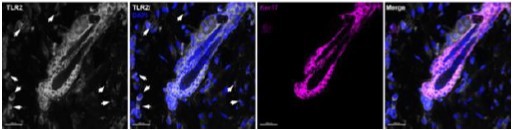
TLR2 regulates hair follicle cycle and regeneration via BMP signaling
Figures
Hair follicle stem cells (HFSCs) downregulate TLR2 in response to stress like a high-fat diet and aging.
(A) Dysregulated pathways in old vs young mouse HFSCs. The top pathways are labeled in bold. (B) Representative confocal images of telogen hair follicles from young and old mice immunostained for TLR2 and CD34 demonstrate decreased TLR2 intensity in HFSC (CD34-positive) of old mice. Scale bars are 50 μm. The middle and right panels show a magnified view of the boxed area. Scale bars are 20 μm. (C) Quantification of TLR2 fluorescent intensity in images from B showing significantly lower TLR2 expression in HFSCs from the old mice. N=6 for each group. (D) GEO2R analysis of published RNA data from sorted follicle populations in the second telogen to anagen transition demonstrates the increased level of Tlr2 mRNA accompanied by the activation of Toll-like receptors (TLRs) signaling downstream. (E) Representative confocal images showing TLR2 expression in hair follicles from mice fed with a normal diet (ND) or high-fat diet (HFD). CD34 is an HFSC marker. Scale bars are 50 μm. Magnified images demonstrate decreased TLR2 intensity in HFSC (CD34-positive) of mice after HFD. Scale bars are 20 μm. (F) Quantification of TLR2 fluorescent intensity in images from E showing significantly lower TLR2 expression in HFSCs from HFD-fed mice. N=7 and 6 for ND and HFD groups, respectively. AU, arbitrary unit. (G) Tlr2 mRNA expression in HFSCs from mice fed with ND or HFD for 4 days or 3 months. Data regenerated from published RNA sequencing dataset GSE131958. N=3 for each group. All bar graphs are mean ± s.e.m. Non-parametric Mann-Whitney test (C, G) or unpaired two-tailed t-test (F) was used to determine statistical difference. A p-value ≤ 0.05 was considered to be statistically significant.

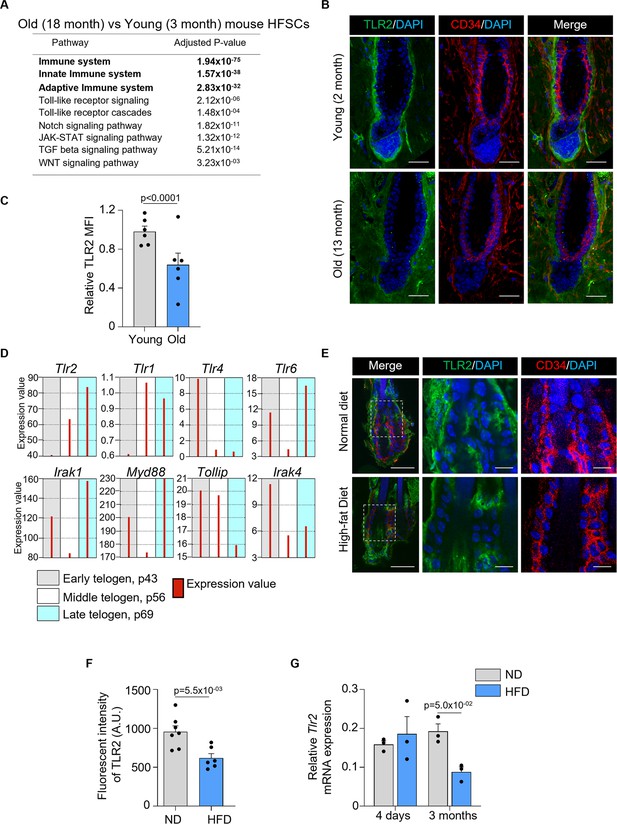
TLR2 is enriched in hair follicle stem cells (HFSCs) and is upregulated during HFSC activation.
TLR2-GFP reporter mouse skin sections were immunostained with anti-GFP to assess TLR2 expression in the hair follicles. (A) Representative confocal images of P21 first telogen hair follicle immunostained for TLR2-GFP, CD34 (bulge stem cells), P-cad (secondary hair germ [sHG]), and DAPI (nuclei). The green color in the surface rendering panel represents TLR2 expression, and other surfaces show co-localization between TLR2 and specific markers. TLR2 is present in bulge, sHG, and dermal papilla (DP) cells. P represents postnatal days. Scale bar is 10 μm. (B) TLR2-GFP in P28 anagen was co-immunostained with CD49f of basement membrane outlining the DP. Scale bar is 10 μm. (C) TLR2 is co-localized to the sHG lineage (P-cad+ layers), DP, and outer root sheath (ORS) lineage. Scale bar is 20 μm. (D) TLR2-GFP in P28 anagen was co-immunostained with CD34 in old bulge (D) and Ker5 in ORS (E) revealing TLR2 localization to the old bulge, ORS, but not inner root sheath (IRS). Scale bars are 20 μm. (F) Co-immunostaining of TLR2-GFP in P38 catagen hair follicle with Ker5 in ORS lineage cells showing co-localization of TLR2 with ORS and bulge. Scale bar is 20 μm. (G) P41 late catagen hair follicle immunostained for TLR2 and CD34 showing co-localization of TLR2 to the old bulge, new bulge, sHG, and DP. Scale bar is 20 μm. (H) P53 second telogen hair follicle immunostained for TLR2, CD34, and P-cad reveals co-localization of TLR2 to the bulge, sHG, and DP. Scale bar is 20 μm. (I) Quantification of TLR2 fluorescent intensity in bulge cells at different phases showing TLR2 upregulation in anagen. N=3 for each group. (J) Quantitative polymerase chain reaction (qPCR) analysis of Tlr2 mRNA expression in FACS-purified mouse HFSCs in anagen, telogen, and catagen. N=3 or 4 per group. (K) qPCR analysis of Tlr2 mRNA expression in mouse epidermal cells and FACS-purified HFSCs showed significantly higher Tlr2 expression in HFSCs compared with raw epidermal cells. N=6 mice per group. All bar graphs are mean ± s.e.m. Two-tailed unpaired t-test (K) or Kruskal-Wallis test with Dunn’s post hoc test (I, J) was used to determine statistical difference. A p-value ≤ 0.05 was considered to be statistically significant.
