhythmic death in failing hearts
Figures

Dantrolene mitigates ventricular tachycardia/fibrillation (VT/VF) and prevents sudden cardiac death (SCD) in vivo.
(A) Schematic of experimental design. (B) Representative Electrocardiogram (ECG) tracings from 24 hr telemetry recordings. Spontaneous incidences of premature ventricular contractions (PVCs), VT, and VF were suppressed in heart failure (HF) animals with chronic dantrolene (DS) treatment. (C) DS therapy lowered PVC burden (p<0.001) in HF animals (N: Control, black = 15, HF, red = 20, HF+DS, blue = 8). (D) Kaplan Meier’s survival plot shows that over 50% of the animals in the vehicle group (HF, N=68, red) group experienced SCD. Chronic DS treatment (HF+DS, N=10, blue; Ctrl, N=10, black) prevented VT and VF in heart failure models and mitigated SCD in 80% of the heart failure animals (p<0.05). A p<0.05 was considered significant; two-tailed log-rank analysis was performed on each group for each measure.
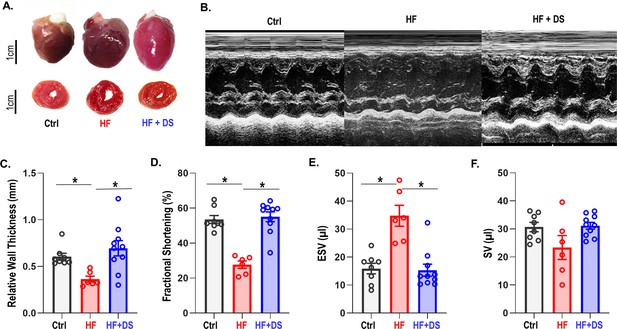
Dantrolene improves cardiac function and reverses heart failure.
(A) The hearts in the vehicle groups are enlarged, as shown in representative photos of gross hearts (top) and matching cross-sections (bottom) after 4 weeks. In the heart failure (HF) animals, the left ventricular (LV) cavity is bigger, and the LV free wall is thinned. Treatment with dantrolene (DS) stops the LV walls from thinning. Chronic DS group hearts were normal in size. (B) Representative M-mode echocardiography images from all groups (C–F) Echocardiography parameters at 4 weeks. HF animals showed significant (p<0.005, N: Control = 15, HF = 20, HF+DS = 8) loss of cardiac function with a decline in fractional shortening and relative wall thickness and an increase in stroke volume (SV) and end systolic volume (ESV). DS therapy prevented pressure overload dependent structural remodeling of the heart (p<0.005).
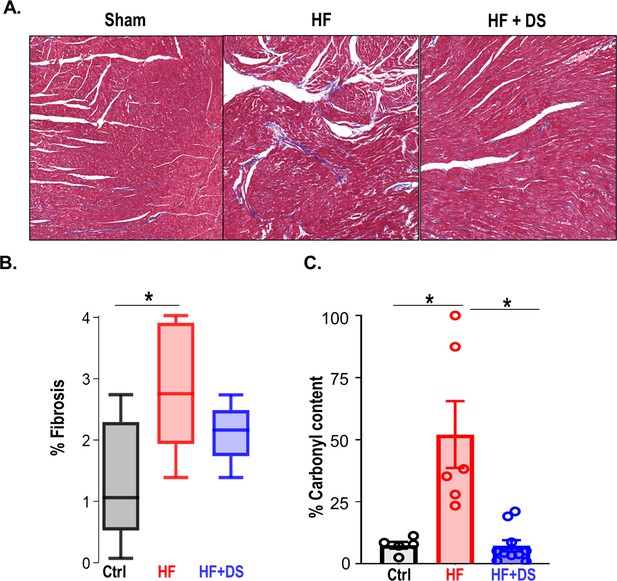
Dantrolene reverses tissue damage and oxidation profile of ryanodine receptor 2 (RyR2).
(A) Representative image of Masson trichrome staining (318 x 344 pixels) in Ctrl, heart failure (HF) & HF+DS hearts (N=3) showing high fibrotic regions as (B) summarized. (C) The percent carbonyl content/mg of protein was lower in dantrolene (DS)-treated heart failure animals, showing reversal of RyR2 oxidation profile with DS therapy in HF animals (p<0.005, N≥4). A p<0.05 was considered significant; two-tailed log-rank analysis was performed on each group for each measure.
