Deficiency of IQCH causes male infertility in humans and mice
Figures
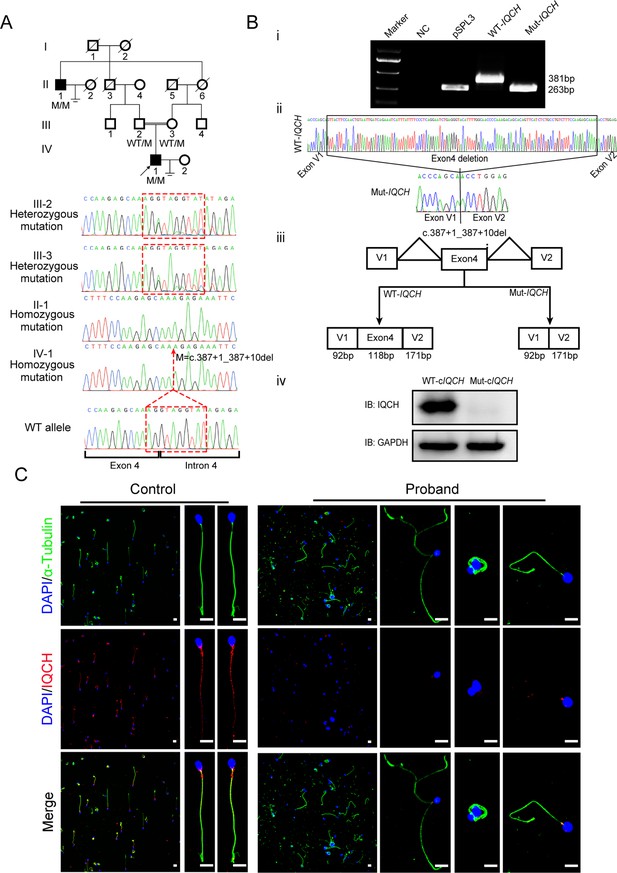
Identification of a homozygous splicing mutation in IQ motif-containing H (IQCH) in a consanguineous family with male infertility.
(A) Pedigree analysis of the consanguineous family with two infertile males (II-1 and IV-1), with the black arrow pointing to the proband. Sanger sequencing revealed that the affected males exhibited a homozygous variant in IQCH. Sequence chromatograms are shown below the pedigree. (B) (i) The electrophoresis results of the minigene assay show a decrease in the molecular weight of the RT-PCR products generated from Mut-IQCH (263 bp) compared with those from WT-IQCH (381 bp). (ii) Sanger sequencing of the complementary DNA of the splicing mutation showing the deletion of exon 4 in IQCH. (iii) The pattern diagram demonstrating the splicing effects caused by the IQCH mutation. (iv) Western blotting results showed that the Mut–cIQCH plasmids did not express IQCH. NC, negative control. Three independent experiments were performed. (C) Immunofluorescence staining showed that the expression of IQCH was barely detected in the proband’s sperm compared with that in the control (blue, DAPI; green, α-Tubulin; red, IQCH; scale bars, 5 μm).
-
Figure 1—source data 1
Primers for Sanger sequencing and Minigene.
- https://cdn.elifesciences.org/articles/88905/elife-88905-fig1-data1-v1.docx
-
Figure 1—source data 2
Original blots and gels of Figure 1.
- https://cdn.elifesciences.org/articles/88905/elife-88905-fig1-data2-v1.zip
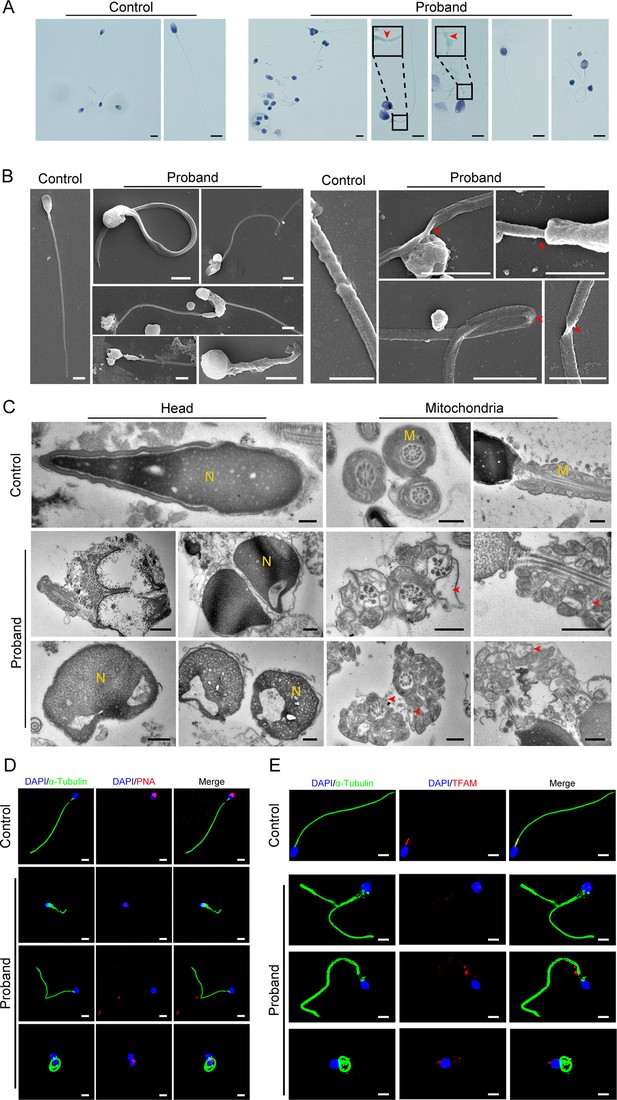
Abnormal flagellar morphology and defective acrosomes and mitochondria in the infertile patient.
(A and B) Papanicolaou staining (A) and scanning electron microscopy (SEM) (B) results show flagellar morphological abnormalities (scale bars in A, 5 μm; scale bars in B, 2.5 μm). The dotted boxes and red arrowheads denote axoneme cracking and exposure. (C) Transmission electron microscopy (TEM) results showing deformed acrosomes and abnormal arrangement and diameter of mitochondria (scale bars, 500 nm). The red arrowheads denote abnormal mitochondria. N, nucleus; M, mitochondria. (D and E) Defects in the acrosome and mitochondria were observed in the proband’s sperm by PNA (D) and TFAM (E) staining (blue, DAPI; green, α-Tubulin; red, PNA or TFAM; scale bars, 5 μm). PNA, peanut agglutinin; TFAM, transcription factor A, mitochondrial.
