EphrinB2 knockdown in cervical spinal cord preserves diaphragm innervation in a mutant SOD1 mouse model of ALS
Figures
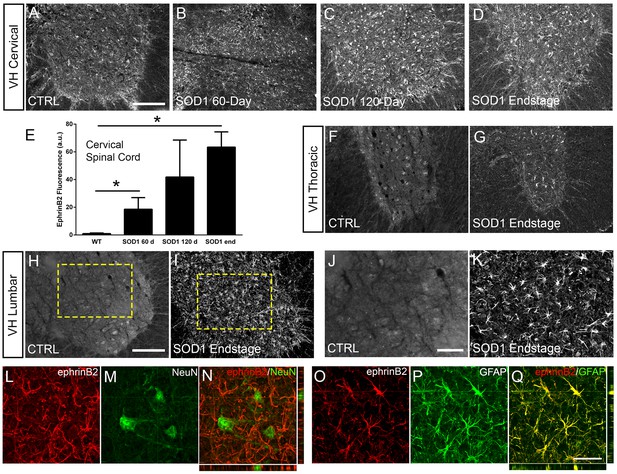
EphrinB2 expression was increased in ventral horn astrocytes.
SOD1G93A mouse ventral horn cervical spinal cord tissue immuinostained for ephrinB2 at 60 days (b), 120 days (c), endstage (d), and WT mouse age-matched control (a), scale bar: 200 µm. Quantification of ephrinB2 expression within the ventral horn shows a progressive increase in expression over time compared to WT controls (e). Endstage ephrinB2 expression in the thoracic (f, g) and lumbar (h–k) regions; scale bar: 200 µm, 100 µm, respectively. Endstage SOD1G93A mouse cervical spinal cord tissue co-immuostained for ephrinB2 (l, n, o, q) and neuronal and astrocyte lineage-specific markers NeuN (m–n) and GFAP (p–q), respectively; scale bar: 30 µm. Analysis in panels A-K: n=3–4 mice per genotype and per time point; 1–2 females and 2 males per condition. Analysis in panels L-O: n=3 mice per genotype and per time point; 1 female and 2 males per condition.
-
Figure 1—source data 1
File contains the raw data for Figure 1, panel E.
- https://cdn.elifesciences.org/articles/89298/elife-89298-fig1-data1-v1.xlsx
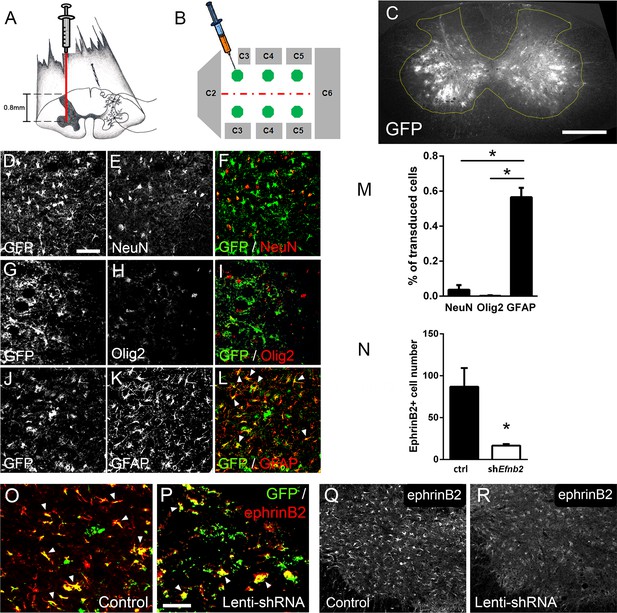
Lenti-shRNA injection reduced ephrinB2 expression in cervical spinal cord astrocytes.