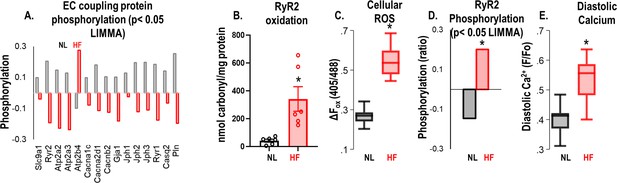
The Guinea Pig model of heart failure (HF)/(sudden cardiac death) SCD shows a remodeled and hyperactive ryanodine receptor 2 (RyR2).
(A) Proteomics data show decreased RyR2 protein expression in HF animal hearts compared to sham (p<0.05 (LIMMA)). (B) Carbonyl content assay performed on left ventricular (LV) tissue indicates that RyR2 protein is highly oxidized in animals with HF compared to sham/normal (p<0.005). (C) Baseline reactive oxygen species (ROS) assay indicates increased cellular ROS in HF cardiomyocytes compared to normal/sham cardiomyocytes (*p<0.005, N>10/group). (D) Phosphorylation profile of electrocardiogram (EC) coupling proteins in HF cardiac tissue indicates hyperphosphorylation of RyR2 (p<0.05 (LIMMA)). (E) HF cardiomyocytes have higher diastolic calcium than normal or sham (p<0.005, N≥10/group for all models). A p<0.05 was considered significant; two-tailed log-rank analysis was performed on each group for each measure.
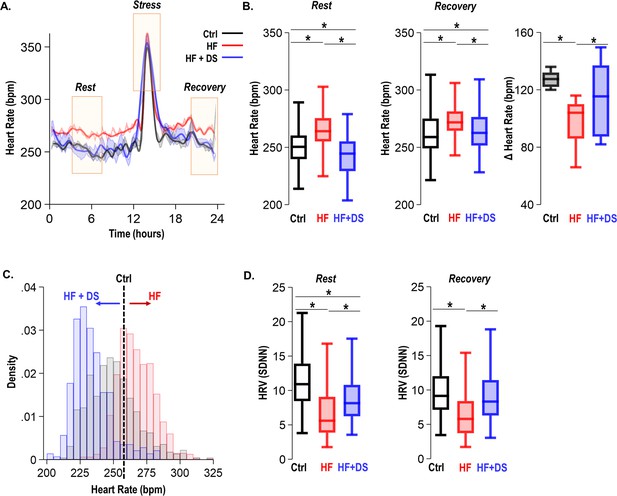
Dantrolene treatment decreases heart rate and improves chronotropic competency in heart failure (HF).
(A) Plot shows heart rate derived from 24 hr continuous electrocardiogram (ECG) recordings. The animals were subjected to mild transient β-AR stress for 1 hr. Continuous ECG analysis was performed at the following time points: resting heart rate (pre-stress); transient stress and, post-stress recovery (4 hr post-stress) (B) The box plots summarize HR during the resting and recovery phases. The dantrolene (DS) treated group (HF+DS) show a lower heart rate (HR) both at rest and post-stress recovery. The Δ Heart Rate (bpm) indicates the net increase in HR from resting HR to peak HR in response to transient β-AR stress. Failing hearts displayed higher resting HR and blunted chronotropic response to stress (p<0.02, N≥7 for all models). (C) Histogram shows resting HR distribution for all groups. Resting heart rate is decreased in HF+DS animals. However, the peak heart rates (during stress) are higher in HF+DS compared to heart failure (HF) (N; Ctrl = 8, HF = 10; HF+DS = 7). (D) DS increases heart rate variability (HRV) both at rest and during stress (p<0.05, N≥7 for all models). A p<0.05 was considered significant; two-tailed log-rank analysis was performed on each group for each measure.

Dantrolene mitigates repolarization abnormalities, normalizes the QT variability, and reduces T-wave heterogeneity.
(A) Representative QT segments recorded over 24 hr from Ctrl, heart failure (HF) and HF+DS show heterogeneity of T-wave repolarization in HF (red), but not in HF+DS (blue) animals. Dantrolene (DS) mitigates increased dispersion of repolarization in HF animals. (B) QTc prolongation in HF animals is significantly shortened by DS treatment (p<0.005), both at rest and post-stress recovery. The dispersion of repolarization was quantified by measurement of QT variability. (C) Increased QT variability predisposes the heart to ventricular tachycardia/fibrillation (VT/VF), which is prevented by DS therapy (p<0.0001). (D) Kernel density plots show that the QT variability index increases during rest, transient stress, and post-stress recovery. QT variability recovers quickly post-stress in the DS-treated group. A p<0.05 was considered significant; two-tailed log-rank analysis was performed on each group for each measure. (N: Control = 15, HF = 20, HF+DS = 8).