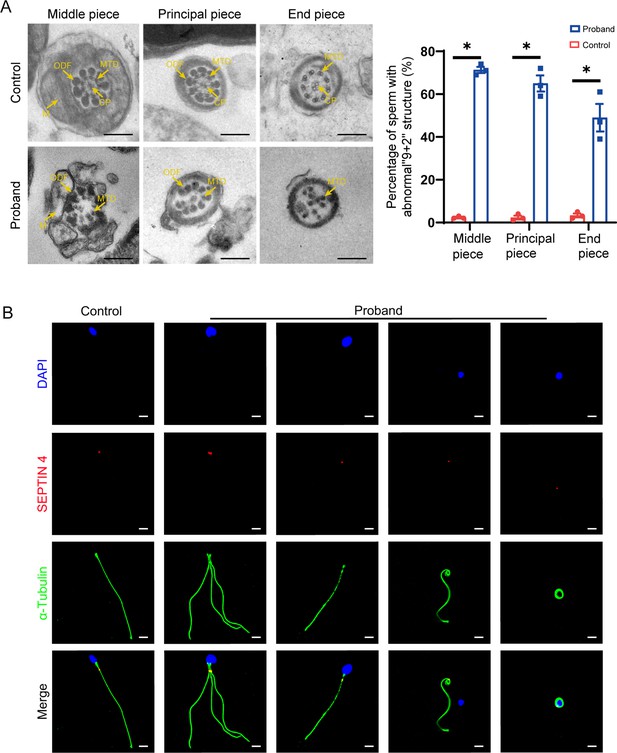
Transmission electron microscopy (TEM) and immunofluorescence analysis of the abnormal phenotype of spermatozoa in the patient.
(A) TEM results show the deranged or incomplete ‘9+2’ microtubule structure of the flagella. The percentage of abnormal ‘9+2’ microtubule structures in the control and proband (scale bars, 500 nm; Student’s t-test; *p<0.05; error bars, s.e.m.). CP, central-pair microtubule; MTD, peripheral microtubule doublet; ODF, outer dense fiber; M, mitochondrion. (B) Immunofluorescence staining of SEPTIN4 showed no significant difference in the annulus between spermatozoa from the control and the proband (blue, DAPI; green, α-Tubulin; red, SEPTIN4; scale bars, 5 µm).
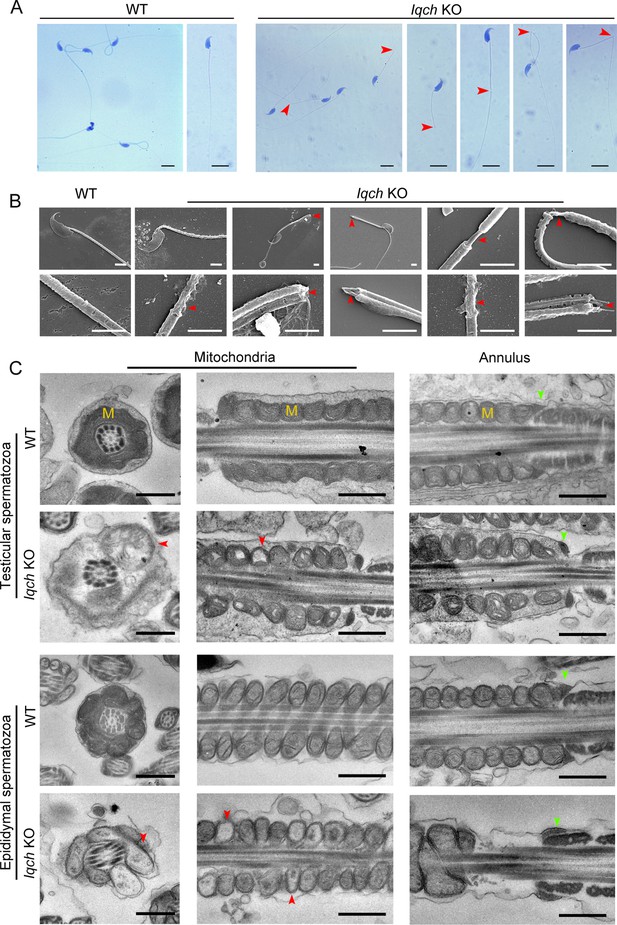
The absence of IQ motif-containing H (Iqch) impaired spermatogenesis in mice.
(A and B) Papanicolaou staining (B) and scanning electron microscopy (SEM) (C) results showing unmasking, bending, or cracking of the axoneme in spermatozoa from Iqch knockout (KO) mice (n=3 biologically independent wild-type (WT) mice and KO mice; scale bars in B, 5 μm; scale bars in C, 2.5 μm). The red arrowheads point to axoneme abnormalities. (C) Transmission electron microscopy (TEM) revealing dilated intermembrane spaces of mitochondria and a normal annulus in the testicular and epididymal spermatozoa of Iqch KO mice (n=3 biologically independent WT mice and KO mice; scale bars, 500 nm). The red arrowheads point to the dilated intermembrane spaces of mitochondria. The green arrowheads point to the normal annulus. M, mitochondria.
-
Figure 3—source data 1
Primers for RT-PCR and sgRNA sequences.