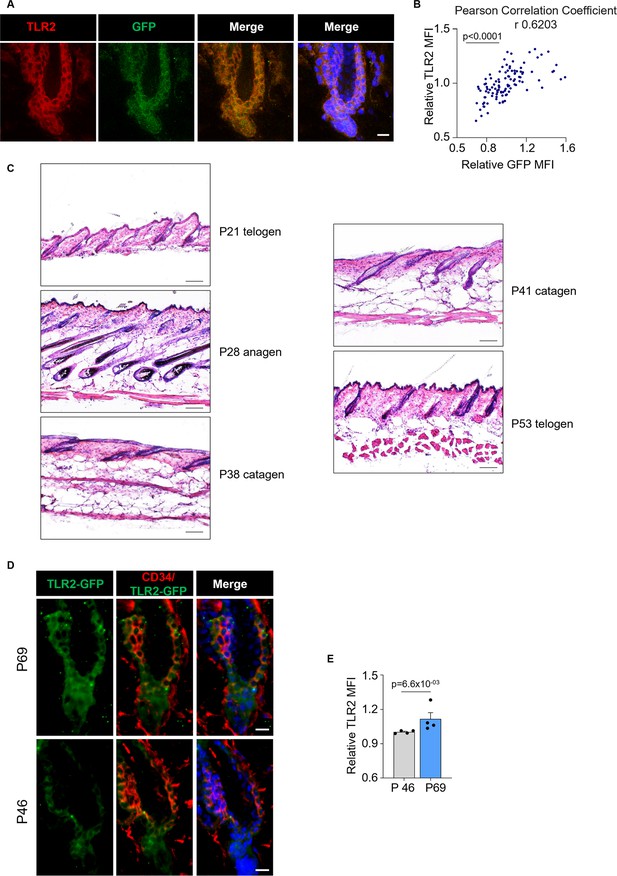
TLR2/GFP correlation in immunostaining.
H&E staining of dorsal skin of wild-type (WT) mice. TLR2 dynamic during the second telogen. (A) Confocal images of dorsal skin hair follicles co-immunostained for TLR2 and GFP. Scale bars are 10 µm. (B) A scatter graph shows a high level of correlation between TLR2 and GFP intensity. N=3. (C) Representative H&E staining of the dorsal skin of WT mice at indicated time points demonstrates the typical changes in hair follicle morphology. Scale bars are 100 µm. (D) Confocal images of dorsal skin hair follicles from p46 and p69 mice immunostained for TLR2. Scale bars are 10 µm. (E) Bar graph showing an elevated level of TLR2 in hair follicle stem cell (HFSC) of p69 mice (late second telogen) compared to p46 (early second telogen). N=4. Correlation analysis and the Mann-Whitney test were used to determine the statistical significance. All data are mean ± s.e.m. A p-value ≤ 0.05 was considered to be statistically significant.
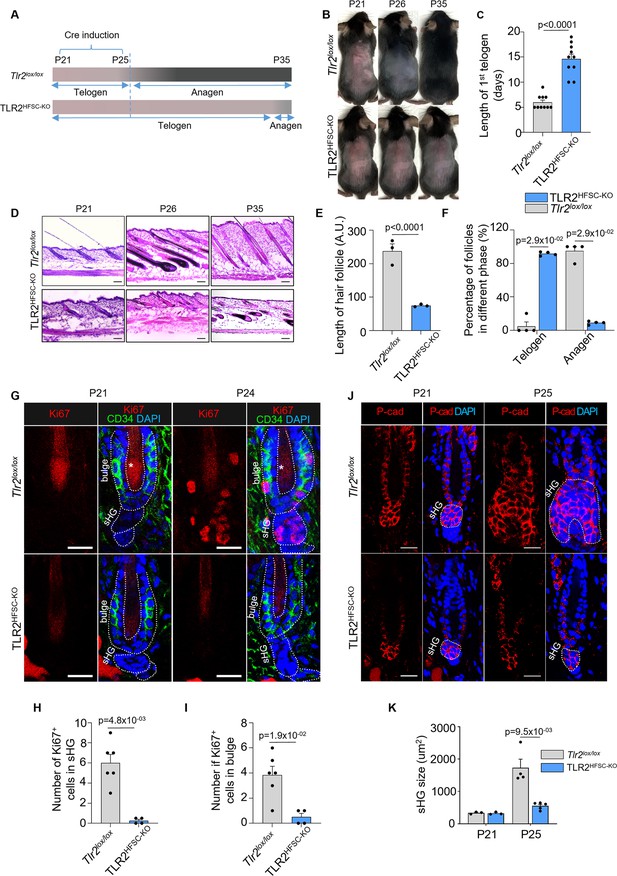
Deletion of TLR2 in hair follicle stem cells delays anagen onset.
(A) Schematic of RU486-mediated Cre induction and dorsal skin pigmentation change (gradient bars) in Tlr2lox/lox and TLR2HFSC-KO mice. (B) Representative images of shaved Tlr2lox/lox and TLR2HFSC-KO mice showing different phases of the hair cycle. The Tlr2lox/lox mouse transitions from telogen (pink skin) to anagen (gray/black skin) at P26 and a full hair coat is developed by P35. The TLR2HFSC-KO mouse exhibits a prolonged telogen (P21–P30–P35). Representative images of at least 10 mice in each group. (C) Bar graph showing the length of first postnatal telogen starting from P21 measured by skin color change from B. N=10 per group. (D) Representative H&E staining of dorsal skin at indicated time points showing prolonged telogen in TLR2HFSC-KO mice. Scale bars are 50 μm. (E) The length of hair follicles at P26 from images in D. 50 hair follicles from three mice per group were used for quantification. (F) Percentages of telogen or anagen hair follicles at P26 from D. N=4 mice per group. (G) Representative confocal images of P21 and P24 first telogen hair follicles from Tlr2lox/lox and TLR2HFSC-KO mice immunostained for CD34, Ki67, and DAPI. Stars label the hair shaft. Scale bars are 20 μm. (H, I) Quantification of images in G shows a diminished number of Ki67+ cells in secondary hair germ (sHG) (H) and in CD34+ bulge (I) in TLR2HFSC-KO mice compared to Tlr2lox/lox at P24. N=6 and 4 mice for Tlr2lox/lox and TLR2HFSC-KO group, respectively. (J) Representative confocal images of P21 and P25 dorsal skin sections from Tlr2lox/lox and TLR2HFSC-KO mice immunostained for P-cad and DAPI showing changes in the size of sHG. Scale bars are 20 μm. (K) Quantification of sHG size in panel K shows enlarged sHG in Tlr2lox/lox mice compared with TLR2HFSC-KO mice. N=4 mice for P25 Tlr2lox/lox, and N=5 mice for TLR2HFSC-KO. Statistical significance was determined using a non-parametric Mann-Whitney test. All data are mean ± s.e.m. A p-value ≤ 0.05 was considered to be statistically significant.