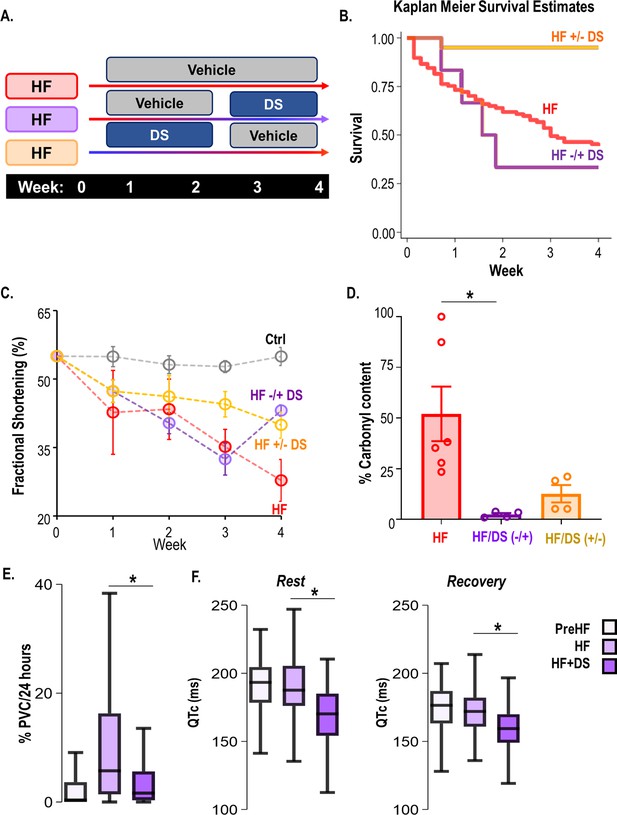
Dantrolene after heart failure (HF) development can mitigate ventricular tachycardia/fibrillation (VT/VF) and prevent sudden cardiac death (SCD).
(A) Schematic illustrates a randomized 2 x 2 cross-over experimental design to examine the impact of dantrolene (DS) therapy. The protocols were as follows (i) only vehicle administered till the endpoint i.e., week 4 (HF), (ii) DS administered from the onset of HF until chronic contractile dysfunction is evident in the untreated control HF group (usually 3 weeks post-banding; HF +/−DS), or (iii) DS administered after HF had already developed in the HF group (3 weeks post-banding; HF−/+DS group). Echocardiography and ECG recordings were taken at week 2/3/4/5. (B) Kaplan Meier’s survival curve showed that 50% of the HF animals experience SCD. Treatment with DS after HF development (around week 3) prevented additional SCDs and improved survival after starting DS therapy in the HFDS−/+group (purple). Discontinuation of DS, however, did not increase mortality in HF +/−DS group(yellow). (C) Serial echocardiography plot shows that DS reversed heart failure in HF−/+DS (purple) animals. The fractional shortening (FS)% in this group initially declined at a pace similar to HF (red) animals, but it quickly normalized as soon as DS therapy was started. However, in the HF +/−DS (yellow) group when the therapy was stopped, the FS% started showing a gradual decline, most likely due to the washout effect without additional SCD. (D) DS treatment pre or post-HF development alters ryanodine receptor 2 (RyR2) oxidation of HF animals (p<0.05). Treatment with DS reduced RyR2 oxidation even when therapy was initiated or discontinued after HF development. (E) Close examination of the HF−/+DS (purple) group, pre and post-DS therapy shows an increase in PVC load with the progression of HF. (G) The box plots summarize QTc pre-HF (light purple), during chronic HF (medium purple) and after DS therapy was initiated (dark purple). The HF−/+DS group displays all symptoms of HF including prolonged QTc after the development of HF (around week 3, medium purple box). After the start of therapy (dark purple box), QTc shortened at rest and recovery. A p<0.05 was considered significant; two-tailed log-rank analysis was performed on each group for each measure.