- https://cdn.elifesciences.org/articles/88905/elife-88905-fig3-data1-v1.docx
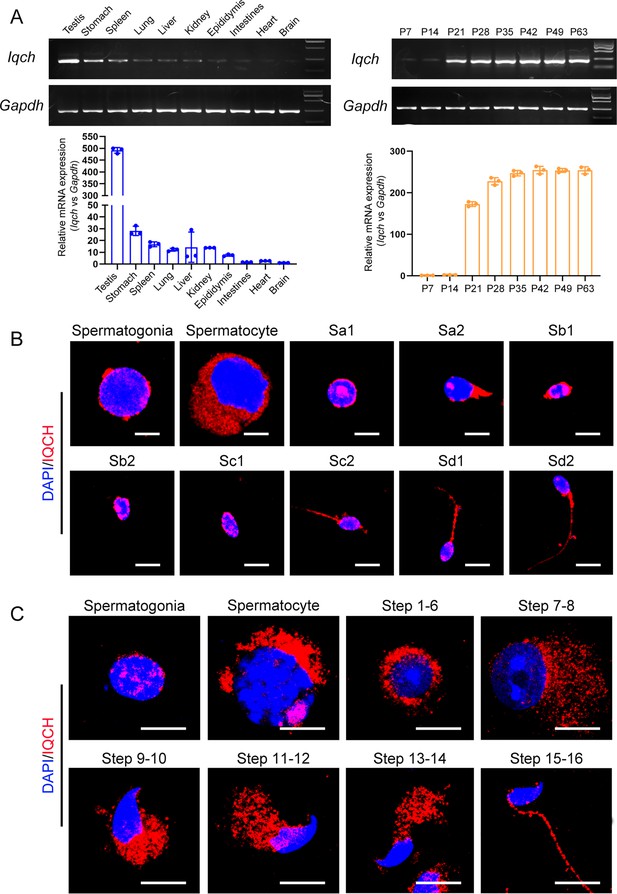
The expression pattern of IQ motif-containing H (IQCH) in testes.
(A) qPCR analysis showed that Iqch was predominantly expressed in the testes, and Iqch began to be significantly expressed on P21, reached its peak on P35, and then showed a stable expression pattern (Student’s t-test; *p<0.05; error bars, SEM). P, postnatal day. (B) Immunofluorescence staining of IQCH in isolated human germ cells (blue, DAPI; red, IQCH; scale bars, 5 µm). (C) Immunofluorescence staining of IQCH in isolated mouse germ cells (blue, DAPI; red, IQCH; scale bars, 5 µm). Three independent experiments were performed.
-
Figure 3—figure supplement 1—source data 1
Original blots and gels of Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/88905/elife-88905-fig3-figsupp1-data1-v1.zip
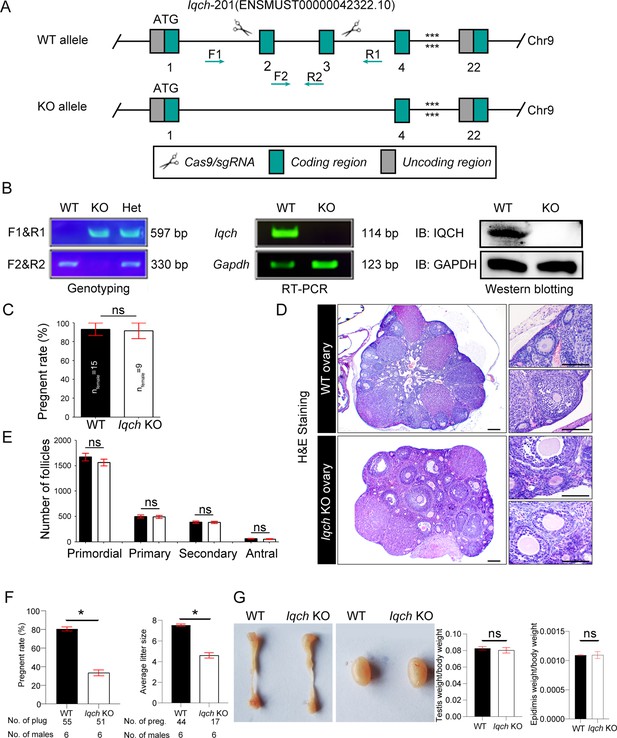
Generation of IQ motif-containing H (Iqch) knockout (KO) mice.
(A) A schematic illustration of the strategy used to generate the Iqch KO mice. Exons 2 through 3 of the Iqch gene were targeted by the CRISPR-Cas9 system. (B) PCR, RT‒PCR, and western blotting were used to determine the genotype and KO efficiency (n=3 biologically independent wild-type (WT) mice and KO mice). (C) Female fertility was unaffected in the Iqch KO mice according to the mouse fertility test. Iqch KO female mice (n=9) and WT female mice (n=15) were mated with WT male mice for 6 months at a male/female ratio of 1:3 (Student’s t-test; ns, not significant; error bars, SEM). (D) Representative images of H&E-stained WT and Iqch KO ovary tissue sections (n=3 biologically independent WT mice and KO mice; scale bars, 75 μm). (E) The number of primordial follicles, primary follicles, secondary follicles, and antral follicles in the ovaries of the WT and Iqch KO mice (n=3 biologically independent WT mice and KO mice; Student’s t-test; ns, not significant; error bars, s.e.m.). (F) Fertility of the WT and Iqch KO males. KO male mice and littermate WT male mice were bred with WT female mice. The average pregnancy rates and litter sizes were measured (n=6 biologically independent WT mice and KO mice; Student’s t-test; *p<0.05; error bars, SEM). preg, pregnancy. (G) The sizes of the epididymides and testes of the WT and Iqch KO mice showed no significantly different. The testis and epididymis/body weight ratio of the Iqch KO mice were not significantly different from those of the WT male mice (n=3 biologically independent WT mice and KO mice; Student’s t-test; ns, not significant; error bars, SEM).
-
Figure 3—figure supplement 2—source data 1
Original blots and gels of Figure 3—figure supplement 2.
- https://cdn.elifesciences.org/articles/88905/elife-88905-fig3-figsupp2-data1-v1.zip
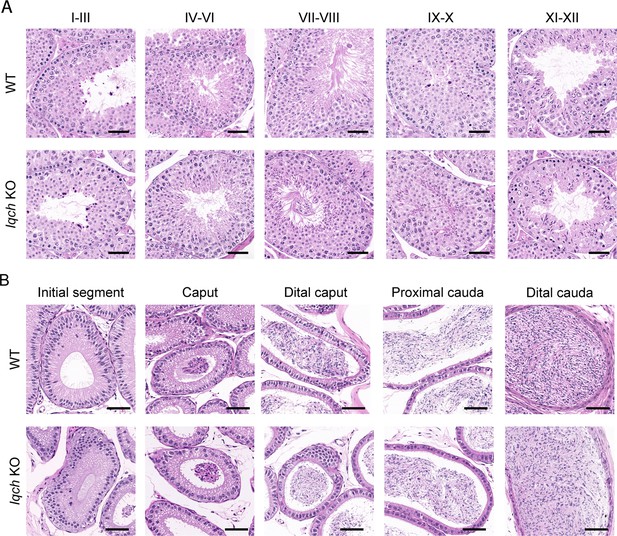
No obvious abnormalities in the histology of the testes or epididymis were observed in the IQ motif-containing H (Iqch) knockout (KO) mice.