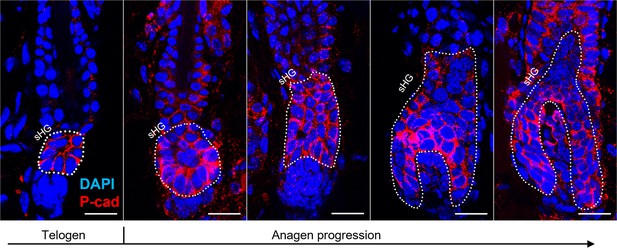
Confocal images of hair follicles immunostained for P-cad secondary hair germ (sHG) enlargement and elongation at anagen onset.
Representative confocal images of hair follicles immunostained for P-cad showing changes in the size of sHG during the progression of the hair cycle. At anagen onset, sHG cells proliferate and grow downward to envelop the dermal papilla. The proliferation of sHG cells results in a larger sHG compared to telogen. Dashed lines outline the sHG. Scale bars are 20 μm.
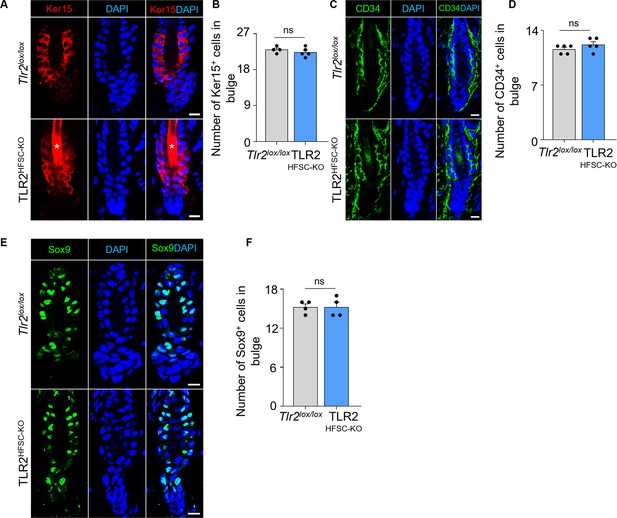
Bulge stem cell marker expression in TLR2HFSC-KO mouse.
(A) Representative confocal images of telogen hair follicles from Tlr2lox/lox and TLR2HFSC-KO mice immunostained for Ker15. Stars label the hair shaft. Scale bars are 10 μm. (B) Bar graph showing no difference in numbers of Ker15+ cells in bulge area in Tlr2lox/lox compared with TLR2HFSC-KO telogen hair follicles from images in A. N=4 and 5 mice for Tlr2lox/lox and TLR2HFSC-KO respectively. (C) Telogen hair follicles from Tlr2lox/lox and TLR2HFSC-KO mice immunostained for CD34. Scale bars are 10 μm. (D) Quantification of CD34+ cell numbers in bulge area in images from C showing no difference in Tlr2lox/lox compared with TLR2HFSC-KO follicles. N=5 per group. (E) Representative confocal images of Tlr2lox/lox and TLR2HFSC-KO telogen hair follicles immunostained for Sox9. Scale bars are 10 μm. (F) Bar graph showing no difference in Sox9+ cell numbers in bulge area in Tlr2lox/lox and TLR2HFSC-KO follicles from images in E. N=4. Mann-Whitney test was used to determine the statistical significance. All data are mean ± s.e.m. A p-value ≤ 0.05 was considered to be statistically significant.

TLR2 interacts with BMP pathway to regulate the hair cycle.
(A) Representative confocal images of BMP7 staining in hair follicles of dorsal skin in early (P46) and late (P69) second telogen. Scale bars are 10 μm. (B) Quantification of BMP7 fluorescent intensity from A showing diminished BMP7 expression during the second telogen from the early to late phases. N=4 per group. (C) Representative confocal images of pSMAD1/5/9 staining in hair follicles of dorsal skin in early (P46) and late (P69) second telogen. Scale bars are 10 μm. (D) Quantification of pSMAD1/5/9+ positive cells in CD34+ bulge stem cells demonstrates a decrease of pSmad1/5/9 expression in late telogen. N=4 per group. (E) Quantitative polymerase chain reaction (qPCR) analysis reveals dysregulation of BMP singling molecules in hair follicle stem cells (HFSCs) lacking Tlr2. N=4 mice for control and Bmp2, N=3 mice for Bmp7 and Bmpr1a. (F) Representative confocal images of BMP7 staining in hair follicles from Tlr2lox/lox or TLR2HFSC-KO mice. Scale bars are 10 μm. Stars label hair shaft. (G) Quantification of BMP7 fluorescent intensity from F showing higher BMP7 expression in TLR2HFSC-KO mice. N=4 per group. (H) P21 and P24 dorsal skin sections from Tlr2lox/lox and TLR2HFSC-KO mice immunostained for CD34, pSmad1/5/9, and DAPI. Scale bars are 10 μm. (I) Quantification of pSmad1/5/9+ cells in CD34+ bulge stem cells in P24 dorsal skin from H. N=4 and 5 for Tlr2lox/lox and TLR2HFSC-KO respectively. (J) Representative confocal images of dorsal skin sections from TLR2HFSC-KO mice treated with BSA or noggin immunostained for CD34, pSmad1/5/9, and DAPI. Star labels the hair shaft. Scale bars are 10 μm. (K) Quantification of pSmad1/5/9+ cells in CD34+ bulge stem cells from images in J. N=5 per group. (L) Immunostaining for Ki67 and DAPI in dorsal skin sections from TLR2HFSC-KO mice treated with BSA or noggin. Scale bars are 10 μm. (M) Quantification of images in L showing an increase in Ki67+ cells in secondary hair germ (sHG) of noggin-treated compared to BSA-treated TLR2HFSC-KO dorsal skin. N=5 per group. (N) Representative confocal images of Ki67 and DAPI immunostaining of dorsal skin sections from TLR2HFSC-KO mice treated with BSA or noggin. Arrows point to hair follicles with Ki67+ cells in the sHG. Scale bars are 20 μm. (O) Quantification of images in N showing percentages of hair follicles with Ki67+ cells in sHG. N=5 per group. (P) BSA- or noggin-treated TLR2HFSC-KO mouse dorsal skin immunostained for P-cad and DAPI. The dashed line outlines the sHG. Scale bars are 10 μm. (Q) Bar graph showing significantly larger sHG in noggin-treated TLR2HFSC-KO mice. N=5 per group. Mann-Whitney test was used to determine the statistical significance. All data are mean ± s.e.m. A p-value ≤ 0.05 was considered to be statistically significant.
