The Na+ leak channel NALCN controls spontaneous activity and mediates synaptic modulation by α2-adrenergic receptors in auditory neurons
Figures
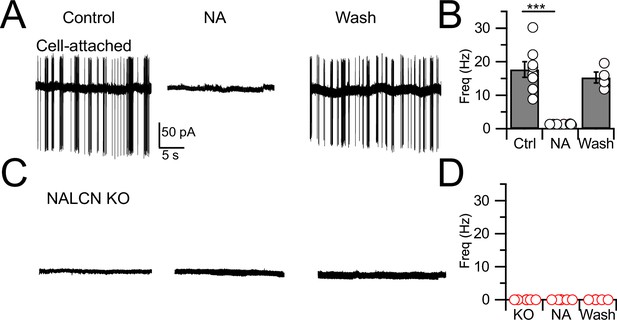
Spontaneous firing is absent in NALCN conditional knockout (cKO) mice.
(A) Representative cell-attached recording from a spontaneously spiking cartwheel cell (CWC) in control. Noradrenaline (NA; 10 µM) applied to the bath in the middle trace, and washout on the right-most trace. (B) Spontaneous spike rate in control, NA, and washout, showing that NA eliminated spontaneous spiking in CWCs (n = 6 cells). (C) Representative trace showing absence of spontaneous firing in a CWC from a NALCN cKO mouse. NA had no effect on firing. (D) Summary of spontaneous firing and lack of NA effect in CWC from NALCN cKO mice (n = 5 cells). See Figure 1—source data 1.
-
Figure 1—source data 1
Source data for Figure 1B, D.
- https://cdn.elifesciences.org/articles/89520/elife-89520-fig1-data1-v1.xlsx
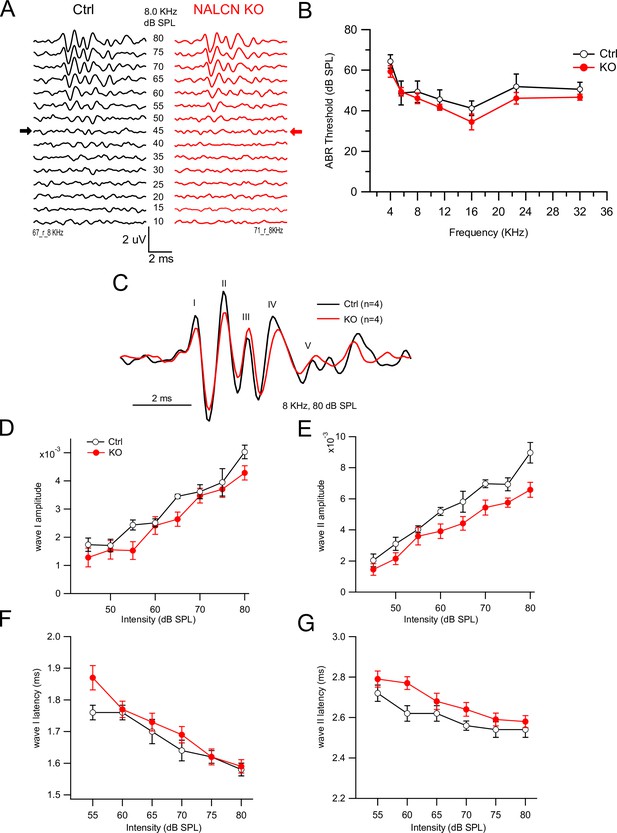
NALCN conditional knockout (cKO) mice show no difference in auditory brainstem response (ABR) from wildtype (WT).
(A) Example traces of WT (left) and NALCN cKO (right: red) ABR responses to 8 kHz pure tones presented at different sound intensities. Arrows indicate ABR wave I threshold. (B) ABR thresholds for WT and NALCN cKO mice were similar for various pure tones tested for mouse hearing (p>0.95 for all frequencies, ANOVA/Sidak’s multiple-comparison test). (C) Example ABR trace from WT (black) and NALCN cKO (red) in response to 8 kHz pure tone at 80 dB SPL. (D–G) Maximum wave I and II amplitude (D, F) and latencies (E, G) to 8 kHz tone stimulation presented at 80 dB SPL were not different between WT (N = 4) and NALCN cKO animals (N = 4). See Figure 1—figure supplement 1—source data 1.