(A) Hematoxylin-eosin (H&E) staining of testicular sections from adult Iqch KO mice shows no obvious abnormalities at different stages of spermatogenesis compared to those from wild-type (WT) mice (n=3 biologically independent WT mice and KO mice; scale bars, 50 μm). (B) H&E staining of epididymal sections from adult WT and Iqch KO mice reveal no significant difference in the lumens of the epididymides (n=3 biologically independent WT mice and KO mice; scale bars, 50 μm).
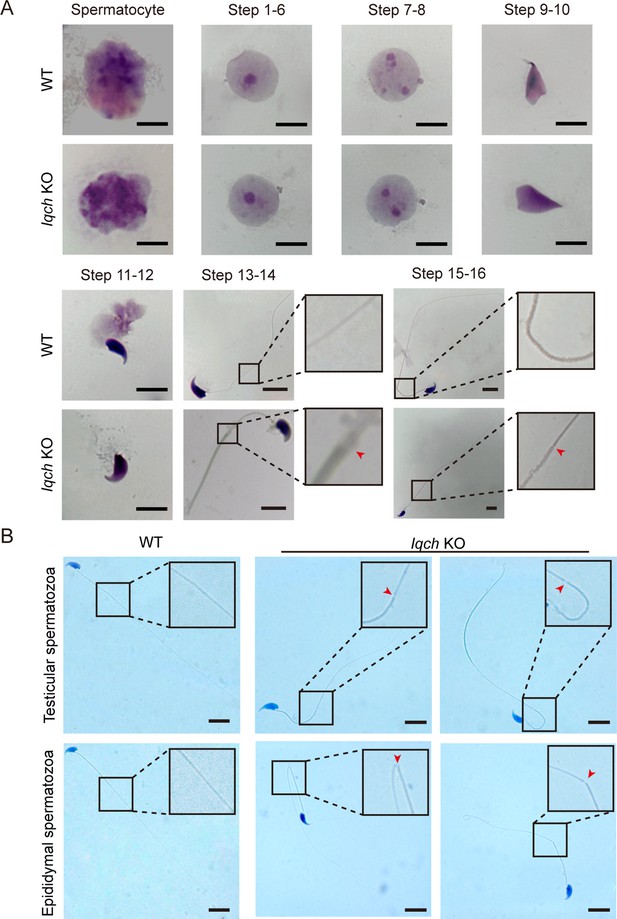
Cracking of the axoneme occurred during spermatogenesis in the IQ motif-containing H (Iqch) knockout (KO) mice.
(A) Papanicolaou staining of the isolated germ cells from the testes of the Iqch KO mice revealed cracking of the axoneme at steps 13-14 and 15-16 (n=3 biologically independent wild-type (WT) mice and KO mice; scale bars, 5 μm). (B) The cracking of the axoneme became more severe in the isolated epididymal spermatozoa than in the testicular spermatozoa (n=3 biologically independent WT mice and KO mice; scale bars, 5 μm). The dotted boxes and red arrowheads denote axoneme cracking and exposure.
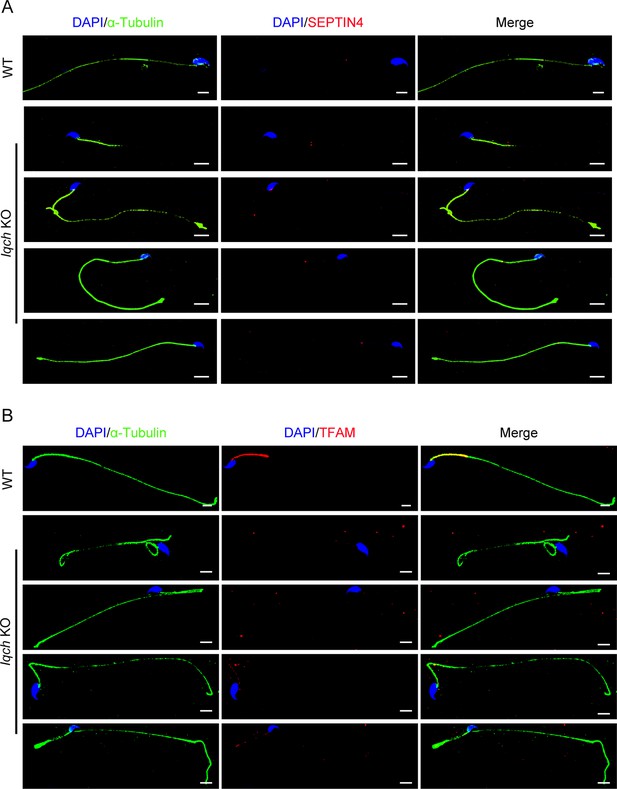
The spermatozoa from the IQ motif-containing H (Iqch) knockout (KO) mice exhibited defective mitochondria.
(A) Immunofluorescence staining of SEPTIN4 revealed no significant differences at the annulus between the spermatozoa from the wild-type (WT) mice and those from the Iqch KO mice (n=3 biologically independent WT mice and KO mice; scale bars, 5 μm). (B) Immunofluorescence staining of TFAM revealed defects in the mitochondria of the spermatozoa from the Iqch KO mice (n=3 biologically independent WT mice and KO mice; scale bars, 5 μm). TFAM, Transcription Factor A, Mitochondrial.
The movie showed the in vitro motility of epididymal sperm from wild-type (WT) mice.
Sperm were collected, incubated, and recorded by video-recording under a phase-contrast microscope. The movie demonstrates normal sperm quantity and motility in the WT mice (n=3 biologically independent WT mice).
Sperm with reduced motility were collected from the cauda epididymis and vas deferens of IQ motif-containing H (Iqch) knockout (KO) male mice and analyzed under a phase-contrast microscope(n=3 biologically independent KO mice).
