Leucine alleviates cytokine storm syndrome by regulating macrophage polarization via the mTORC1/LXRα signaling pathway
Figures
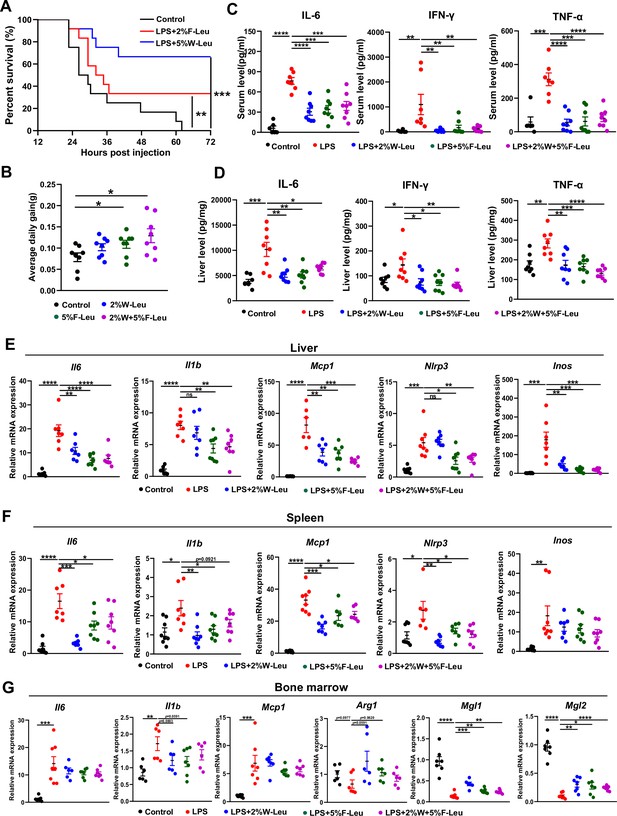
Leucine ameliorates lipopolysaccharide (LPS)-induced inflammation.
(A) Kaplan–Meier curve showing survival of the mice (n = 12). (B) Average daily weight gain of mice (n = 8). (C, D) Measurement of IL-6, IFN-γ, and TNF-α secretion in mouse serum and liver by enzyme-linked immunosorbent assay (ELISA) after treatment with LPS for 6 hr. (E–F) mRNA expression of Il6, Il1β, Nlrp3, Mcp1, and Inos, measured by real-time PCR in the liver and spleen. (G) mRNA expression of Il6, Il1b, Mcp1, Arg1, Mgl1, and Mgl2, measured by real-time PCR in the bone marrow. Student’s t-test was used to determine statistical significance, defined as *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
-
Figure 1—source data 1
Mouse survival curves (Figure 1A).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig1-data1-v1.zip
-
Figure 1—source data 2
Mouse body weight (Figure 1B).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig1-data2-v1.zip
-
Figure 1—source data 3
Mouse serum inflammatory levels (Figure 1C).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig1-data3-v1.zip
-
Figure 1—source data 4
Mouse liver inflammatory levels (Figure 1D).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig1-data4-v1.zip
-
Figure 1—source data 5
mRNA expression of genes in liver (Figure 1E), spleen (Figure 1F), and bone marrow (Figure 1G).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig1-data5-v1.zip
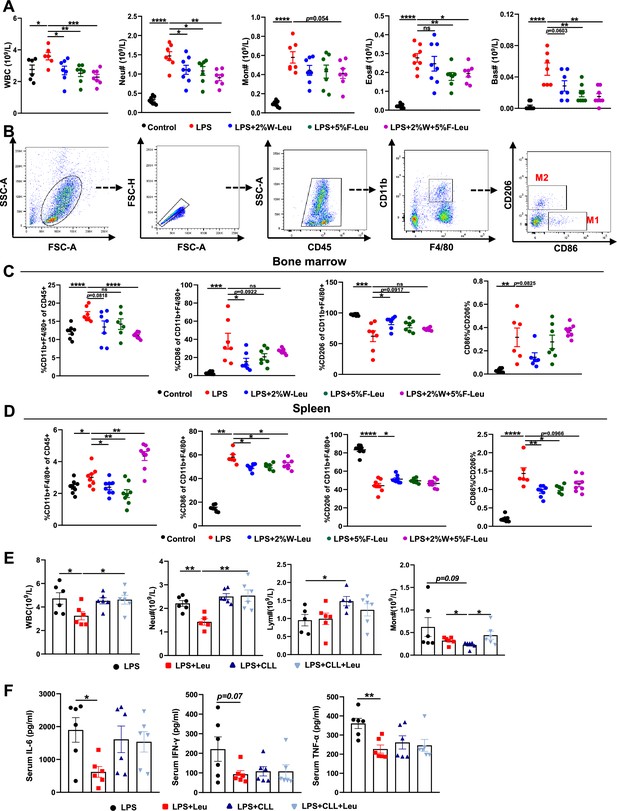
Leucine inhibits M1 polarization and promotes M2 polarization in mice.