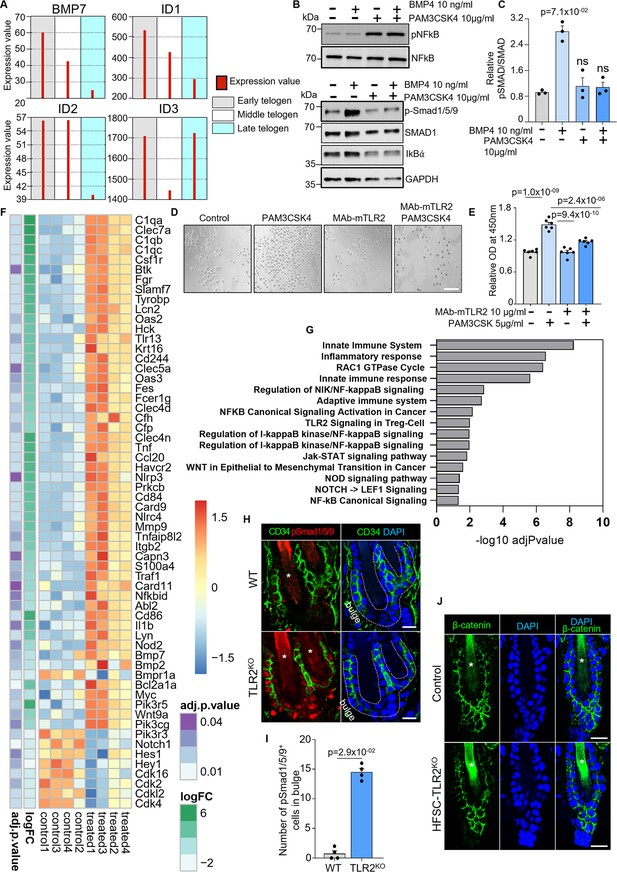
TLR2-BMP axis in hair follicle cells.
BMP signaling in the hair bulge of wild-type (WT) and TLR2HFSC-KO mice. (A) GEO2R analysis of published RNA data from sorted follicle populations in the second telogen to anagen transition demonstrates the declined expression of Bmp7 mRNA and key transcriptional target of the BMP signaling pathway in the skin, Id1, Id2, and Id3 mRNA. (B) Western blot analysis of pNFkB, NFkB, pSMAD1/5/9, SMAD1, ikBα protein level in human epidermal keratinocytes treated with/without 10 µg/ml Pam3SCK4 and 10 ng/ml BMP4. (C) Bar graphs show the activation effect of BMP4 treatment on the BMP signaling which was abolished in the presence of TLR2 ligand Pam3SCK4. N=3 independent experiments. (D) Representative microphotographs of human hair follicle stem cells (HFSCs) pre-treated with 10 µg/ml MAb-mTLR2 or DMSO and co-cultured with/without 5 µg/ml Pam3SCK4. Representative images from at least three independent assays are shown. Scale bar 50 µm. (E) Bar graphs show increased proliferation of HFSC in the presence of TLR2 ligand Pam3SCK4 with no effect in MAb-mTLR2 pre-treated cells compared to control. N=6 independent experiments. (F) Dysregulated genes were confirmed by RNA sequencing or quantitative polymerase chain reaction (qPCR) of FACS-sorted HFSCs from the first telogen dorsal skin of Tlr2lox/lox and TLR2HFSC-KO mice. HFSCs from four mice in each group were sorted and sequenced. Dysregulated genes in RNA sequencing were those with an adjusted p-value <0.05. (G) Dysregulated pathways in HFSCs lacking TLR2. (H) Representative confocal images of competent telogen follicles from WT or TLR2KO mice immunostained for CD34 and pSmad1/5/9. Stars label hair shaft. Scale bars are 10 μm. (I) Quantification of pSmad1/5/9+ bulge stem cell number in images from H shows significantly more pSmad1/5/9+ cells in WT hair follicle bulge region compared with TLR2KO hair follicles. N=4 for each group. (J) Representative confocal images of Tlr2lox/lox and TLR2HFSC-KO telogen hair follicles immunostained for β-catenin showed no difference between the two groups. Stars label the hair shaft. Scale bars are 20 μm. All bar graphs are mean ± s.e.m. Non-parametric Mann-Whitney test (I), or Kruskal-Wallis test with Dunn’s multiple comparisons test (C), or one-way ANOVA with Tukey’s multiple comparisons test (E) was used to determine statistical differences. A p-value ≤ 0.05 was considered to be statistically significant. For the western blot (WB) quantification with experiments with n=3, the minimum achievable p-value for the non-parametric tests is 0.1000.
-
Figure 4—figure supplement 1—source data 1
Uncropped WB gels.