We injected 60-day-old SOD1G93A mice with Lenti-GFP control or Lenti-shRNA-Efnb2 into cervical ventral horn (a). Injections were made bilaterally into six sites throughout C3-C5 to target the PhMN pool (b).Thirty µm transverse tissue sections of the cervical spinal cord show robust expression of the GFP reporter bilaterally within the ventral horn (c); scale bar: 500 µm. Cell lineage of viral transduction was assessed using three markers: neuronal marker NeuN, oligodendrocyte lineage marker Olig2, and astrocyte marker GFAP. Spinal cord tissue collected from SOD1G93A injected with Lenti-GFP vector was sectioned at 30 µm and immunostained for NeuN (d-f), Olig2 (g–i) and GFAP (j–l); scale bar: 150 µm. Quantification of transduction lineage was assessed by counting the total numbers of GFP+ cells that were co-labeled with each lineage-specific marker and expressing this as a percentage of the total number of GFP+ cells (m). We assessed the amount of knockdown achieved by the Lenti-shRNA-Efnb2 vector by immunostaining endstage SOD1G93A cervical spinal cord tissue with an anti-ephrinB2 antibody in both Lenti-GFP control (o, q) and Lenti-shRNA-Efnb2 (p, r) tissue; scale bar: 100 µm. Knockdown was quantified by counting the total number of GFP+ cells expressing ephrinB2+ within the cervical ventral horn (n). Analyses in all panels: n=3 mice per condition; 1 female and 2 males per condition.
-
Figure 2—source data 1
File contains the raw data for Figure 2, panels M and N.
- https://cdn.elifesciences.org/articles/89298/elife-89298-fig2-data1-v1.xlsx
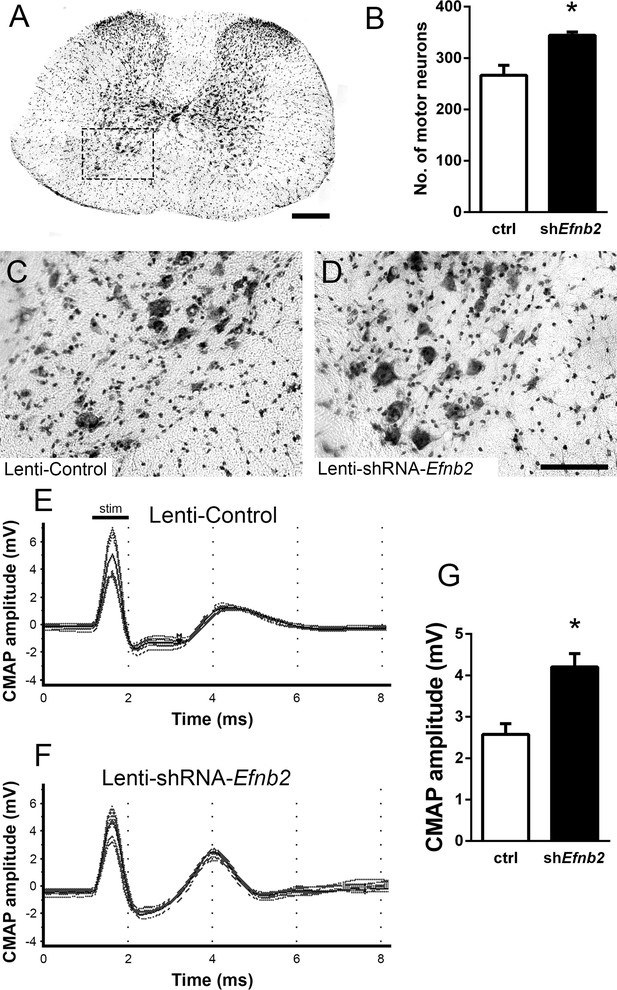
EphrinB2 knockdown protected cervical spinal cord motor neurons and preserved functional innervation of the diaphragm in SOD1G93A mice.
SOD1G93A mice injected with Lenti-GFP control or Lenti-shRNA-Efnb2 were cresyl violet stained and MN counts were performed at 117 days of age. Thirty µm transverse cervical spinal cord tissue sections were stained with cresyl violet (a); scale bar: 250 µm. The dotted box outlines the ventral horn and area of the image shown in (c). MN populations within the ventral horn were quantified (b). Representative images show a greater loss of MNs in Lenti-Control (c) compared to the Lenti-shRNA-Efnb2 (d) group; scale bar: 100 µm. SOD1G93A mice injected with Lenti-GFP control or Lenti-shRNA-Efnb2 were assessed in vivo for PhMN-diaphragm innervation by electrophysiological analysis at 117 days of age. CMAP amplitudes were recorded from each hemi-diaphragm following ipsilateral phrenic nerve stimulation. Representative traces of Lenti-GFP (e) and Lenti-shRNA-Efnb2 (f) recordings show a larger CMAP amplitude in the Lenti-shRNA-Efnb2 group. Quantification of maximal CMAP amplitude shows significant preservation in the Lenti-shRNA-Efnb2-treated group compared to control (g). Analysis in panels A-D: n=4 mice per condition; 2 females and 2 males per condition. Analysis in panels E-G: n=4 mice per genotype and per time point; 2 females and 2 males per condition.