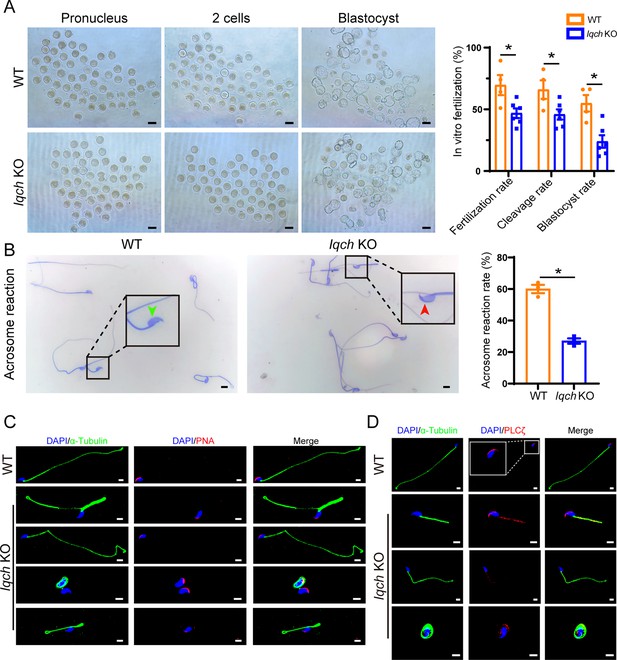
Poor in vitro fertilization (IVF) treatment outcomes resulting from the use of sperm from IQ motif-containing H (Iqch) knockout (KO) mice.
(A) Representative pronucleus embryos, two-cell embryos, and blastocysts were obtained from wild-type (WT) mice and Iqch KO mice. Iqch KO mice exhibited significantly lower fertilization rates, cleavage rates, and blastocyst formation rates than WT mice (n=3 biologically independent WT mice and KO mice; scale bars, 100 μm; Student’s t-test; *p<0.05; error bars, SEM). (B) The acrosome reaction rates in the capacitated spermatozoa from the WT mice and Iqch KO mice were determined by Coomassie brilliant blue staining. The acrosome reaction rates were reduced in the spermatozoa from the Iqch KO mice (n=3 biologically independent WT mice and KO mice; scale bars, 5 μm; Student’s t-test; *p<0.05; error bars, SEM). The green arrowheads indicate the reacted acrosomes. The red arrowheads indicate intact acrosomes. (C and D) PNA (C) and PLCζ (D) staining showing abnormal acrosome morphology and aberrant PLCζ localization and expression in spermatozoa from Iqch KO mice (n=3 biologically independent WT mice and KO mice; scale bars, scale bars, 5 μm). The dotted box indicates the typical pattern of PLCζ localization and expression in spermatozoa from WT mice. PNA, peanut agglutinin; PLCζ, phospholipase C zeta 1.
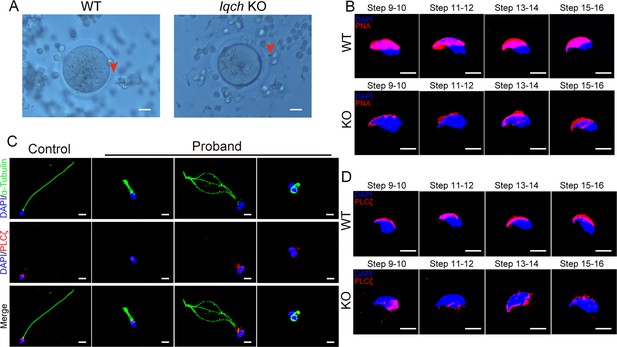
The loss of function of IQ motif-containing H (IQCH) resulted in deficiencies in oocyte activation and acrosomes.
(A) Representative images of the in vitro fertilization (IVF) treatments show that the spermatozoa from the Iqch knockout (KO) mice did not penetrate the zona pellucida (n=3 biologically independent wild-type (WT) mice and KO mice; scale bars, 50 μm). The red arrowheads indicate the spermatozoa penetrating the zona pellucida. (B) Immunofluorescence staining of the germ cells at different developmental stages revealed that the development of the PNA was impaired in Iqch KO mice (n=3 biologically independent WT mice and KO mice; blue, DAPI; purple, PNA; scale bars, 50 μm). (C) Immunofluorescence staining suggested that the loss of function of IQCH resulted in the abnormal location and expression of PLCζ in the sperm of the proband (blue, DAPI; green, α-Tubulin; red, PLCζ; scale bars, 50 μm). (D) Immunofluorescence staining of germ cells at different developmental stages from Iqch KO mice revealed the aberrant expression of PLCζ compared to that in WT mice (n=3 biologically independent WT mice and KO mice; blue, DAPI; green, α-Tubulin; red, PLCζ; scale bars, 50 μm). PNA, peanut agglutinin; PLCζ, phospholipase C zeta 1.
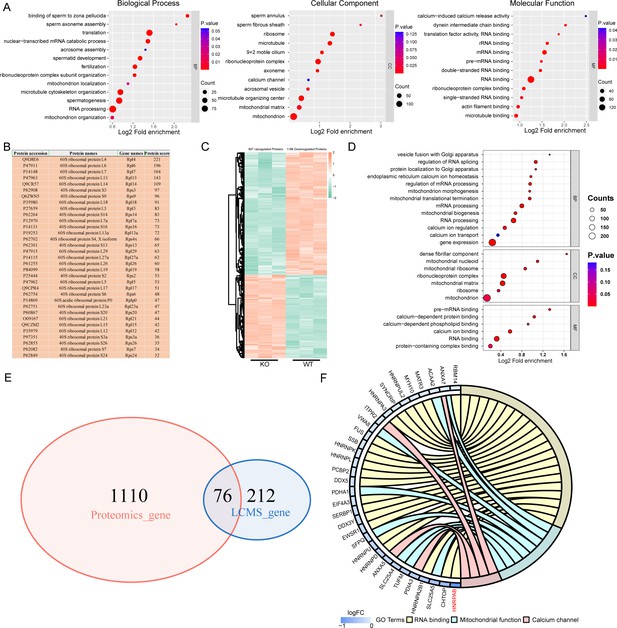
IQ motif-containing H (IQCH) bound and upregulated male reproduction-related proteins in mouse sperm.