- https://cdn.elifesciences.org/articles/89335/elife-89335-fig4-figsupp1-data1-v1.pptx
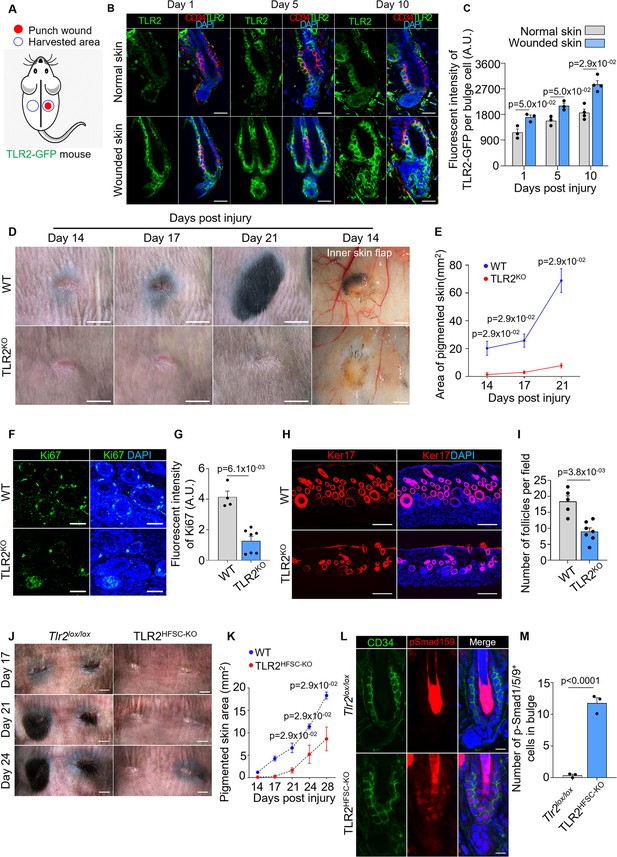
Hair follicle stem cell (HFSC) TLR2 is crucial for wound-induced hair follicle regeneration.
(A) Schematic of wound healing assay using TLR2-GFP reporter mouse. Full-thickness wounds on the dorsal skin of TLR2-GFP mice were created. Normal unwounded skin and the skin adjacent to the wound were harvested at different time points. (B) Representative confocal images of normal and wounded skin from TLR2-GFP mice at different time points post-injury immunostained for TLR2-GFP and CD34. Scale bars are 20 μm. (C) Quantification of TLR2 fluorescent intensity per bulge cell in hair follicles from B shows increased TLR2 level in hair follicles from wounded skin as compared to normal skin. N=3 for day 1 and day 5, and N=4 for day 10 per group. (D) Representative photographs showing hair regeneration on the dorsal skin and inner skin flaps at indicated time post-injury in wild-type (WT) and TLR2 global knockout (TLR2KO) mice. Diminished hair growth around the wound is apparent in TLR2KO skin from day 14 through 21 post-injury compared to WT skin. The inner skin flaps from TLR2KO at day 14 post-injury show an absence of pigmented hair bulbs and skin pigmentation. Scale bars are 5 mm for dorsal skin and 1 mm for inner skin flaps. (E) Quantification of the pigmented dorsal skin area around the wound from images in D shows diminished pigmentation in TLR2KO skin compared with WT skin at all time points post-injury. N=4 per group. (F) Representative confocal images of skin adjacent to wound immunostained for Ki67 and DAPI. Scale bars are 50 μm. (G) Bar graph showing diminished Ki67 fluorescent intensity in the skin adjacent to wound in TLR2KO mouse compared to WT mouse from images in F. N=4 and 7 for WT and TLR2KO respectively. (H) Representative confocal images of skin adjacent to wound immunostained for Ker17 and DAPI. Scale bars are 100 μm. (I) Quantification of hair follicle numbers from images in H reveals a significant decrease in regenerated hair follicles in TLR2KO skin compared with WT skin. N=5 and 7 for WT and TLR2KO respectively. (J) Representative photographs showing a lack of hair regeneration and skin pigmentation around the wound on the dorsal skin of TLR2HFSC-KO mice compared with Tlr2lox/lox mice on day 17, day 21, and day 24 post-injury. Scale bars are 2 mm. (K) Quantification of pigmented skin area around the wound during 14–28 days post-injury showing significantly smaller pigmented skin area in TLR2HFSC-KO mice compared with Tlr2lox/lox mice. N=4 per group. (L) Representative confocal images of wounded skin from Tlr2lox/lox and TLR2HFSC-KO mice stained for CD34 and pSmad1/5/9. Scale bars are 10 μm. (M) Quantification of images from L showing more pSmad1/5/9+ cells in TLR2HFSC-KO wounded skin. N=3 per group. Mann-Whitney test was used to determine the statistical significance. All data are mean ± s.e.m. A p-value ≤ 0.05 was considered to be statistically significant.

Oxidation-dependent TLR2 ligand carboxyethylpyrrole (CEP) is present in hair follicles and promotes hair regeneration via hair follicle stem cell (HFSC) TLR2.