(A) White blood cell composition and proportion in mice (n = 8). (B) Gating strategy for macrophage flow cytometry in the bone marrow. (C) Percentages of CD45+, CD86+, CD206+, and CD86+/CD206+, detected by flow cytometry in the bone marrow (n = 8). (D) Percentages of CD45+, CD86+, CD206+, and CD86+/CD206+, detected by flow cytometry in the spleen (n = 8). (E) White blood cell composition and proportion in mice (n = 5–6). (F) Measurement of IL-6, IFN-γ, and TNF-α secretion in mouse serum by enzyme-linked immunosorbent assay (ELISA) after treatment with lipopolysaccharide (LPS) for 6 hr (n = 6). Student’s t-test was used to determine statistical significance, defined as *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
-
Figure 2—source data 1
Blood biochemical indicators (Figure 2A).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig2-data1-v1.zip
-
Figure 2—source data 2
Flow cytometry of bone marrow macrophages (Figure 2C).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig2-data2-v1.zip
-
Figure 2—source data 3
Flow cytometry of spleen macrophages (Figure 2D).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig2-data3-v1.zip
-
Figure 2—source data 4
Blood biochemical indices in clodronate-containing liposome-treated mice (Figure 2E).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig2-data4-v1.zip
-
Figure 2—source data 5
Serum and peritoneal fluid inflammatory factor levels in clodronate-containing liposome-treated mice (Figure 2F).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig2-data5-v1.zip
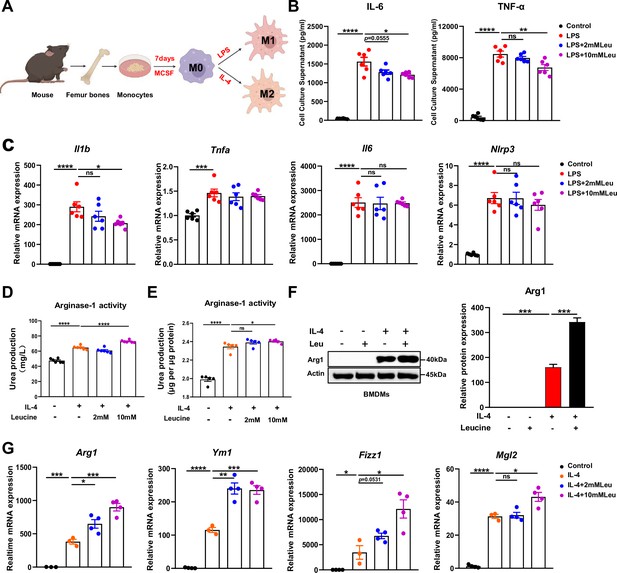
Leucine promotes M2 polarization in BMDMs.