-
Figure 1—figure supplement 1—source data 1
Source data for Figure 1—figure supplement 1B.
- https://cdn.elifesciences.org/articles/89520/elife-89520-fig1-figsupp1-data1-v1.xlsx
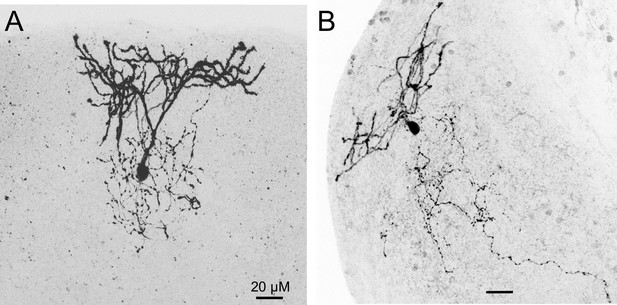
Biocytin-filled cartwheel cells (CWCs) from NALCN conditional knockout (cKO) mice.
(A, B) Two examples of CWC that were labeled with biocytin imaged using confocal microscopy. Characteristic of CWC, spiny dendrites extend up to the ependymal layer while a fine axon ramifies within the molecular and cell body layers of the dorsal cochlear nucleus (DCN).
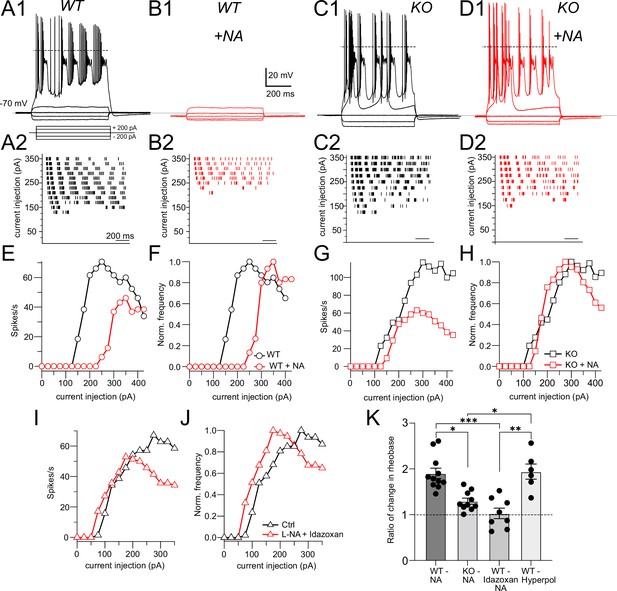
NALCN is required for noradrenaline (NA)-mediated shift in rheobase.
(A1) Profile of voltage responses to current steps. Simple and complex spikes evoked by +200 pA. (A2) Raster plot of spike timings during different level of current injection. (B1, B2) Same cell as in (A) but in the presence of 10 μM NA (indicated by red traces and markers). (C, D) As in (A, B) but for recordings from a cartwheel cell (CWC) from a NALCN conditional knockout (cKO) mouse. (E) Spike rate calculated from data in (A2) and (B2) plotted as a function of current level. (F) Data from (E) normalized to peak firing rate. (G) Spike rate calculated from data in (C2) and (D2) plotted as a function of current level. (H) Data from (G) normalized to peak firing rate. (I), (J), raw and normalized frequency-intensity plots (respectively) for current responses from one WT cell in control solutions and in presence of 10 mM NA + 1 mM idazoxan. (K) Summary data (mean ± SEM) for ratio of change in rheobase of CWC from WT and KO mice with 10 μM NA, NA + idazoxan, and hyperpolarization conditions. Significance: *<0.05; **<0.01; ***<0.001. Dashed line in (A1, C1, D1) indicates 0 mV. See Figure 2—source data 1.
-
Figure 2—source data 1
Source data for Figure 2K.