-
Figure 3—source data 1
File contains the raw data for Figure 3, panels B and G.
- https://cdn.elifesciences.org/articles/89298/elife-89298-fig3-data1-v1.xlsx
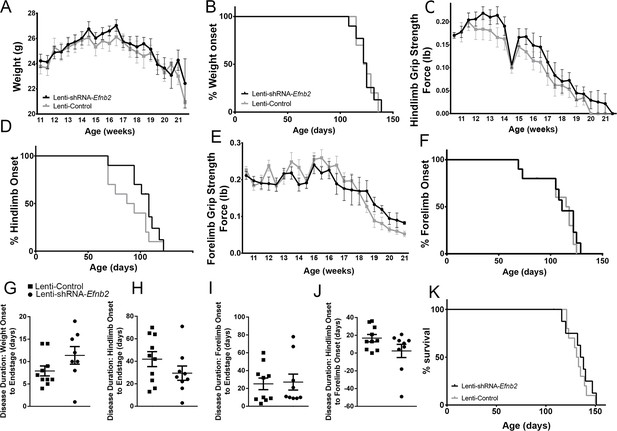
Knockdown of ephrinB2 in the cervical spinal cord ventral horn did not extend survival or delay onset of disease in SOD1G93A mice.
Biweekly weights of Lenti-shRNA-Efnb2 and Lenti-Control mice were recorded until endpoint sacrifice (a), and disease onset was measured for each animal when there was a 10% drop in body weight (b). Biweekly individual hindlimb grip strengths were assessed for each animal (c), and disease onset was recorded when the animal had a 10% decline in hindlimb grip strength (d). Each animal was also tested for forelimb grip strength (e), and disease onset was recorded when the animal had a 10% decline in forelimb grip strength (f). Weights, forelimb grip strength and hindlimb grip strength were taken biweekly starting one week prior to injection of Lenti-shRNA-Efnb2 or Lenti-GFP control, and all force measurements plotted were the average force (lb) of all animals combined in each group. Disease duration was determined by time from weight onset to endstage (g), hindlimb disease onset to endstage (h), and forelimb diseaseonset to endstage for each animal (i). Disease duration was also measured from the time of forelimb disease onset to time of hindlimb disease onset (j). Survival was measured as the day each animal reached endstage, which was determined by the righting reflex (k). Analyses in all panels: n=8–10 mice per genotype and per time point; 4–5 females and 4–5 males per condition.
-
Figure 4—source data 1
File contains the raw data for Figure 4, panels A-K.
- https://cdn.elifesciences.org/articles/89298/elife-89298-fig4-data1-v1.xlsx
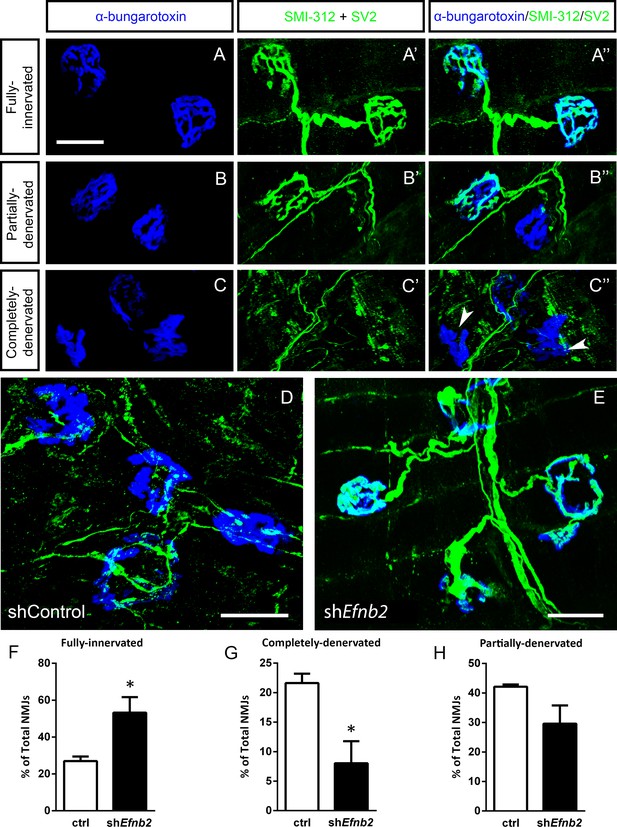
EphrinB2 knockdown preserved morphological innervation of the diaphragm in SOD1G93A mice.