(A) Schematic diagram of macrophage polarization. (B) Measurement of IL-6 and TNF-α secretion in cell culture supernatants by enzyme-linked immunosorbent assay (ELISA) (n = 6). (C) mRNA expression of Il1b, Tnfa, Il6, and Nlrp3, measured by real-time PCR in BMDMs (n = 6). (D, E) Detection of arginase-1 activity in the medium and BMDMs (n = 5–6). (F) BMDMs isolated from mice were stimulated with leucine, IL-4, or both, and the protein expression of Arg1 was determined. (G) mRNA expression of Arg1, Ym1, Fizz1, and Mgl2, measured by real-time PCR in BMDMs (n = 3–4). Student’s t-test was used to determine statistical significance, defined as *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
-
Figure 3—source data 1
Levels of inflammatory factors in cell culture supernatants (Figure 3B).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig3-data1-v1.zip
-
Figure 3—source data 2
mRNA expression of inflammatory genes in BMDMs (Figure 3C).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig3-data2-v1.zip
-
Figure 3—source data 3
Arginase-1 activity in culture media and macrophages (Figure 3D,E).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig3-data3-v1.zip
-
Figure 3—source data 4
Original file for the western blot analysis in Figure 3F.
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig3-data4-v1.zip
-
Figure 3—source data 5
mRNA expression of anti-inflammatory genes in BMDMs (Figure 3G).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig3-data5-v1.zip
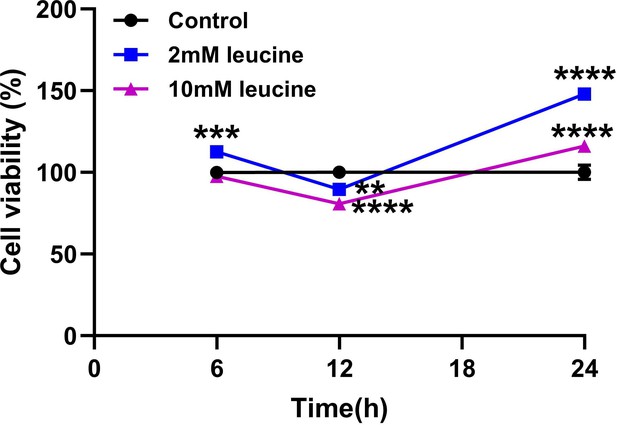
Cell viability.
(A) Cell viability of 2 and 10 mM leucine treatments detected by CCK8 (n = 5). Student’s t-test was used to determine statistical significance, defined as **p < 0.01, ***p < 0.001, and ****p < 0.0001.
-
Figure 3—figure supplement 1—source data 1
The raw data for cell viability with different levels of leucine.
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig3-figsupp1-data1-v1.zip
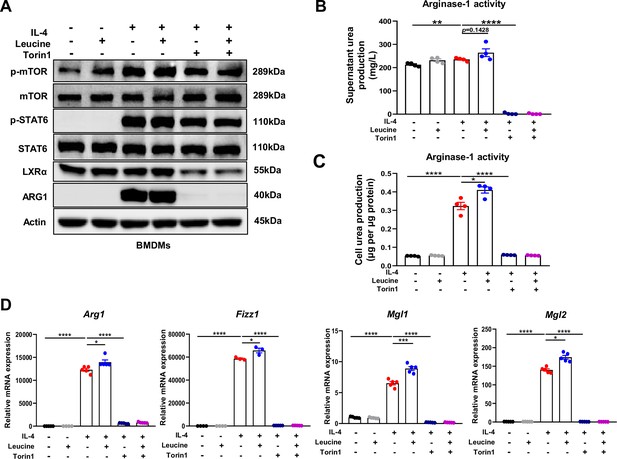
Mechanistic target of rapamycin complex 1 (mTORC1) signaling is necessary for M2 polarization.
(A) Protein levels of ARG1, p-STAT6, STAT6, p-mTOR, and mTOR, determined by western blotting. (B, C) Detection of arginase-1 activity in the medium and BMDMs (n = 4). (D) mRNA expression of Arg1, Fizz1, Mgl1, and Mgl2, measured by real-time PCR in BMDMs (n = 3–5). Student’s t-test was used to determine statistical significance, defined as *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
-
Figure 4—source data 1
Original file for the western blot analysis in Figure 4A.
