Cortical plasticity is associated with blood–brain barrier modulation
Figures
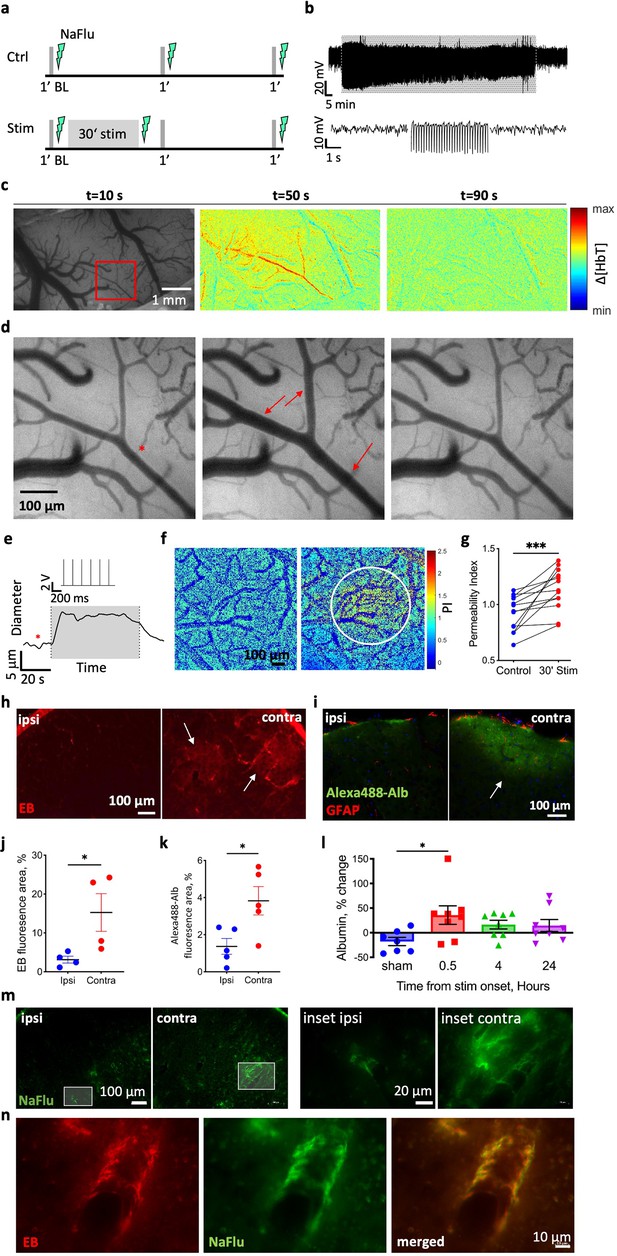
Limb stimulation modulates blood–brain barrier (BBB) permeability.
(a) The experimental paradigm in control animals (Ctrl, top panel) and animals that underwent a 30 min stimulation (Stim, bottom panel). Gray lines indicate 1 min test stimulations, lightning indicates the timing of BBB imaging (using injection of sodium fluorescein, NaFlu). (b) Top: local field potential (LFP) trace from the corresponding region in L2/3 sensorimotor cortex before, during (grayed area), and after 30 min limb stimulation. Bottom: 5 s excerpts of the above trace (left-to-right: before during and after stimulation). (c) Image of the cortical window over the rat sensorimotor cortex, followed by the change in total hemoglobin (Δ[HbT]) concentration maps showing the evolution of the hemodynamic response during and after stimulation (t states the time point of the image; 120 s total: 30 s before and after a 60 s stimulation). Red rectangle marks the responding region magnified in (d). (d) Images of the responding arteriole (dilated parts marked with red arrows) in the rat’s cortex before (left) during (middle) and after stimulation (right). The red asterisk denotes the measured arteriole on panel (e). (e) The diameter of the responding arteriole during 1 min stimulation. Gray area corresponds to time of stimulation (the stimulation trace is shown above the grayed area). (f) NaFlu permeability maps before (left) and after 30 min stimulation (right) showing tracer accumulation around a responding arteriole (marked with a white circle). (g) Permeability index (see ‘Materials and methods’, under in vivo imaging) is higher after stimulation compared to baseline (n = 13 rats, mean ± SEM, Wilcoxon, p<0.001). (h, i) Fluorescence images of cortical sections of stimulated rats injected with the albumin-binding dye Evans blue (EB) (h) and Alexa-488-Alb (i). White arrows point to areas with tracer accumulation. (j, k) Total fluorescence of EB (j) and Alexa488-Alb (k) after stimulation in the contralateral hemisphere compared to the ipsilateral hemisphere. (EB n = 4 rats, 32 sections, nested t-test, p=0.0296; Alexa488-Alb n = 5 rats, 20 sections, nested t-test, p=0.0229; mean ± SEM). (l) Albumin concentration in the contralateral hemisphere relative to the ipsilateral in three different time points after stimulation compared to sham stimulation. (0.5, 4 and 24 hr post-stimulation n = 8, sham n = 7, mean ± SEM, Kruskal–Wallis with false discovery rate (FDR) correction, p=0.0242, q = 0.0406). (m) Cortical sections of the area of limb representation from both hemispheres of a stimulated rat (left) and higher magnification images (right). (n) In an animal injected with both EB and NaFlu post stimulation, fluorescence imaging shows extravascular accumulation of both tracers along a cortical small vessel in the stimulated hemisphere. *p<0.05, ***p<0.001.
-
Figure 1—source data 1
All data measured and analyzed for Figure 1.
- https://cdn.elifesciences.org/articles/89611/elife-89611-fig1-data1-v1.xlsx
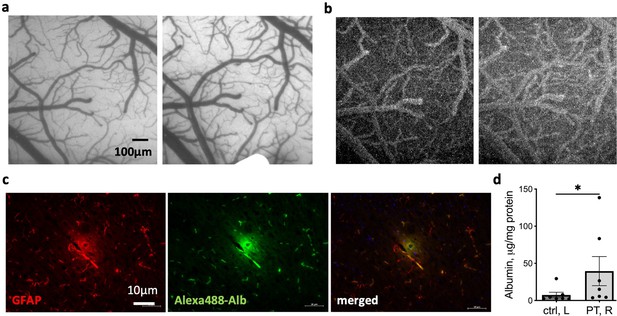
Increased blood–brain barrier (BBB) permeability following stimulation.
(a) Images of the responding region of the rats’ cortex before (left) and after stimulation (right). (b) Images depicted in (a) after NaFlu injection before (left) and after (right) stimulation. (c) Representative image of a small vessel in the stimulated hemisphere stained for astrocytes (GFAP, left), albumin (middle), and the merged image (right) showing co-localization of the two markers and albumin accumulation surrounding the vessel. (d) Albumin concentration in the contralateral hemisphere relative to the ipsilateral in rats following PT-induced BBB dysfunction. *p<0.05, Wilcoxon, n = 7, mean ± SEM.
-
Figure 1—figure supplement 1—source data 1
All data measured and analyzed for Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/89611/elife-89611-fig1-figsupp1-data1-v1.xlsx
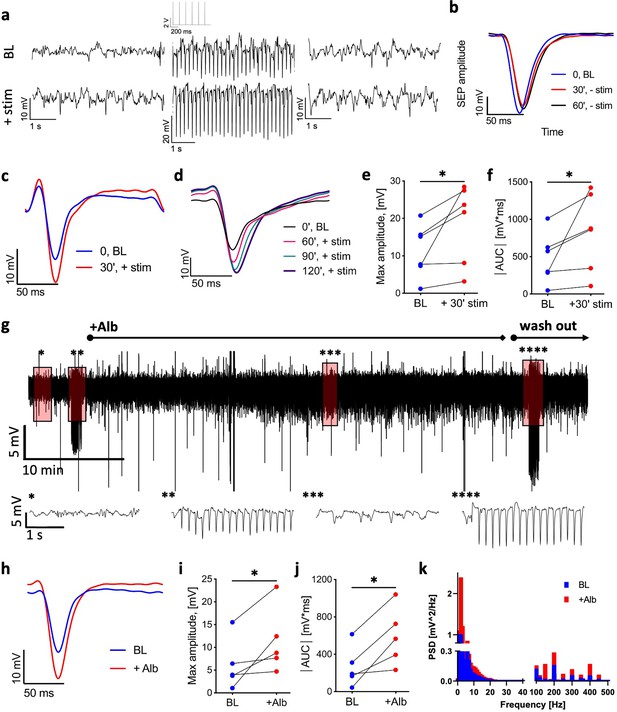
Stimulation and cortical perfusion of serum albumin induce long-term potentiation (LTP).