(A) Bubble plots of the GO analysis show that the IQCH-interacting proteins are significantly enriched in spermatogenesis and RNA processing in three categories: biological process, cellular component, and molecular function. GO, Gene Ontology. (B) Thirty-three ribosomal proteins interacted with IQCH in mouse sperm. (C) A heatmap showing the differential protein results from the proteomic analysis of the sperm from the wild-type (WT) and Iqch KO mice. (D) Bubble plots showing the decreased enrichment of proteins related to the spermatogenetic process and RNA processing according to the GO analysis of the Iqch KO mice compared to the WT mice. (E) Venn diagram depicting the 76 overlapping proteins among the 1186 downregulated proteins in sperm from Iqch KO mice and the 288 proteins that bind to IQCH. (F) The Chord diagram shows 21 proteins involved in RNA binding, eight proteins involved in mitochondrial function, and four proteins involved in calcium channel activity among the 76 overlapping proteins. HNRPAB showed the greatest reduction in expression in the Iqch KO mice.
-
Figure 5—source data 1
List of 288 interactors of IQ motif-containing H (IQCH) about LC-MS/MS analysis of mouse sperm lysates.
- https://cdn.elifesciences.org/articles/88905/elife-88905-fig5-data1-v1.xlsx
-
Figure 5—source data 2
List of differentially down-regulated proteins about proteomic analysis of sperm from IQ motif-containing H (Iqch) knockout (KO) and wild-type (WT) mice.
- https://cdn.elifesciences.org/articles/88905/elife-88905-fig5-data2-v1.xlsx
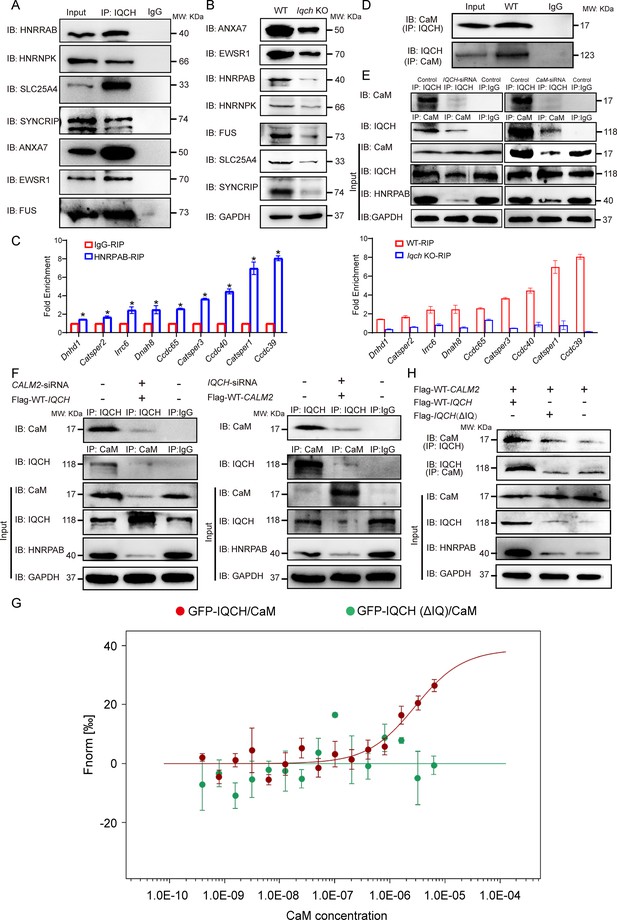
IQ motif-containing H (IQCH) interacted with calmodulin (CaM) to regulate HNRPAB expression and spermatogenesis.
(A) Co-immunoprecipitation (co-IP) of mouse sperm lysates revealed that the seven proteins most relevant to the phenotype of the Iqch knockout (KO) mice were associated with IQCH. (B) Western blotting shows the reduced expression of the seven proteins in the sperm from the Iqch KO mice compared to the wild-type (WT) mice. (C) qPCR analysis of RNA immunoprecipitation (RIP) using HNRPAB antibodies and IgG antibodies on mouse sperm lysates showed that HNRPAB interacted with several RNAs associated with fertilization and axoneme assembly. qPCR analysis of RIP in the sperm lysates from the Iqch KO mice revealed a decrease in the interaction between HNRPAB and the RNA targets compared to that in the WT mice (Student’s t-test; *p<0.05; error bars, SEM). (D) co-IP assays showing the binding of IQCH and CaM in WT sperm. (E) co-IP assays showed that the decreased expression of HNRPAB was due to the reduced binding of IQCH to CaM resulting from the knockdown of IQCH or CaM. (F) The overexpression of IQCH and the simultaneous knockdown of CaM or the overexpression of CaM and the simultaneous knockdown of IQCH in K562 cells further confirmed that the downregulation of HNRPAB was due to the diminished interaction between IQCH and CaM, as determined by western blotting analysis. (G) Analysis of the binding affinity between GFP-IQCH from the cell lysates (target) and recombinant CaM (ligand) by a microscale thermophoresis (MST) assay showing the interaction between IQCH and CaM. Their interaction was disrupted after the deletion of the IQ motif within IQCH. (H) co-IP of HEK293 cells cotransfected with the WT-IQCH and WT-CALM2 plasmids, cotransfected with the IQCH (△IQ) and WT- CALM2 plasmids, or cotransfected with the control and WT- CALM2 plasmids showed that CaM interacted with the IQ motif. The downregulation of HNRPAB was due to the disrupted interaction between IQCH and CaM. Three independent experiments were performed.
-
Figure 6—source data 1
Primers for qPCR and siRNA sequences.
- https://cdn.elifesciences.org/articles/88905/elife-88905-fig6-data1-v1.docx
-
Figure 6—source data 2
Overview of the antibodies or dyes used in this study.
- https://cdn.elifesciences.org/articles/88905/elife-88905-fig6-data2-v1.docx
-
Figure 6—source data 3
Original blots of Figure 6.