Diaphragm muscles were labeled with SMI-312 (green), SV2, (green) and alpha-Bungarotoxin (blue). Representative images of fully-innervated (a), partially-denervated (b) and completely-denervated ((c): arrowheads denote completely-denervated NMJs) NMJs are shown; all scale bars: 30 µm. Compared to SOD1G93A mice treated with Lenti-GFP (d), animals injected with Lenti-shRNA-Efnb2 (e) showed greater preservation of PhMN innervation of the diaphragm NMJ. Quantification revealed a significant increase in the percentage of fully-innervated NMJs (f) and a decrease in the percentage of completely-denervated NMJs (g) in the Lenti-shRNA-Efnb2 group compared to Lenti-GFP controls. The percentage of partially-denervated NMJs was not statistically different between the two groups (h). Analyses in all panels: n=4 mice per condition; 2 females and 2 males per condition.
-
Figure 5—source data 1
File contains the raw data for Figure 5, panels F-H.
- https://cdn.elifesciences.org/articles/89298/elife-89298-fig5-data1-v1.xlsx
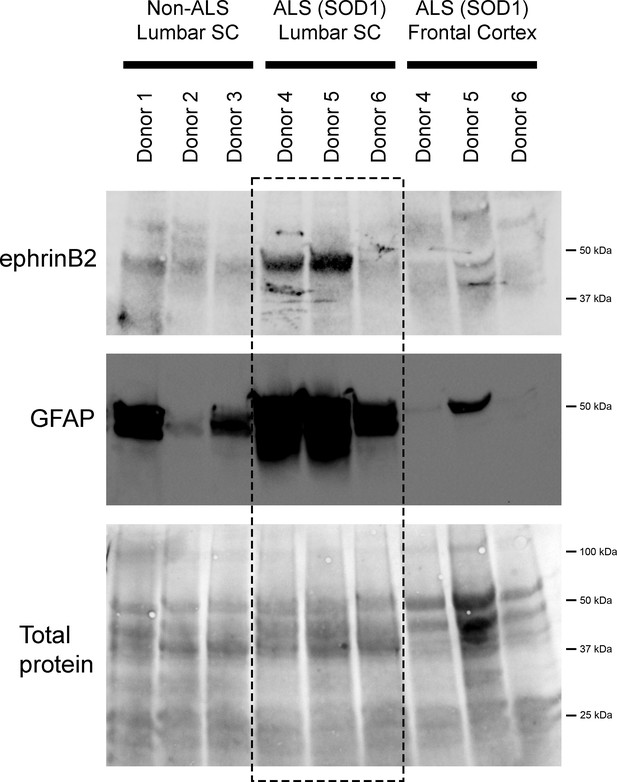
EphrinB2 upregulation in human spinal cord from ALS donors with an SOD1 mutation.
Immunoblotting analysis on postmortem samples from human ALS donors with an SOD1 mutation and also non-diseased human samples. In the lumbar enlargement, there was a large increase in ephrinB2 protein expression in the SOD1 mutation ALS samples compared to the non-diseased controls (top blot). GFAP immunoblotting on the same lumbar spinal cord samples shows a robust increase in GFAP protein levels in the two samples with increased ephrinB2 expresison (middle blot). There was not increased ephrinB2 expression in a disease unaffected region in these same three ALS donor samples, as ephrinB2 protein levels were not elevated in the frontal cortex (top blot). Immunoblot for total protein (bottom blot). Demographic information: Donor 1 – death at 67 years; male; non-ALS; Donor 2 – death at 70 years; male; non-ALS; Donor 3 – death at 70 years; female; non-ALS; Donor 4 – death at 42 years; female; SOD1-D102H mutation; absence of C9orf72 repeat expansion; Donor 5 – death at 55 years; male; SOD1-A4V mutation; absence of C9orf72 repeat expansion; Donor 6 – death at 58 years; male; SOD1-V87A mutation; absence of C9orf72 repeat expansion.