(a) Top: LFP trace from the rat L2/3 sensorimotor cortex before (left), during (middle), and after (right) test stimulation (1 min, 6 Hz, 2 mA, an excerpt of the stimulation trace is shown above the middle LFP trace). Bottom: LFP trace from the same rat before (left), during (middle), and after (right) test stimulation (1 min, 6 Hz, 2 mA), administered following a 30 min stimulation (6 Hz, 2 mA). (b) The somatosensory-evoked potential (SEP) of test stimulation at baseline (0 min, BL) and after 30 and 60 minutes (blue, red, and black, respectively, each averaged over 360 stimuli). (c) Somatosensory-evoked potential (SEP) in response to test stimulation at baseline (blue) and following a 30 min stimulation (red). (d) SEP in response to test stimulation at baseline (blue) and three time points following a 30 min stimulation (60 min, red; 90 min, green; 120 min, purple). (e) Maximum amplitude of the SEP (absolute values) following a 30 min stimulation compared to baseline (n = 6 rats, mean ± SEM, Wilcoxon, p=0.0312). (f) Area under the curve (AUC) of the SEP following a 30 min stimulation compared to baseline (n = 6 rats, mean ± SEM, Wilcoxon, p=0.0312). (g) Top: 1 hr LFP trace from a representative rat. Bottom: 5 s magnifications of the above trace at selected time points (noted by asterisks). Left to right: baseline activity; during test stimulation; following cortical application of 0.1 mM albumin (Alb); during test stimulation post-Alb. (h) SEP amplitude during test stimulation at baseline (normal aCSF, blue) and following 0.1 mM Alb (red). (i) Maximum amplitude of the SEP during test stimulation post-Alb compared to baseline (n = 5 rats, mean ± SEM, Wilcoxon, p=0.0312). (j) AUC of the SEP post-Alb compared to baseline. (n = 5 rats, mean ± SEM, Wilcoxon, p=0.0312). (k) Power spectrum density of 10 min spontaneous activity before (blue) and post-Alb (red) (p=0.0035, paired t-test). *p<0.05.
-
Figure 2—source data 1
All data measured and analyzed for Figure 2.
- https://cdn.elifesciences.org/articles/89611/elife-89611-fig2-data1-v1.xlsx
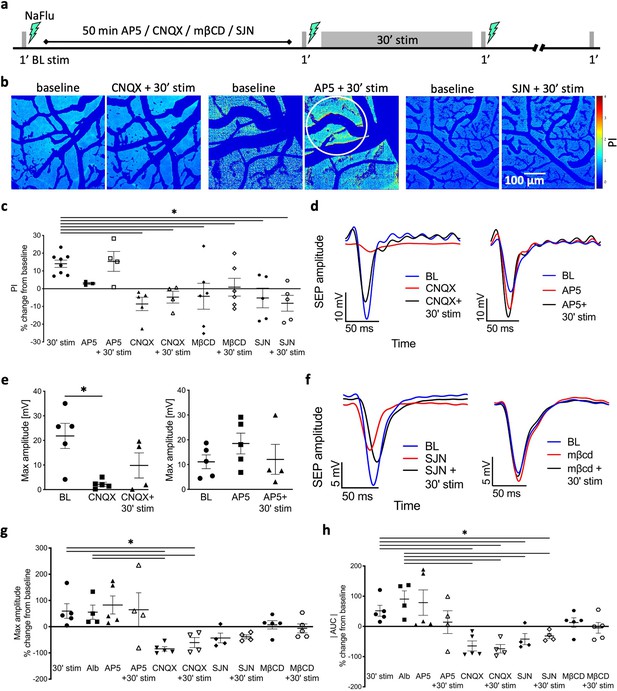
Stimulation-induced plasticity is associated with blood–brain barrier (BBB) modulation.