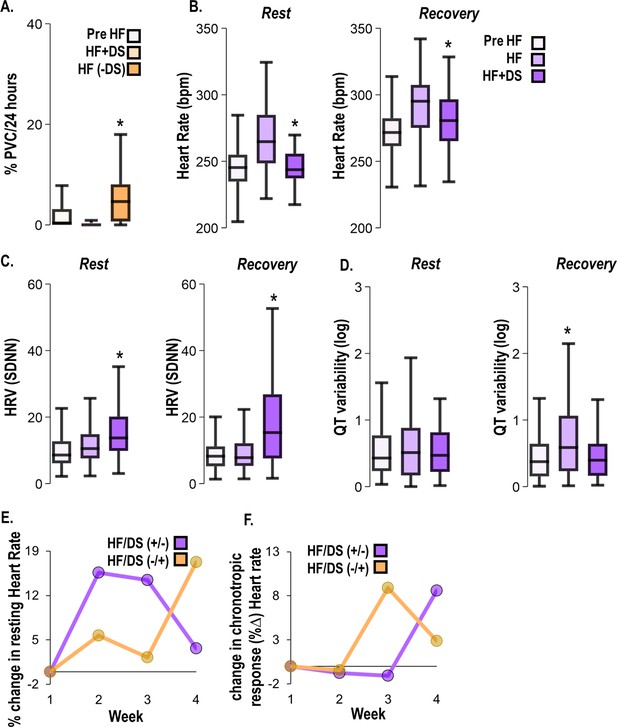
Dantrolene after heart failure (HF) development alleviates HF symptoms.
(A) In the HFDS+/− (yellow) group, premature ventricular contractions (PVC) burden decreased with chronic dantrolene (DS) treatment and increased after DS treatment was stopped. (B) HFDS−/+ (purple) group displays all symptoms of HF including increased heart rate (HR), (C) decreased heart rate variability (HRV), and (D) increased QT variability at week 3 (HF). After the start of therapy (week 4), the HR decreased, HRV increased, and QT variability increased at rest and recovery. (E) Paired analysis shows a % decrease in resting heart rate (bpm) before and after DS therapy in DS+/− (yellow) and DS−/+ (purple) groups. (F) Paired analysis indicates that response to β-adrenergic stimulation is blunted in without therapy phase but recovers once DS therapy starts (purple). Vice versa in the observed in DS +/− group (yellow). A p<0.05 was considered significant; two-tailed log-rank analysis was performed on each group for each measure.
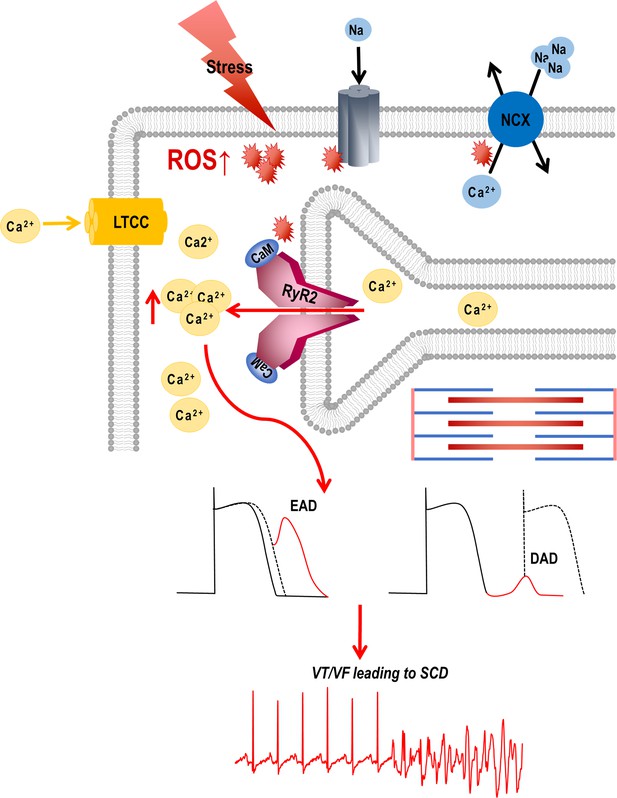
Graphical abstract.
Stress-induced reactive oxygen species (ROS) increases Ca2+ overload via leaky ryanodine receptor 2 (RyR2) channels causing ventricular tachycardia/fibrillation (VT/VF) and SCD. External or internal stress releases substantial amounts of ROS in the cardiovascular system. This ROS can alter the oxidation/phosphorylation profiles of several electrocardiogram (EC) coupling proteins including RyR2. The altered RyR2 is leaky causing spontaneous calcium release into the cytosol causing early afterdepolarizations (EADs) and diastolic calcium overload causing delayed afterdepolarizations (DADs). This can ultimately lead to triggered activity and cause ventricular tachyarrhythmias (VT/VF) leading to sudden cardiac death (SCD).