- https://cdn.elifesciences.org/articles/89520/elife-89520-fig2-data1-v1.xlsx
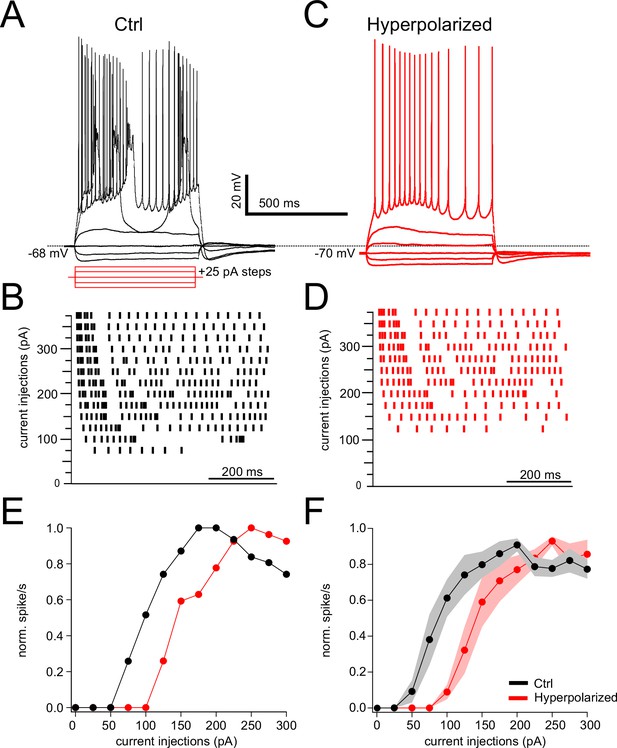
Noradrenaline (NA) effect on rheobase and spiking is voltage dependent.
(A) Profile of voltage responses to current steps. Simple and complex spikes evoked in response to depolarizing current steps. (B) Raster plot of spike timings during different level of current injection. (C, D) Same cell as in (B) but membrane hyperpolarized by 2 mV (see dashed line). (E) Normalized spike rate plotted as a function of current level of cells A and C. (F) Average data for spike rate from Ctrl and bias current injected conditions. Hyperpolarizing current injection show increase in rheobase and suppression of evoked firing (rheobase Ctrl = 70.88 ± 7.68 pA, hyperpolarized = 125 ± 12.90 pA, N = 6, p=0.0009).
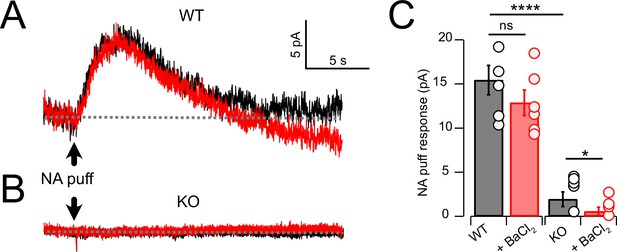
Noradrenaline (NA) evoked a Ba2+-resistant outward current that required NALCN.
(A) Outward current in response to NA puff (100 µM, 50 ms) in whole-cell voltage-clamp mode (– 65 mV) before (black) and after (red) block of GIRK channels by bath application of 100 μM Ba2+. (B) As in (A), but from a cartwheel cell (CWC) from a NALCN conditional knockout (cKO) mouse. (C) Average data showing responses to NA puff in control conditions and after the block of GIRK channels from control (n = 5) and NALCN cKO mice (n = 6). In the KO, the NA response was markedly reduced and was Ba2+ sensitive. Dashed line indicates initial current level. See Figure 3—source data 1.
-
Figure 3—source data 1
Source data for Figure 3C.
- https://cdn.elifesciences.org/articles/89520/elife-89520-fig3-data1-v1.xlsx
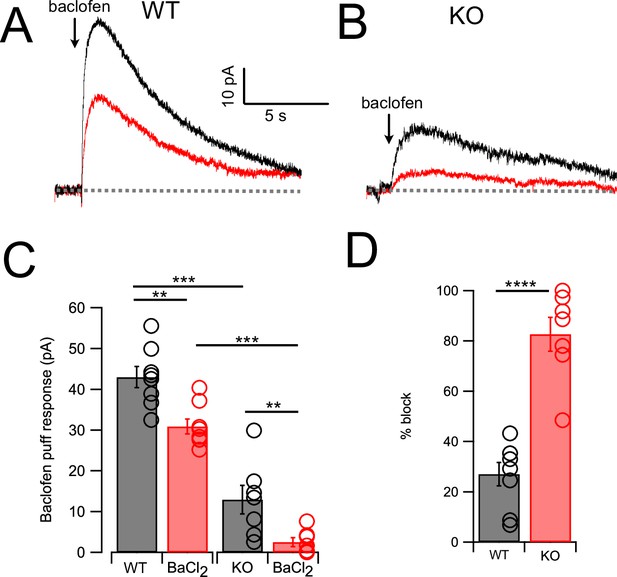
GABAB receptors activate outward current mediated by NALCN and GIRK channels.