(a) Timeline of the experimental protocol for stimulations and imaging with blockers application (CNQX/AP5 [50 μM]/mβCD [10 μM]/SJN [0.3 mM]). (b) NaFlu permeability maps of the cortical window before (control, left) and after CNQX/AP5/SJN + 30 min stimulation (right). Tracer accumulation area is marked with a white circle. (c) Permeability index (PI) following blockers application before and after stimulation compared to a 30 min stimulation. (% change from baseline, 30 min stim n = 8, AP5 n = 4, CNQX n = 5, mβCD n = 6, SJN n = 5; mean ± SEM, Kruskal–Wallis with false discovery rate [FDR] correction, *p<0.05). (d) Somatosensory-evoked potential (SEP) amplitude in response to test stimulation, baseline (blue); following application of CNQX (left, red) or AP5 (right, red); following CNQX + 30 min stimulation (left, black); and following AP5 + 30 min stimulation (right, black); (e) Left: max SEP amplitude to test stimulation following CNQX compared to baseline (mean ± SEM, n = 5, paired t-test, p=0.0176). Right: max SEP amplitude following AP5 compared to baseline. (f) SEP amplitude in response to test stimulation, baseline (blue); following SJN (red); following SJN + 30 min stimulation (black); and following mβCD (right). (g) Max SEP amplitude following 30 min stimulation or albumin application compared to blockers, and blockers + 30 min stimulation. (h) Area under the curve (AUC) of the SEP for test stimulation following 30 min stimulation or albumin application compared to blockers and blockers + 30 min stimulation. (g, h) % change from baseline (mean ± SEM, 30 min stim n = 5, Alb n = 4, AP5 n = 5, CNQX n = 5, SJN n = 4, mβCD n = 5; Kruskal–Wallis with FDR correction, *p<0.05).
-
Figure 3—source data 1
All data measured and analyzed for Figure 3.
- https://cdn.elifesciences.org/articles/89611/elife-89611-fig3-data1-v1.xlsx
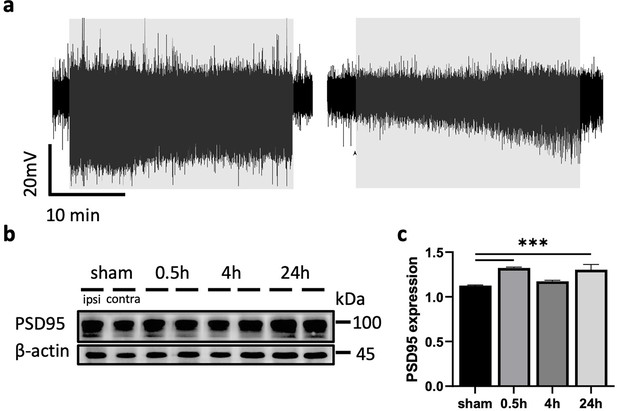
Modulation of blood–brain barrier (BBB) permeability associated with synaptic plasticity is activity dependent.
(a) Representative local field potential (LFP) trace from the responding area in L2/3 of the somatosensory cortex during a 30 min stimulation following AP5 (left) or CNQX application (right); grayed rectangles mark stimulation. (b) Representative western blot for PSD-95 expression in the ipsi- and contralateral cortex of sham and stimulated rats at different time points. Normalized to β-actin (loading control). (c) Quantification of PSD-95 expression levels at different time points post stimulation (n = 8 each group, one-way ANOVA with false discovery rate [FDR] correction, ***p<0.001).