-
Figure 6—source data 1
File contains the raw data for Figure 6.
- https://cdn.elifesciences.org/articles/89298/elife-89298-fig6-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Mus musculus) | Transgenic SOD1G93A mice: C57BL/6 J congenic line B6.Cg-Tg(SOD1*G93A)1Gur/J | The Jackson Laboratory | RRID: IMSR_JAX:004435 | Both female and male mice used |
| Genetic reagent (Mus musculus) | Transgenic SOD1G93A mice: B6SJL-Tg(SOD1*G93A)1Gur/J | The Jackson Laboratory | RRID:IMSR_JAX:002726 | Both female and male mice used |
| Biological sample (Homo sapiens) | Spinal cord and cortex samples from ALS donors | Biorepository of the Jefferson Weinberg ALS Center (1 of 3 ALS samples) Project ALS (2 of 3 ALS samples) | All three ALS donors had an SOD1 mutation (donor 1: D102H mutation; donor 2: A4V; donor 2: V87A), and all three donors did not have a C9orf72 repeat expansion. These three donors succumbed to ALS at 42 (female), 55 (male), or 58 (male) years of age. | |
| Biological sample (Homo sapiens) | Spinal cord and cortex from non-ALS donors | NIH NeuroBioBank | Age of death for these three non-ALS donors was 67, 70, and 70 years. | |
| Antibody | Mouse monoclonal Anti-SV2 (Used for IHC) | Developmental Studies Hybridoma Bank, Iowa City, IA | RRID: AB_2315387 | 1:10 |
| Antibody | Mouse monoclonal Anti-SMI312 (Used for IHC) | Covance, Greenfield, IN | RRID: AB_2314906 | 1:1000 |
| Antibody | Mouse monoclonal Anti-NeuN (Used for IHC) | EMD-Millipore, Temecula, CA | RRID: AB_2298772 | 1:200 |
| Antibody | Rabbit polyclonal Anti-GFAP (Used for IHC) | Dako, Carpinteria, CA | RRID: AB_10013482 | 1:400 |
| Antibody | Mouse monoclonal Anti-GFAP (Used for Westerns) | BD Bioscience, Franklin Lakes, NJ | Cat. #610566 | 1:2000 |
| Antibody | Rabbit polyclonal Anti-Olig2 (Used for IHC) | EMD-Millipore, Temecula, CA | RRID: AB_2299035 | 1:200 |
| Antibody | Goat polyclonal Anti-ephrinB2 (Used for IHC) | R&D Systems, Minneapolis, MN | RRID: AB_2261967 | 1:50 |
| Antibody | Rabbit polyclonal Anti-ephrinB2 antibody (Used for Westerns) | Abcam, Cambridge, MA | RRID: AB_11156896 | 1:500 |
| Antibody | Goat polyclonal Anti-EphA4 (Used for IHC) | R&D Systems, Minneapolis, MN | RRID: AB_2099371 | 1:100 |
| Antibody | Rabbit polyclonal Anti-GFP (Used for IHC) | Aves Labs, Davis, CA | RRID: AB_10000240 | 1:500 |
| Recombinant DNA reagent | Lenti-shRNA-Efnb2; VSVG.HIV.SIN.cPPT.U6. SbRmEphrinB2.4.CMV.EGFP | This study | Vector generated by the Dalva lab | |
| Recombinant DNA reagent | Lenti-Control; VSVG.HIV.SIN.cPPT. U6.Empty.CMV.EGFP | This study | Vector generated by the Dalva lab | |
| Software | Scope 3.5.6 software | ADInstruments, Colorado Springs, CO | RRID: SCR_001620 | |
| Software | ImageJ/Fiji software | RRID: SCR_003070 | ||
| Software | Bio-Rad Image Lab software | Bio-Rad, Hercules, CA | RRID:SCR_014210 | |
| Software | Graphpad Prism 6 | Graphpad Software Inc, LaJolla, CA | RRID: SCR_002798 | |
| Other (Microscope) | Olympus FV1000 confocal microscope | Olympus, Center Valley, PA | RRID: SCR_014215 | ‘NMJ analysis’ subsection of the Materials and methods section |
| Other (Microscope) | Zeiss Axio M2 Imager confocal microscope | Zeiss, Hebron, KY | RRID:SCR_020922 | ‘Motor neuron counts’ subsection of the Materials and methods section |