- https://cdn.elifesciences.org/articles/88905/elife-88905-fig6-data3-v1.zip
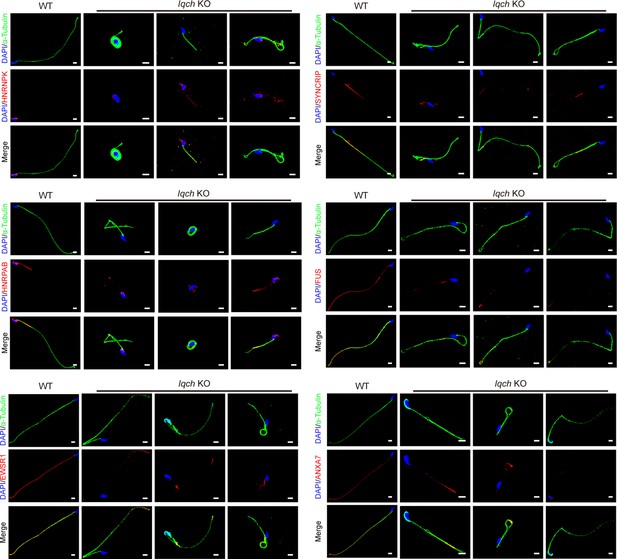
The reduced expression levels of the seven proteins were most relevant to the phenotype of the spermatozoa of the IQ motif-containing H (Iqch) knockout (KO) mice.
Immunofluorescence staining shows that the expression levels of the seven proteins most relevant to the phenotype of the Iqch KO mice, including HNRNPK, HNRPAB, EWSR1, SYNCRIP, FUS, and ANXA7, were reduced in the spermatozoa of the Iqch KO mice compared to those of the wild-type (WT) mice (n=3 biologically independent WT mice and KO mice; blue, DAPI; green, α-Tubulin; red, HNRNPK, HNRPAB, EWSR1, SYNCRIP, FUS, or ANXA7; scale bars, 5 μm).
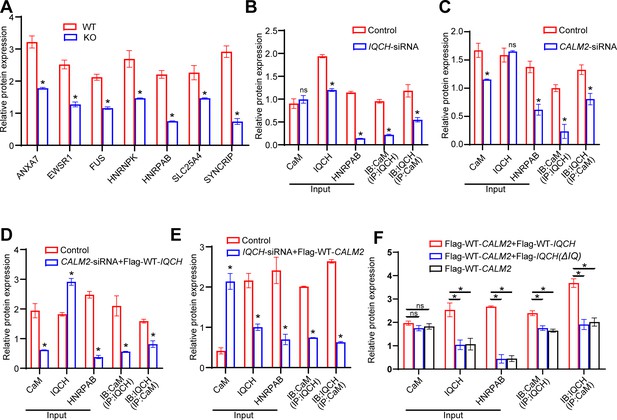
Statistics results about protein expression levels by western blotting analysis.
(A) Densitometry analysis was performed on corresponding Western blotting to assess the expression levels of seven proteins most relevant to the IQ motif-containing H (Iqch) knockout (KO) mouse phenotype in wild-type (WT) and Iqch KO mice (n=3 biologically independent WT mice and KO mice; Student’s t-test; *p<0.05; error bars, SEM). (B and C) Statistical analysis was performed on the protein expression levels of calmodulin (CaM), IQCH, and HNRPAB, as well as on the interaction between IQCH and CaM in K562 cells with IQCH (B) or CaM (C) knockdown (Student’s t-test; *p<0.05; error bars, SEM). siRNA, small interfering RNA. (D and E) Quantitative analysis was conducted on the protein expression levels of CaM, IQCH, and HNRPAB, as well as on the interaction between IQCH and CaM in K562 cells overexpressing IQCH with simultaneous knockdown of CaM (D) or overexpressing CaM with simultaneous knockdown of IQCH (E) (Student’s t-test; *p<0.05; error bars, SEM.). siRNA, small interfering RNA. (F) Statistical analysis was conducted on the protein expression levels of CaM, IQCH, and HNRPAB, as well as on the interaction between IQCH and CaM in HEK293 cells cotransfected with the WT-IQCH and WT-CALM2 plasmids, cotransfected with the IQCH (△IQ) and WT- CALM2 plasmids, or cotransfected with the control and WT- CALM2 plasmids (Student’s t-test; *p<0.05; error bars, SEM). Three independent experiments were performed.
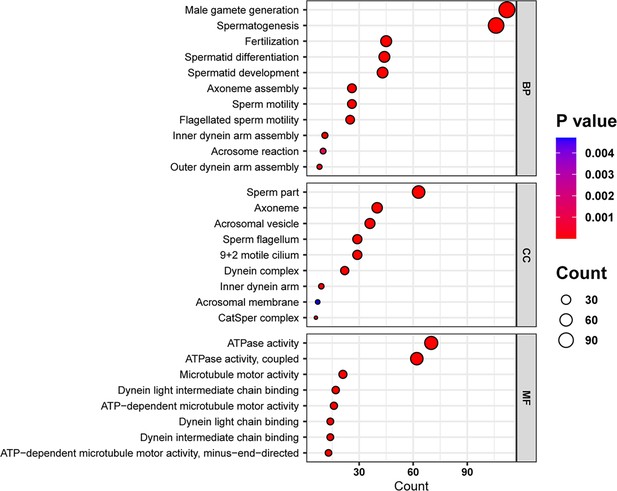
Gene Ontology (GO) analysis of the downregulated genes of the IQ motif-containing H (Iqch) knockout (KO) mice through RNA sequencing.
Bubble plots of the GO analysis revealed the effects of IQCH deficiency on male fertility in three categories: BP, biological process; CC, cellular component; and MF, molecular function.