(A) Traces from one neuron showing baclofen puff (100 µM, 50 ms) evoked outward current in control solution (black) and with 100 μM Ba2+ in bath (red). (B) As in (A) but for a cartwheel cell (CWC) from a NALCN conditional knockout (cKO) mouse. (C) Averaged data showing Ba2+ sensitivity of the noradrenaline (NA) response in wildtype (WT) and knockout (KO) tissue. Ba2+ produced a significant block in all cases, while KO CWC showed significantly smaller baclofen responses. (D) The degree of block by Ba2+ was significantly greater in KO mice, indicating that more of the baclofen response is mediated by GIRK channels after KO of NALCN. Dashed line indicates initial current level. See Figure 4—source data 1.
-
Figure 4—source data 1
Source data for Figure 4C, D.
- https://cdn.elifesciences.org/articles/89520/elife-89520-fig4-data1-v1.xlsx
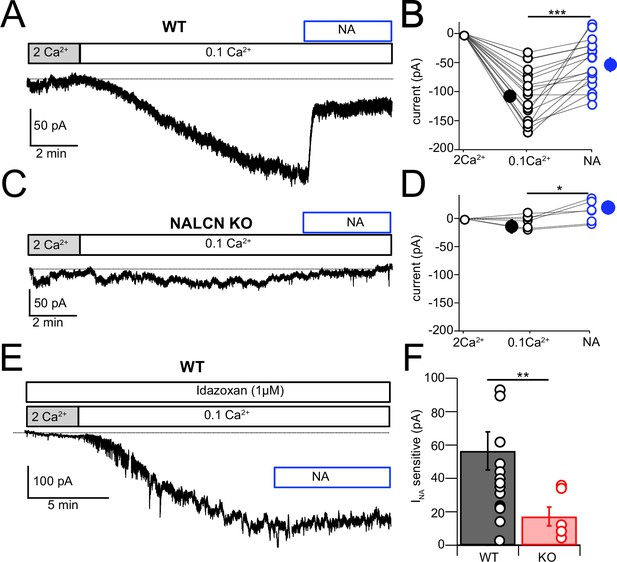
NALCN current evoked by Ca2+ reduction is inhibited by α2 receptors.
(A) Shifting bath Ca2+ from 2 mM to 0.1 mM evokes a slow inward current that is then rapidly reduced by subsequent wash-in of 10 μM noradrenaline (NA). (B) Group data showing the magnitude of inward current shift in 0.1 mM Ca2+ (black) and the significantly smaller shift in 0.1 mM Ca2+ plus NA (blue). N = 18 cells. (C) Experiment as in (A) but for a cartwheel cell (CWC) from a NALCN conditional knockout (cKO) mouse. (D) As in (B), but for CWC from knockout (KO) tissue. N = 6 cells. (E) Experiment as in (A) but in continuous presence of 1 µM idazoxan. NA failed to block the low-Ca2+-evoked current. (F) The magnitude of inward current blocked by NA was significantly greater in wildtype (WT) compared to KO cells. All neurons voltage-clamped to –70 mV. Statistical significance: *p<0.05; **p<0.01; ***p<0.001. Extracellular solution contained TTX, NBQX, MK-801, strychnine, SR95331, and apamin. Dashed line indicates initial current level. See Figure 5—source data 1.
-
Figure 5—source data 1
Source data for Figure 5B, D, F.