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig4-data1-v1.zip
-
Figure 4—source data 2
Urea production in medium (Figure 4B).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig4-data2-v1.zip
-
Figure 4—source data 3
Urea production in BMDMs treated with torin1 (Figure 4C).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig4-data3-v1.zip
-
Figure 4—source data 4
mRNA expression of anti-inflammatory genes in BMDMs treated with torin1 (Figure 4D).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig4-data4-v1.zip
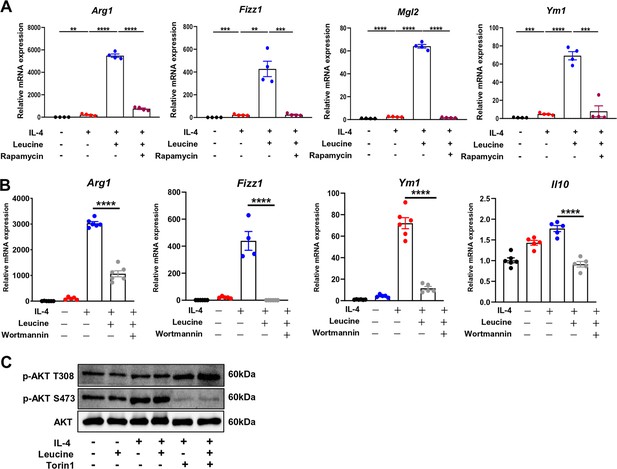
Inhibition of M2 polarization by rapamycin and wortmannin.
(A) mRNA expression of Arg1, Fizz1, Mgl1, and Mgl2 in rapamycin-treated BMDMs measured by real-time PCR (n = 4). (B) mRNA expression of Arg1, Fizz1, Mgl1, and Mgl2 in wortmannin-treated BMDMs measured by real-time PCR (n = 4–6). (C) Protein levels of p-AKT (T308) and p-AKT (S473), determined by western blotting. Student’s t-test was used to determine statistical significance, defined as **p < 0.01, ***p < 0.001, and ****p < 0.0001.
-
Figure 4—figure supplement 1—source data 1
Inhibition of M2 polarization by rapamycin.
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig4-figsupp1-data1-v1.zip
-
Figure 4—figure supplement 1—source data 2
Inhibition of M2 polarization by wortmannin.
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig4-figsupp1-data2-v1.zip
-
Figure 4—figure supplement 1—source data 3
Torin1 affects the phosphorylation of AKT.
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig4-figsupp1-data3-v1.zip
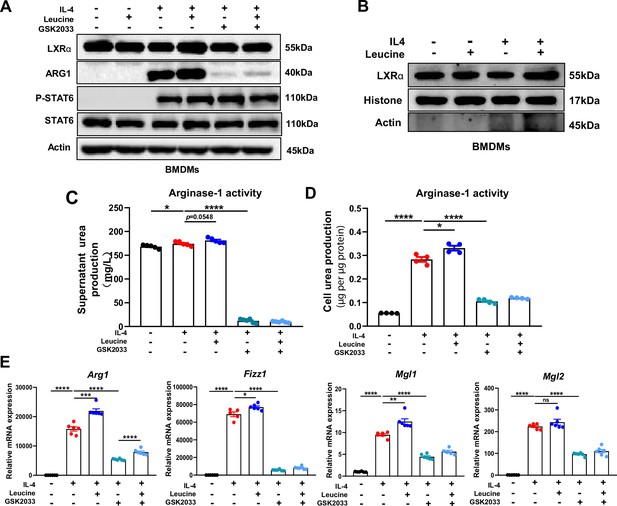
Leucine promotes M2 polarization via mechanistic target of rapamycin complex 1 (mTORC1)/liver X receptor α (LXRα) signaling.
(A) Protein levels of LXRα, ARG1, p-STAT6, and STAT6, determined by western blotting. (B) The nuclear proteins of BMDMs were extracted, and the protein levels of histones and LXRα were determined by western blotting. (C, D) Detection of arginase-1 activity in the medium and BMDMs (n = 4). (E) mRNA expression of Arg1, Fizz1, Mgl1, and Mgl2, measured by real-time PCR in BMDMs (n = 5–6). Student’s t-test was used to determine statistical significance, defined as *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
-
Figure 5—source data 1
Original file for the western blot analysis in Figure 5A.