(A) Representative images of H&E and CEP immunostaining of consecutive skin sections from wild-type (WT) anagen mouse. Scale bars are 1 mm. (B) Representative confocal images of P5 WT whole-mount skin immunostained for CEP and Ker17. The merged image shows the co-localization of CEP to anagen hair follicles (Ker17+). Scale bar is 200 μm. (C) Longitudinal and cross-sections of anagen and telogen hair follicles from WT mice immunostained for CEP and Ker17. The lower left panel shows a magnified view of the boxed area. Scale bars are 100 μm for anagen, 50 μm for telogen. (D) Quantification of CEP fluorescent intensity at a different distance from the root of anagen hair follicles in longitudinal and cross-sections immunostaining images in images from C. A gradual decrease in CEP levels is observed from the proximal to the distal part of anagen hair follicles. N=50 follicles from 3 mice per group. (E) Line chart showing a sharp decrease of CEP fluorescent intensity with the distance from HF in telogen (from the lower right panel in C). N=10 follicles from 3 mice per group. (F) Representative confocal images of telogen hair follicles from young and old mice immunostained for CEP and Ker17. Scale bars are 20 μm. (G) Quantification of CEP fluorescent intensity from images in F. N=6 mice per group. (H) Representative photographs of dorsal skin (two left panels) and inner skin flaps (two right panels) from WT and TLR2KO mice after irradiation and bone marrow transplantation of WT bone marrow demonstrate an increased number of pigmented hair bulbs and skin pigmentation around wounds in CEP-treated wounds compared to control in WT mice with no differences in TLR2KO transplanted with WT bone marrow. Scale bars are 1 mm for the dorsal skin and 500 μm for the inner skin flap. (I) Quantitative results from H show an increased density of hair follicles upon CEP application around wounds of WT>WT transplanted mice with no changes in WT>TLR2KO mice. N=5 for each group. (J) Representative photographs of dorsal skin (upper panels) and inner skin flaps (lower panels) from Tlr2lox/lox and TLR2HFSC-KO mice treated with CEP show a lack of pigmentation around TLR2HFSC-KO wounds compared with Tlr2lox/lox wounds treated with CEP. The inner skin flap of TLR2HFSC-KO mice demonstrates an absence of pigmented hair bulbs after the CEP treatment. Scale bars are 3 mm. (K) Representative confocal images of skin adjacent to wound immunostained for Ker17. Scale bars are 100 μm. (L) Quantification of hair follicle numbers in images from K reveals a significant decrease in regenerated hair follicles in TLR2HFSC-KO skin compared with Tlr2lox/lox skin. N=7 for Tlr2lox/lox. N=4 for TLR2HFSC-KO. (M) Representative confocal images of skin adjacent to wound immunostained for Ki67. Scale bars are 50 μm. (N) Bar graph showing Ki67 fluorescent intensity in the skin adjacent to wound from images in M. N=7 for Tlr2lox/lox. N=4 for TLR2HFSC-KO. (O) Representative microphotographs of primary keratinocytes isolated from WT or TLR2KO mouse skin co-cultured with CEP or control (PBS or BSA). Representative images from at least three independent assays are shown. Scale bar 50 µm. (P) Cell proliferation of primary keratinocytes in O indicates increased proliferation by CEP in WT but not in TLR2KO keratinocytes. N=3 independent experiments. (Q) Quantitative polymerase chain reaction (qPCR) analyses of Nfkb2, Il1b, and Il6 mRNA levels in FACS-purified mouse HFSCs treated with BSA control or CEP. N=3 per group. (R) qPCR analyses of Bmp7 mRNA levels in FACS-purified mouse HFSCs treated with BSA control or CEP. N=3 per group. (S) Summary of the main findings of this study. Unpaired t-test (G, P) or Mann-Whitney test (I, L, N, Q, R) was used to determine the statistical significance. All data are mean ± s.e.m. A p-value ≤ 0.05 was considered to be statistically significant.
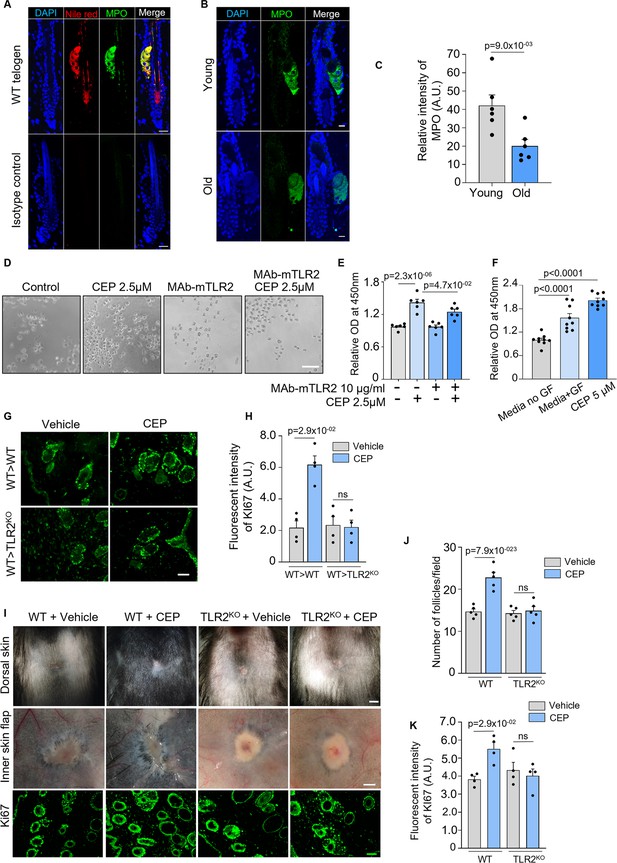
Myeloperoxidase (MPO) expression in sebaceous gland of hair follicles from old vs young mice; effect of carboxyethylpyrrole (CEP) in vitro and in vivo on hair follicle growth.