- https://cdn.elifesciences.org/articles/89520/elife-89520-fig5-data1-v1.xlsx
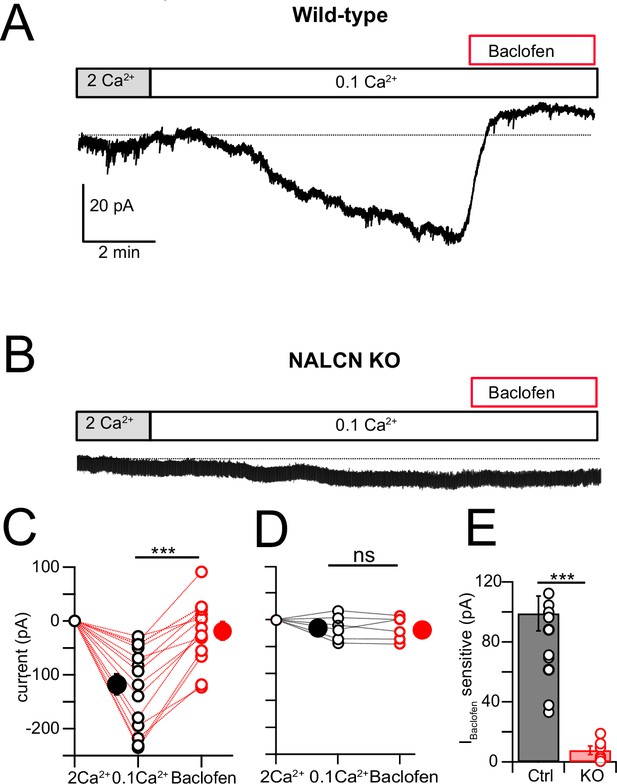
NALCN current evoked by Ca2+ reduction is inhibited by GABAB receptors.
(A) Shifting bath Ca2+ from 2 mM to 0.1 mM evokes a slow inward current that is then rapidly reduced by subsequent wash-in of 10 μM baclofen. (B) Experiment as in (A) but for a cartwheel cell (CWC) from a NALCN conditional knockout (cKO) mouse. (C) Group data showing the magnitude of inward current shift in 0.1 mM Ca2+ (black) and the significantly smaller shift in 0.1 mM Ca2+ plus baclofen (red). N = 18 cells. (D) As in (C), but for CWC from knockout (KO) tissue. N = 6 cells. (E) The magnitude of inward current blocked by baclofen was significantly greater in wildtype (WT) compared to KO cells. All neurons voltage-clamped to –70 mV. Statistical significance: *p<0.05; **p<0.01; ***p<0.001. Extracellular solution contains TTX, NBQX, MK-801, strychnine, SR95331, and apamin. Dashed line indicates initial current level. See Figure 6—source data 1.
-
Figure 6—source data 1
Source data for Figure 6C, D, E.
- https://cdn.elifesciences.org/articles/89520/elife-89520-fig6-data1-v1.xlsx
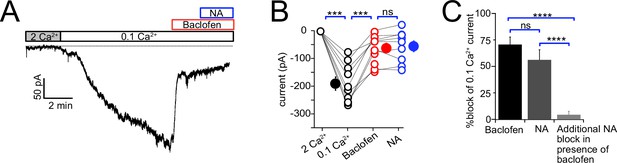
Baclofen and noradrenaline (NA) act on the same population of NALCN channels.
(A) Representative example of NALCN current evoked by shift from 2 mM Ca2+ to 0.1 mM Ca2+, followed by bath application of baclofen (20 µM) and subsequent application of NA (10 µM) with baclofen still present. In the presence of baclofen, the rapid decline in inward current normally evoked by NA was absent. (B) Summary plot of NALCN current amplitude evoked by 0.1 mM Ca2+ and after baclofen and subsequent NA application (wildtype [WT], N = 9). NA failed to produce a significant change after baclofen application. (C) Percentage block of 0.1 Ca2+ current in baclofen, or NA alone, compared to the block of current by NA in a background of baclofen. Baclofen completely occluded response to subsequent application of NA. Statistical significance, ***p<0.001. Dashed line indicates initial current level. See Figure 7—source data 1.
-
Figure 7—source data 1
Source data for Figure 7B, C.