-
Figure 3—figure supplement 1—source data 1
All data measured and analyzed for Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/89611/elife-89611-fig3-figsupp1-data1-v1.xlsx
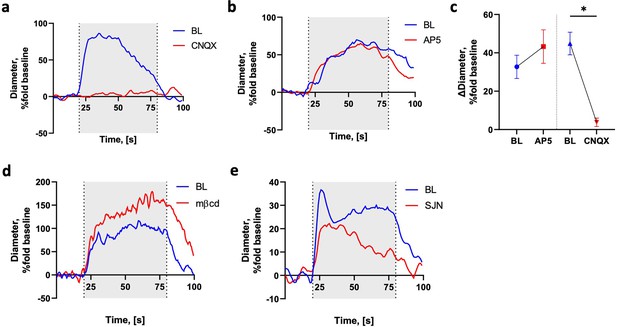
Stimulation-induced blood–brain barrier (BBB) modulation can be prevented with or without affecting the vascular response.
(a, b) Representative examples of the responding arteriole diameter during 1 min stimulation. Presented as % change from a 20 s baseline segment before stimulation. Blue line, baseline (BL); red line, following application of CNQX (a) or AP5 (b). (c) Mean arteriolar diameter during 1 min baseline is similar compared to AP5 and significantly larger compared CNQX stimulations. Presented as % change from a 20 s baseline segment before stimulation (AP5, n = 2; CNQX, n = 3; p=0.011 paired t-test). (d, e) Representative examples of the responding arteriole diameter during 1 min stimulation, presented as % change from a 20 s baseline segment before stimulation. Blue line, baseline (BL); red line, following application of mβCD (d) and SJN (e). *p<0.05.
-
Figure 3—figure supplement 2—source data 1
All data measured and analyzed for Figure 3—figure supplement 2.
- https://cdn.elifesciences.org/articles/89611/elife-89611-fig3-figsupp2-data1-v1.xlsx
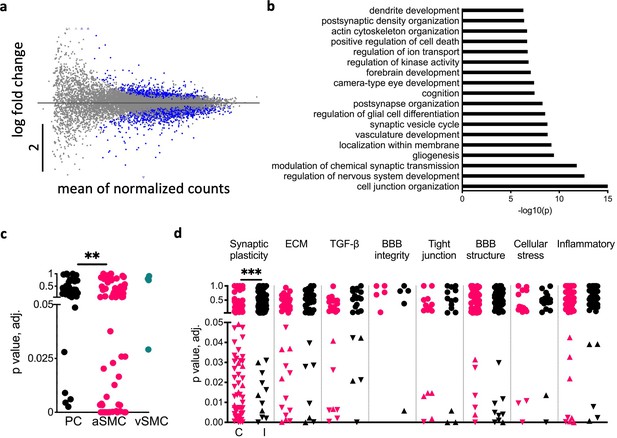
Neuronal activity regulates blood–brain barrier (BBB) transport and synaptic plasticity genes.
(a) Scatter plot of gene expression from RNA-seq in the contralateral somatosensory cortex 24 vs. 1 hr after 30 min stimulation. The y-axis represents the log fold change, and the x-axis represents the mean expression levels (see ‘RNA sequencing and bioinformatics’). Blue dots indicate statistically significant differentially expressed genes (DEGs) by Wald test (n = 8 rats per group). (b) Top Gene Ontologies (GO) enriched terms in the contralateral cortices of rats 24 vs. 1 hr after stimulation. (c) Vascular cell-specific DEGs to pericytes (PC), arterial smooth muscle cells (aSMC), and venous smooth muscle cells (vSMC) (PC n = 42, aSMC n = 70, p=0.0017, chi-square). (d) Scatter plot of DEGs divided by groups of interest: BBB properties, neurovascular unit (NVU) properties, Synaptic plasticity, and inflammatory-related genes in the contralateral (red) vs. ipsilateral (black) cortices of stimulated rats. Circles represent genes with no significant differences between 1 and 24 hr post-stimulation. Upward and downward triangles indicate significantly up- and downregulated genes, respectively. The contralateral somatosensory cortex was found to have a significantly higher number of DEGs related to synaptic plasticity than the ipsilateral side (***p<0.001, chi-square).