The promotion of hair follicle regeneration after wound healing is dependent on TLR2. (A) Representative confocal images of Nile red-labeled (sebaceous gland) wild-type (WT) telogen hair follicles co-immunostained for MPO showing complete co-localization of MPO to the sebaceous gland. The isotype control panel shows the images of hair follicles stained with MPO isotype control antibody. Scale bars are 20 μm. (B) Representative confocal images of hair follicles from old vs young mice stained for MPO. Scale bars are 10 μm. (C) Quantification of MPO fluorescent intensity in B showing significantly less MPO in hair follicles from older mice. N=6 for each group. (D) Representative microphotographs of human hair follicle stem cells (HFSCs) pre-treated with 10 µg/ml MAb-mTLR2 or DMSO and co-cultured with/without 2.5 µM of CEP. Representative images from at least three independent assays are shown. Scale bar 50 µm. (E) Bar graphs show increased proliferation of HFSC in the presence of TLR2 endogenous ligand CEP compared to control, which was abolished in the presence of TLR2 blocking antibody. N=6 independent experiments. (F) Bar graphs show increased proliferation of human hair follicle dermal papilla cells incubated with 5 µM of CEP compared to the control. N=9 independent experiments. (G) Representative confocal images of Ki67 immunostaining of dorsal skin adjacent to wound of CEP-treated WT bone marrow transplanted WT and TLR2KO mice. Scale bars are 50 μm. (H) Quantitative results showed increased Ki67 intensity in hair follicles around wounds of CEP-treated WT bone marrow transplanted WT mice with no differences in TLR2KO with WT bone marrow. N=4 per group. (I) Representative photographs of dorsal skin (upper panels), inner skin flaps (middle panels), and representative confocal images of Ki67 immunostaining (lower panels) of vehicle- or CEP-treated WT or TLR2KO skin. Scale bars are 1 mm for dorsal skin, 500 μm for skin flaps, and 50 μm for confocal images. (J) Bar graph showing quantification of hair follicle numbers of vehicle or CEP-treated skin from I. N=5 per group. (K) Bar graph showing quantification of Ki67 fluorescent intensity of Ki67 staining of vehicle or CEP-treated skin from I. N=4 per group. Unpaired two-tailed t-test (C), or non-parametric Mann-Whitney test (H, J, K), or Kruskal-Wallis test with Dunn’s multiple comparisons test (F), or one-way ANOVA with Tukey’s multiple comparisons test (E) was used to determine statistical differences. All bar graphs are mean ± s.e.m. A p-value ≤ 0.05 was considered to be statistically significant.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus) | K15-CrePR1 | The Jackson Laboratory | Cat.# 005249; RRID:IMSRJAX:005249 | RU 486-inducible Cre recombinase driven by the mouse keratin complex 1, acidic, gene 15 promoter. When induced, Cre activity is observed in epithelial stem cells in the bulge region of the hair follicle |
| Strain, strain background (Mus musculus) | TLR2-GFP | The Jackson Laboratory | Cat.# 031822; RRID:IMSR_JAX:031822 | Tlr2KI knock-in mice have an HA tag and an IRES-EGFP sequence placed at the 3’ end of the Toll-like receptor 2 (Tlr2) gene |
| Strain, strain background (Mus musculus) | TLR2KO | The Jackson Laboratory | Cat.# 004650; RRID:IMSR_JAX:004650 | Global Tlr2 KO |
| Strain, strain background (Mus musculus) | Tlr2flox/flox | Taconic Laboratory | c57BL/6NTacTlr2^tm3243Arte | loxP sites on either side of exon 3 of the targeted TLR2 gene |
| Strain, strain background (Mus musculus) | TLR2HFSC-KO | Described previously; Xiong et al., 2022b | c57BL/6NTacTlr2^tm3243Arte -B6;SJL-Tg(Krt1-15-cre/PGR)22Cot/J | RU 486-inducible hair follicle stem cells-specific Tlr2 KO |
| Strain, strain background (Mus musculus) | C57BL/6J | The Jackson Laboratory | Cat.# 000664 RRID:IMSR_JAX:000664 | |
| Cell line (Mus musculus) | Skin keratinocytes | Described previously; Xiong et al., 2022b | Freshly isolated from the mouse dorsal skin of WT and TLR2KO mice | |
| Cell line (Mus musculus) | Hair follicle stem cells | Described previously; Xiong et al., 2022b | Freshly isolated from the mouse dorsal skin of WT and TLR2HFSC-KO mice | |
| Cell line (human) | Hair follicle dermal papilla cells | Cell Applications, Inc | Cat.# 602-05a | Normal human scalp hair follicle papilla cells |
| Cell line (human) | Hair follicle stem cells | Celprogen | Cat.# 36007-08 | Human frontal region scalp extracted from hair follicle bulge |
| Cell line (human) | Epidermal keratinocytes, neonatal, pooled | Lonza Reagents | Cat.# 192906 | Cryopreserved normal human epidermal keratinocytes from pooled donors |
| Antibody | Mouse monoclonal anti-keratin 17 | Santa Cruz Biotechnology | Cat.# sc-393002- AF647; RRID:AB_2893006 | IF 1:200 |
| Antibody | Rabbit polyclonal anti-keratin 15 | Abclonal | Cat.# A2660; RRID:AB_2764526 | IF 1:100 |
| Antibody | Mouse monoclonal anti-myeloperoxidase | Santa Cruz Biotechnology | Cat.# sc-390109; RRID:AB_2892996 | IF 1:100 |
| Antibody | Mouse monoclonal anti-TLR2 | Santa Cruz Biotechnology | Cat.# sc-21759 RRID:AB_628363 | IF 1:100 |
| Antibody | Rabbit polyclonal anti-GFP | Thermo Fisher Scientific | Cat.# sc-390109; RRID:AB_10709851 | IF 1:100 |
| Antibody | Rabbit monoclonal anti-Ki67 | Abcam | Cat.# ab16667; RRID:AB_302459 | IF 1:250 |
| Antibody | Goat polyclonal anti-P-cadherin | R&D Systems | Cat.# AF761-SP; RRID:AB_355581 | IF 1:50 |
| Antibody | Rabbit polyclonal anti-CEP | Pacific Immunology | Custom | IF 1:200 |
| Antibody | Rabbit polyclonal anti-β-catenin | Cell Signaling Technology | Cat.# 8480; RRID:AB_11127855 | IF 1:80 |
| Antibody | Rat monoclonal anti-CD34 | eBioscience | Cat.# 11-0341-82; RRID:AB_465021 | IF 1:200 FACS 1 µg/test |