- https://cdn.elifesciences.org/articles/89520/elife-89520-fig7-data1-v1.xlsx
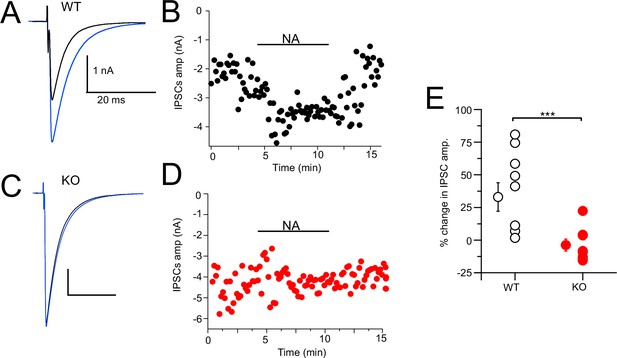
Noradrenaline (NA) enhances cartwheel cell (CWC)-mediated IPSCs in wildtype (WT) but not in NALCN cKO cells.
(A) Example average trace of evoked IPSCs recorded from a CWC with a CsCl pipette fill, before (black) and after (blue) bath application of 10 μM NA. (B) Diary plot of effect of NA application (applied during black bar) of the IPSCs amplitude in cell (A). (C) Example average trace of evoked IPSCs recorded from a CWC as in (A), but from a NALCN cKO mouse. Data shown before (black) and after (blue) bath application of 10 μM NA. (D) Diary plot of effect of NA application in (C). (E) Summary plot of percent change of IPSCs amplitude after NA application in WT and knockout (KO) mice (WT, N = 8; KO, N = 10). See Figure 8—source data 1.
-
Figure 8—source data 1
Source data for Figure 8E.
- https://cdn.elifesciences.org/articles/89520/elife-89520-fig8-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus) | C57BL/6J | Jackson Laboratory | RRID:JAX:000664 | |
| Strain, strain background (M. musculus) | GlyT2-EGFP, Tg(Slc6a5-EGFP)1Uze | MGI, Zeilhofer et al., 2005 | RRID:MGI:J:145521 | |
| Strain, strain background (M. musculus) | B6(Cg)- Nalcntm1c(KOMP)Wtsi/ DrenJ | MGI and Jackson Laboratory | RRID:JAX_030718 | |
| Strain, strain background (M. musculus) | Tg(Slc6a5-cre)KF109Gsat | MGI | RRID:MGI:4367229 | |
| Chemical compound, drug | Strychnine hydrochloride | Sigma | Cat# S8753 | |
| Chemical compound, drug | SR-95531 hydrobromide | Tocris Bioscience | Cat# 1262 | |
| Chemical compound, drug | NBQX disodium salt | Tocris Bioscience | Cat# 1044 | |
| Chemical compound, drug | (+)-MK-801 hydrogen maleate | Sigma | Cat# M107 | |
| Chemical compound, drug | L-(-)-Norepinephrine (+)-bitartrate salt monohydrate | Sigma | Cat# A9512 | |
| Chemical compound, drug | (±)-Norepinephrine (+)-bitartrate salt | Sigma | Cat# A0937 | |
| Chemical compound, drug | Idazoxan hydrochloride | Sigma | Cat# I6138-100MG | |
| Chemical compound, drug | Apamin | Alomone Labs | Cat# STA-200 | |
| Chemical compound, drug | D-AP5 | Tocris Bioscience | Cat# 0106 | |
| Chemical compound, drug | Barium chloride | Sigma | Cat# 202738 | |
| Software, algorithm | pClamp 10 | Molecular Devices | RRID:SCR_011323 | |
| Software, algorithm | Igor Pro 8 | WaveMetrics | RRID:SCR_000325 | |
| Software, algorithm | NeuroMatic | Rothman and Silver, 2018; DOI:10.3389/fninf.2018.00014 | RRID:SCR_004186 | |
| Software, algorithm | Axograph | Axograph | RRID:SCR_014284 | |
| Software, algorithm | Prism 9 | GraphPad | RRID:SCR_002798 | |
| Software, algorithm | Excel | Microsoft | RRID:SCR_016137 | |
| Software, algorithm | Affinity Designer | Serif | RRID:SCR_016952 |