-
Figure 4—source data 1
All data measured and analyzed for Figure 4.
- https://cdn.elifesciences.org/articles/89611/elife-89611-fig4-data1-v1.xlsx
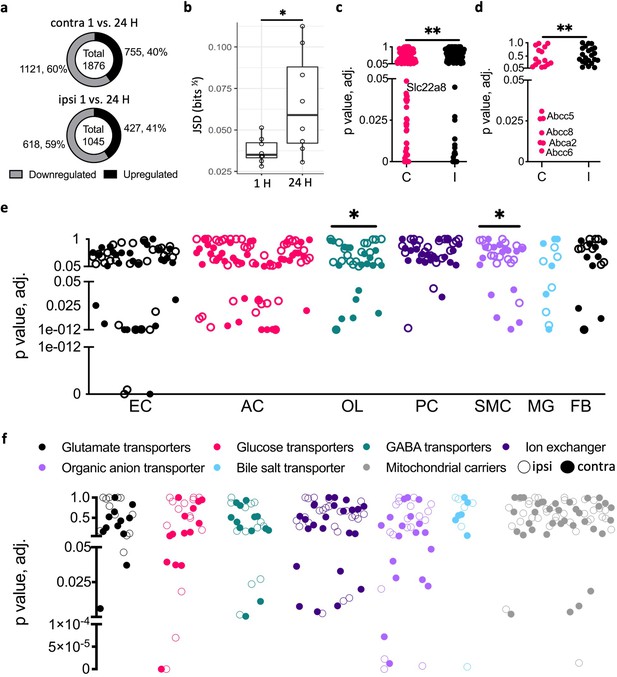
Neuronal activity regulates blood–brain barrier (BBB) transport and neurovascular unit (NVU) cells-specific genes.
(a) Significant differentially expressed genes (DEGs) in the contralateral cortex 24 vs. 1 hr after stimulation (top) and same for the ipsilateral cortex (bottom). (b) Jensen–Shannon divergence (JSD) calculations using normalized counts indicate statistically significant difference between paired contra- and ipsi cortical RNA expression of the rats 24 vs. 1 hr after stimulation (n = 8 each, mean ± SD, p=0.034). (c, d) Scatter plots indicating solute carrier transporters (Slc, c) and ATP-binding cassette transporters (ABC, d) gene expression in the contra- vs. ipsi cortices (C, contra; I, ipsi; Slc n = 331, p=0.0069, ABC n = 45, p=0.0059, chi-square). (e) Scatter plot of NVU cell-specific DEGs in the contra- vs. ipsi cortices of stimulated rats (OL n = 19, p=0.0182, SMC n = 16, p=0.035, chi-square). (f) Scatter plots of Slc transporter genes grouped by families from the ipsi (empty circles) and contra (filled circles) cortices. *p<0.05, **p<0.01.
-
Figure 4—figure supplement 1—source data 1
All data measured and analyzed for Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/89611/elife-89611-fig4-figsupp1-data1-v1.xlsx
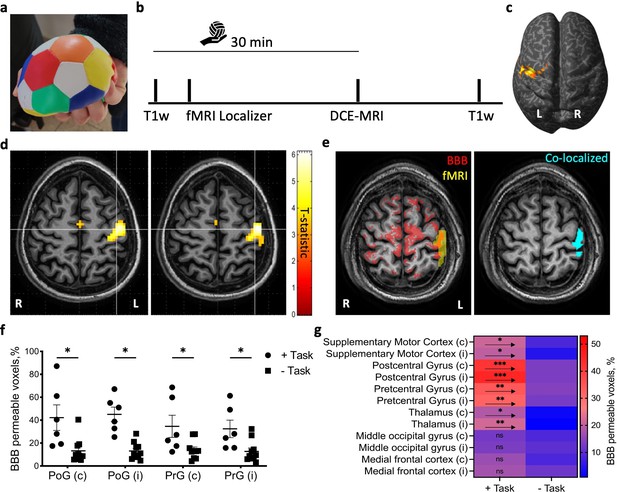
Cortical activation in fMRI co-localizes with blood–brain barrier (BBB) modulation.