| Antibody | Rat monoclonal anti-CD49f | BD Biosciences | Cat.# 562473; RRID:AB_11153684 | IF 1:100 FACS 5 µl/test |
| Antibody | Rabbit monoclonal anti-Sox9 | Cell Signaling Technology | Cat.# 82,630T; RRID:AB_2665492 | IF 1:200 |
| Antibody | Chicken polyclonal anti-keratin 5 | BioLegend | Cat.# 905903; RRID:AB_2721742 | IF 1:200 |
| Antibody | Rabbit polyclonal anti-BMP7 | Proteintech | Cat.# 12221-1-AP; RRID:AB_2063960 | IF 1:200 |
| Antibody | Rabbit monoclonal anti-pSmad1/5/9 | Cell Signaling Technology | Cat.# 13,820P; RRID:AB_2493181 | IF 1:200 WB 1:1000 |
| Antibody | Anti-murine TLR2 (clone T2.5) Detection and Neutralizing mouse monoclonal | Invivogen | Cat.# mab2-mtlr2 RRID N/A | Blocking experiment 0.66 µg/ml |
| Antibody | Smad1 (D59D7) XP Rabbit monoclonal | Cell Signaling Technology | Cat.# 6944 | WB 1:1000 |
| Antibody | NF-κB p65 (D14E12) XP Rabbit monoclonal | Cell Signaling Technology | Cat.# 8242 | WB 1:1000 |
| Antibody | Phospho-NF-κB p65 (Ser536) (93H1) Rabbit monoclonal | Cell Signaling Technology | Cat.# 3033 | WB 1:1000 |
| Antibody | Anti-GAPDH antibody EPR16884 Loading Control Rabbit monoclonal | Abcam | Cat.# 181603 | WB 1:6000 |
| Antibody | Anti-rabbit IgG, HRP-linked Antibody goat anti-rabbit IgG Polyclonal | Cell Signaling Technology | Cat.# 7074S | WB 1:3000 |
| Antibody | Normal mouse IgG2b-PE isotype control | Santa Cruz Biotechnology | Cat.# sc-2868 RRID:AB_737259 | According to immune antibody concentration |
| Antibody | Normal goat IgG isotype control | R&D | Cat.# AB-108-C RRID:AB_354267 | According to immune antibody concentration |
| Antibody | Normal mouse IgG isotype control | Santa Cruz Biotechnology | Cat.# sc-2025 RRID:AB_737182 | According to immune antibody concentration |
| Antibody | Normal rabbit IgG isotype control | Cell Signaling Technology | Cat.# 2729S RRID:AB_1031062 | According to immune antibody concentration |
| Antibody | Normal rat IgG isotype control | Santa Cruz Biotechnology | Cat.# sc-2026 RRID:AB_737202 | According to immune antibody concentration |
| Antibody | Chicken IgY Isotype Control | Novus Biologicals | Cat.# AB-101-C RRID:AB_354263 | According to immune antibody concentration |
| Antibody | Goat anti-Rat IgG Polyclonal (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 | Invitrogen | Cat.# A-11007 RRID:AB_10561522 | IF 1:300 |
| Antibody | Goat anti-Rabbit IgG Polyclonal (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat.# A-11008 RRID:AB_143165 | IF 1:300 |
| Antibody | Goat anti-Rat IgG Polyclonal (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Invitrogen | Cat.# A-11006 RRID:AB_2534074 | IF 1:300 |
| Antibody | Alexa Fluor Plus 594 Goat anti-rabbit Polyclonal Secondary Antibody | Thermo Fisher Scientific | Cat.# A-32740 RRID:AB_2762824 | IF 1:300 |
| Antibody | Goat anti-Mouse IgG (H+L) Cross-Adsorbed Polyclonal Secondary Antibody, Alexa Fluor 568 | Thermo Fisher Scientific | Cat. # A-11004 RRID:AB_2534072 | IF 1:300 |
| Antibody | Goat anti-Mouse IgG (H+L) Cross-Adsorbed Polyclonal Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat.# A-11001 RRID:AB_2534069 | IF 1:300 |
| Antibody | Donkey anti-Goat IgG (H+L) Cross-Adsorbed Polyclonal Secondary Antibody, Alexa Fluor 594 | Thermo Fisher Scientific | Cat.# A-11058 RRID:AB_142540 | IF 1:300 |
| Antibody | Goat anti-Chicken IgY (H+L) Secondary Antibody, Polyclonal Alexa Fluor 488 | Invitrogen | Cat.# A-11039 RRID:AB_2534096 | IF 1:300 |
| Other | DAPI Solution | BD Biosciences | Cat#564907 RRID:AB_2869624 | Fluorescent stain IF 1:300 |
| Other | Nile Red | ATT BioQuest | Cat.# 22190 | Lipophilic stain IF 10 µM |
| Other | 7-AAD | BD Biosciences | Cat.# 559925 RRID:AB_2869266 | Membrane impermeant dye 0.25 µg/test |
| Sequence-based reagent | TLR2_F | This paper | PCR primers | TCTAAAGTCGATCCGCGACAT |
| Sequence-based reagent | TLR2_R | This paper | PCR primers | CTACGGGCAGTGGTGAAAACT |
| Sequence-based reagent | BMP7_F | This paper | PCR primers | ACGGACAGGGCTTCTCCTAC |
| Sequence-based reagent | BMP7_R | This paper | PCR primers | ATGGTGGTATCGAGGGTGGAA |
| Sequence-based reagent | BMP2_F | This paper | PCR primers | GGGACCCGCTGTCTTCTAGT |
| Sequence-based reagent | BMP2_R | This paper | PCR primers | TCAACTCAAATTCGCTGAGGAC |
| Sequence-based reagent | BMPr1A_F | This paper | PCR primers | AACAGCGATGAATGTCTTCGAG |
| Sequence-based reagent | BMPr1A_R | This paper | PCR primers | GTCTGGAGGCTGGATTATGGG |
| Sequence-based reagent | NFkB2_F | This paper | PCR primers | GGCCGGAAGACCTATCCTACT |
| Sequence-based reagent | NFkB2_R | This paper | PCR primers | CTACAGACACAGCGCACACT |
| Sequence-based reagent | IL1b_F | This paper | PCR primers | GCAACTGTTCCTGAACTCAACT |
| Sequence-based reagent | IL1b_R | This paper | PCR primers | ATCTTTTGGGGTCCGTCAACT |
| Sequence-based reagent | IL6_F | This paper | PCR primers | TAGTCCTTCCTACCCCAATTTCC |
| Sequence-based reagent | IL6_R | This paper | PCR primers | TTGGTCCTTAGCCACTCCTTC |
| Chemical compound, drug | Pam3CSK4 | Invivogen | Cat.# tlrl-pms | 10 µg/ml |
| Chemical compound, drug | Recombinant Human BMP-4 Animal-Free Protein | R&D Systems | Cat.# AFL314E-010 | 20 ng/ml |
| Chemical compound, drug | CEP (carboxyethylpyrrole) | Custom | Custom | Cell experiments 2.5–5 µM Skin treatment 5 µg/ml |
| Software, algorithm | Imaris V9.7.2 | Bitplane | ||
| Software, algorithm | ImageJ, Fiji V1.53t | National Institutes of Health | ||
| Software, algorithm | GraphPad Prism 9 | GraphPad by Dotmatics | ||
| Software, algorithm | Flow Jo | Becton, Dickinson & Company |
Additional files
-
Supplementary file 1
Quantitative polymerase chain reaction (qPCR) primers.
- https://cdn.elifesciences.org/articles/89335/elife-89335-supp1-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/89335/elife-89335-mdarchecklist1-v1.docx