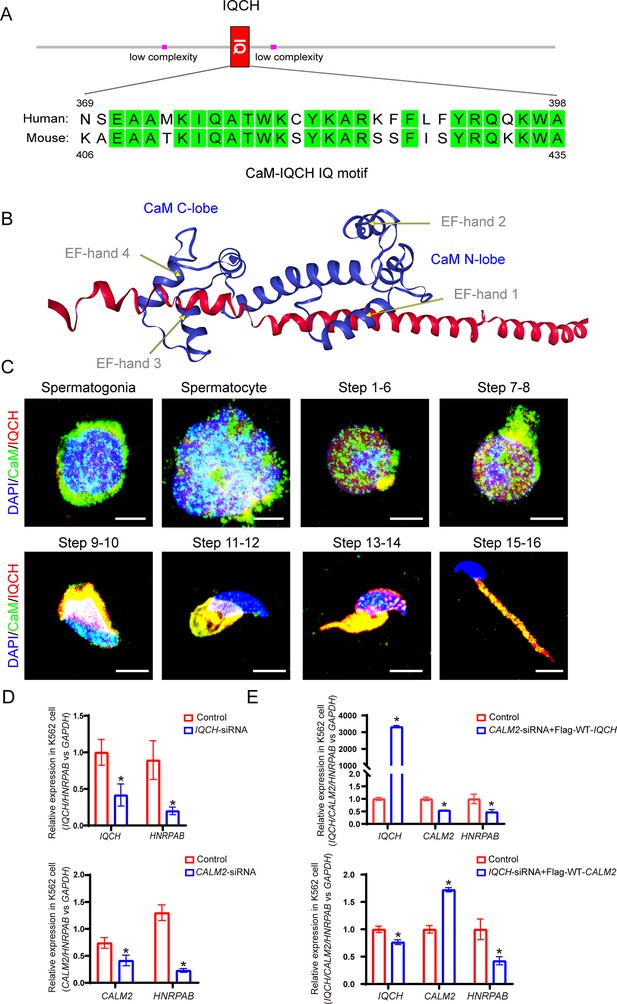
IQ motif-containing H (IQCH) interacted with calmodulin (CaM) to regulate spermatogenesis.
(A) A schematic representation of IQCH showing an IQ motif conserved between humans and mice. The domains were predicted via SMART (http://smart.embl-heidelberg.de/). (B) The protein-protein docking between IQCH and CaM was predicted by the AlphaFold Protein Structure Database (https://AlphaFold.ebi.ac.uk/) and LZerD website (https://lzerd.kiharalab.org/). (C) Immunofluorescence staining of germ cells at different developmental stages suggested the colocalization of CaM and IQCH in elongating spermatids in wild-type (WT) mice. As expected, IQCH was not detected in the testes of the Iqch KO mice, and only CaM was expressed in elongating spermatids in the Iqch KO mice (n=3 biologically independent WT mice and KO mice; scale bars, 50 μm). (D) qPCR analysis demonstrated reduced IQCH and CALM2 expression levels in K562 cells following transfection with IQCH-siRNA and CALM2-siRNA, respectively, compared to those in K562 cells transfected with the negative control siRNA. The downregulation of HNRPAB resulted from decreased IQCH or CALM2 expression (Student’s t-test; *p<0.05; error bars,SEM). (E) qPCR analysis of K562 cells overexpressing IQCH with simultaneous knockdown of CALM2 or overexpressing CALM2 with simultaneous knockdown of IQCH revealed significant downregulation of HNRPAB (Student’s t-test; *p<0.05; error bars, SEM). siRNA, small interfering RNA. Three independent experiments were performed.
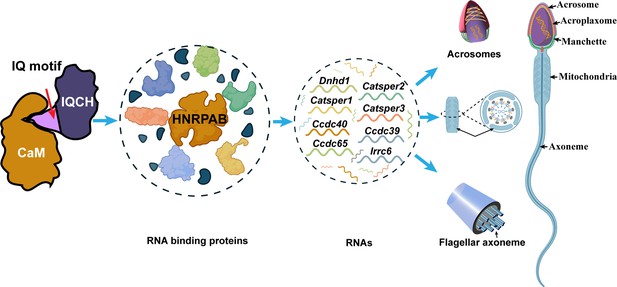
Proposed model for the mechanisms underlying the involvement of IQ motif-containing H (IQCH) in spermatogenesis.
IQCH interacts with calmodulin (CaM) via the IQ motif to regulate the expression of RNA-binding proteins. RNA binding proteins, particularly HNRPAB, bind and regulate several RNAs that influence the development of the acrosome, mitochondria, and axoneme, thereby playing a critical role in spermatogenesis.
Tables
Results of the semen analysis and sperm morphology examination of the patient.
| Parameter | Patient | Reference* |
|---|---|---|
| Sperm volume, ml | 2.8 | ≥1.5 |
| Sperm concentration, million/ml | 10.0 | ≥15 |
| Vitality, % | 30 | ≥58 |
| Motility, % | 4 | ≥32 |
| Abnormal morphology (%) | 99.5 | - |
-
*
The reference values were based on the guidelines provided in the WHO manual fifth edition (2010).
Semen analysis using computer-assisted sperm analysis (CASA) in the mouse model of IQ motif-containing H (Iqch) knockout (KO).
| Adult Male Mice | |||
|---|---|---|---|
| WT | KO | p† value | |
| Semen parameters | |||
| Sperm concentration (106 /ml) * | 94.51±10.68 | 67.75±3.70 | 0.038 |
| Motility (%) | 57.08±2.18 | 5.49±3.25 | <0.001 |
| Progressive motility (%) | 57.00±2.05 | 5.49±3.25 | <0.001 |
| Sperm locomotion parameters | |||
| Curvilinear velocity (VCL) (μm/s) | 78.29±6.23 | 9.94±4.03 | <0.001 |
| Straight-line velocity (VSL) (μm/s) | 36.18±2.09 | 2.90±1.98 | <0.001 |
| Average path velocity (VAP) (μm/s) | 45.10±0. 19 | 4.17±2.49 | <0.001 |
| Amplitude of lateral head displacement (ALH) (μm) | 0.77±0. 02 | 0.13±0.03 | <0.001 |
| Linearity (LIN) | 0.46±0.01 | 0.27±0.08 | 0.047 |
| Wobble (WOB,=VAP/VCL) | 0.58±0.04 | 0.40±0.08 | 0.033 |
| Straightness (STR,=VSL/VAP) | 0.80±0.05 | 0.67±0.07 | 0.069 |
| Beat-cross frequency (BCF) (Hz) | 4.33±0.19 | 0.65±0.26 | <0.001 |
-
n = 3 biologically independent wild-type (WT) mice or knockout (KO) mice.
-
*
Epididymides and vas deferens.
-
†
two-tailed Student’s t-test.