(a) Subjects were given an elastic stress ball to squeeze continuously for the length of the session (30 min). (b) Timeline of the experimental protocol for task performance (the hand holding a ball represents the duration of the ball-squeezing task), and MRI sequences (T1-weighted, functional [fMRI], and dynamic contrast-enhanced [DCE] MRI). (c) Activation map (voxels with statistically significant activation [t-statistic]) for the localizer task displayed over the inflated brain of an exemplary subject (p<0.05, family-wise error [FWE] corrected, neurological convention). (d) Activation maps for the localizer task displayed over anatomical axial slices of an exemplary subject (p<0.05, FWE corrected, radiological convention). White lines point to voxels of highest activation (t-statistic). (e) Left: superimposed masks of BBB modulated voxels (red) and fMRI activation (yellow). Right: co-localized voxels map on the same slice (cyan). (f) The percent of voxels with BBB leakage in the primary motor cortex (M1, precentral gyrus, PrG) and primary somatosensory cortex (S1, postcentral gyrus, PoG) for subjects performing the task compared to controls (Task n = 6, controls n = 10, i, ipsi, c, contra, mean ± SEM, *p<0.05, two-way ANOVA with false discovery rate [FDR] correction). (g) Heatmap of BBB modulated voxels percentage (as in f) in motor/sensory-related areas and some non-activated cortical regions of task vs. controls (+Task n = 6, -Task n = 10, i, ipsi, c, contra, mean ± SEM, *p<0.05, **p<0.001, ***p<0.0001, ns, nonsignificant, two-way ANOVA with FDR correction).
-
Figure 5—source data 1
All data measured and analyzed for Figure 5.
- https://cdn.elifesciences.org/articles/89611/elife-89611-fig5-data1-v1.xlsx
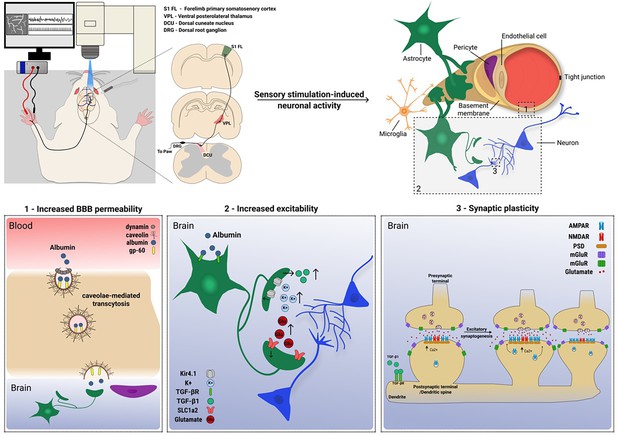
Activity-dependent modulation of blood–brain barrier (BBB) permeability associated with synaptic plasticity.
Top left: experimental procedure illustrating stimulation of the forepaw to elicit neuronal activity in the somatosensory cortex of the rat. Top right: illustration of the neurovascular unit (NVU). Labeled rectangles indicate the hypothesized processes induced by the stimulation. Bottom: (1) stimulation-induced increased BBB permeability manifested by increased CMT of serum albumin. (2) Increased excitability is induced by albumin binding to TGF-β receptors on astrocytes (Weissberg et al., 2015), leading to the secretion of TGF-β1 (Allen and Eroglu, 2017), and downregulation of Kir4.1 channels and glutamate transporters (David et al., 2009; Frigerio et al., 2012), which in turn result in elevated extracellular K+ and glutamate (David et al., 2009; Ivens et al., 2007). (3) Increased excitability leads to synaptic plasticity, indicated by excitatory synaptogenesis (Patel and Weaver, 2021).