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig5-data1-v1.zip
-
Figure 5—source data 2
Original file for the western blot analysis in Figure 5B.
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig5-data2-v1.zip
-
Figure 5—source data 3
Urea production in medium treated with GSK2033 (Figure 5B).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig5-data3-v1.zip
-
Figure 5—source data 4
Urea production in BMDMs treated with GSK2033 (Figure 5D).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig5-data4-v1.zip
-
Figure 5—source data 5
mRNA expression of anti inflammatory genes in BMDMs treated with GSK2033 (Figure 5E).
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig5-data5-v1.zip
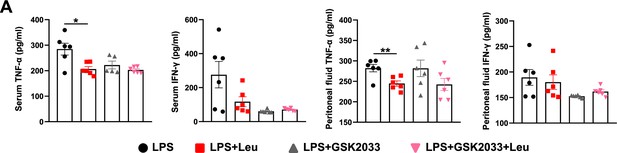
Levels of inflammatory factors in serum and peritoneal fluid of GSK2033-treated mice.
(A) Measurement of IFN-γ and TNF-α secretion in mouse serum and peritoneal fluid by enzyme-linked immunosorbent assay (ELISA) after treatment with lipopolysaccharide (LPS) for 6 hr (n = 6). Student’s t-test was used to determine statistical significance, defined as *p < 0.05 and **p < 0.01.
-
Figure 5—figure supplement 1—source data 1
Levels of inflammatory factors in serum and peritoneal fluid.
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig5-figsupp1-data1-v1.zip

Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels.
(A) AST and ALT levels in serum and liver (n = 6). Student’s t-test was used to determine statistical significance, defined as *p < 0.05, **p < 0.01, and ***p < 0.001.
-
Figure 5—figure supplement 2—source data 1
AST and ALT levels.
- https://cdn.elifesciences.org/articles/89750/elife-89750-fig5-figsupp2-data1-v1.zip
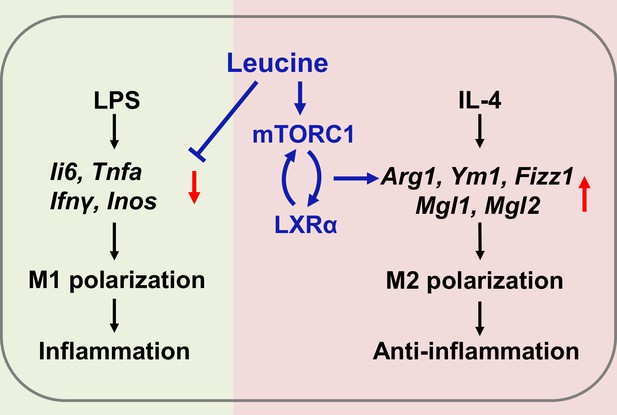
Mechanism of leucine alleviating lipopolysaccharide (LPS)-induced cytokine storm syndrome (CSS) by modulating mechanistic target of rapamycin complex 1 (mTORC1)/liver X receptor α (LXRα) signaling.
In macrophages, LPS promotes M1 polarization to promote the secretion of inflammatory factors leading to inflammation, and IL-4 promotes M2 polarization to alleviate inflammation. Leucine further promotes IL-4-induced M2 polarization by activating mTORC1/LXRα to alleviate inflammation and repair damaged tissues, while leucine also inhibits LPS-mediated M1 polarization and reduces the expression and secretion of inflammatory factors in the organism.
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/89750/elife-89750-mdarchecklist1-v1.docx
-
Supplementary file 1
qPCR primer sequences.
- https://cdn.elifesciences.org/articles/89750/elife-89750-supp1-v1.docx
-
Supplementary file 2
Antibody information.
- https://cdn.elifesciences.org/articles/89750/elife-89750-supp2-v1.docx