Smith–Magenis syndrome protein RAI1 regulates body weight homeostasis through hypothalamic BDNF-producing neurons and neurotrophin downstream signalling
Figures
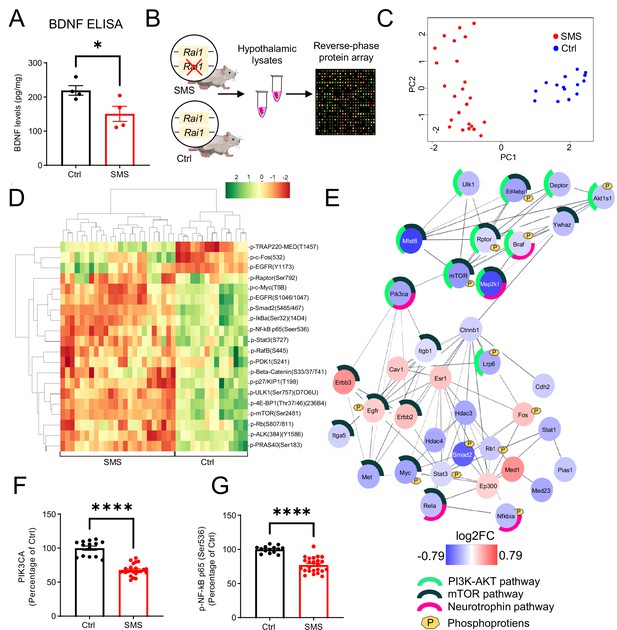
Rai1 haploinsufficiency disrupts hypothalamic proteomic profile in mice.
(A) ELISA showing that brain-derived neurotrophic factor (BDNF) protein levels in hypothalamic tissues were significantly reduced in Smith–Magenis syndrome (SMS) mice compared to controls (n = 4/genotype). *p<0.05, unpaired t-test, two-tailed. (B) A schematic diagram showing the experimental strategy. Hypothalamic tissue lysates from 7-week-old control (n = 5, technical triplicates/sample) and SMS (n = 8, technical triplicates/sample) mice were subjected to reverse-phase protein array (RPPA) that utilizes a cocktail of 240 validated antibodies to probe several key intracellular signalling pathways by quantifying the expression levels of total and phosphorylated proteins. (C) Principal component analysis segregates the SMS and Ctrl groups where PC1 explains 47% of the variance, and PC2 explains 25% of the variance. (D) Heat map showing hierarchical clustering of differentially expressed phospho-proteins: most showed reduced levels in SMS samples. (E) Protein–protein interaction (PPI) analysis showing differentially expressed proteins and phospho-proteins in a crosstalk network. PPI enrichment p-value:<1.0e-16. The nodes represent the proteins/phospho-proteins and the lines indicate physical interactions. Associations with specific molecular pathways are shown as colour-coded outer rings. (F, G) The protein levels of two neurotrophin pathway-related nodes, PIK3CA (F) and phospho-NF-kB (G), were significantly reduced in SMS samples. ****p<0.0001, unpaired t-test, two-tailed.
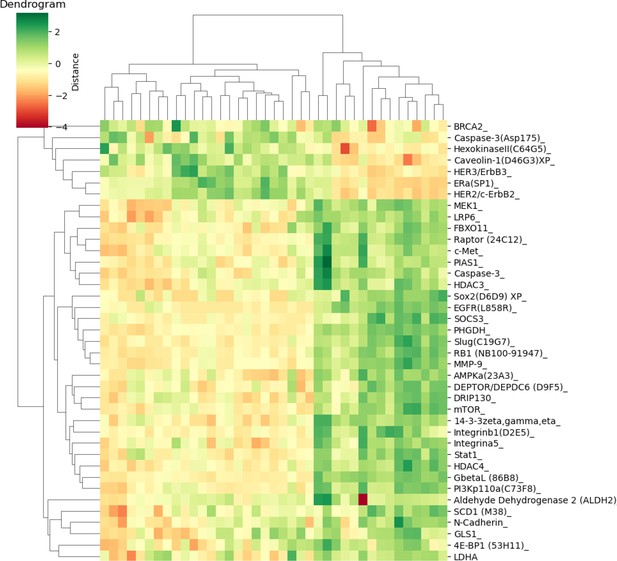
Differentially expressed proteins in the hypothalamus of Smith–Magenis syndrome (SMS) mice.
A heat map showing hierarchical clustering of differentially expressed proteins in the hypothalamus of SMS mice.
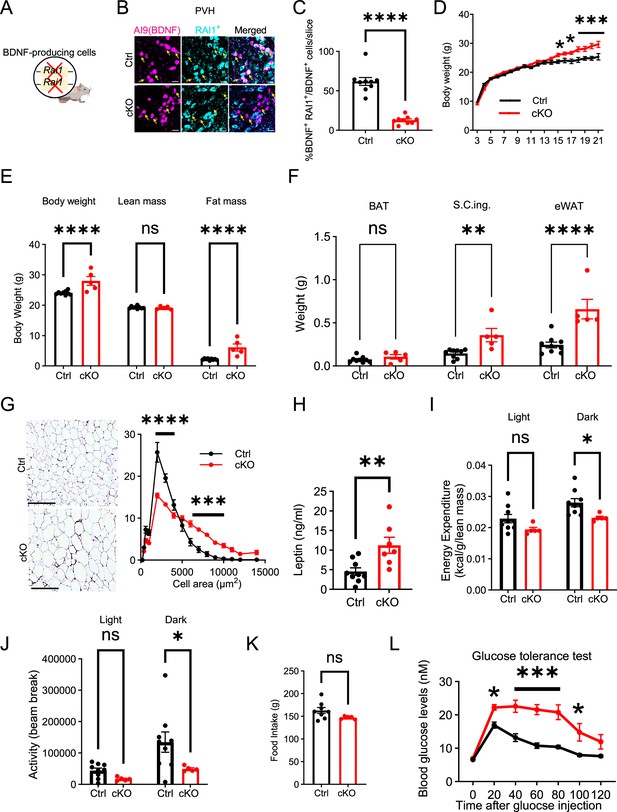
RAI1 is required in brain-derived neurotrophic factor (BDNF)-producing cells to regulate energy metabolism.
(A) A schematic diagram showing selective deletion of Rai1 from BDNF-producing cells in mice. (B) Representative images showing that in Ctrl mice (BdnfCre/+; Ai9), many PVHBDNF neurons (magenta) express RAI1 (cyan, double-positive cells are indicated with yellow arrows). By contrast, PVHBDNF neurons lack RAI1 expression in the conditional knockout (cKO) group (BdnfCre/+; Rai1fl/fl; Ai9). Scale bars = 20 µm. (C) Percentage of PVHBDNF neurons co-expressing RAI1 in Ctrl (n = 3) and cKO (n = 3) mice. unpaired t-test, two-tailed, ****p<0.0001. (D) Female cKO mice (n = 12) showed a significant weight gain when compared to female Ctrl mice (n = 10). Two-way ANOVA with Šidák’s multiple comparisons test. *p<0.05, ***p<0.001. (E) Body composition was measured with echo-MRI, showing an increased fat mass in 26-week-old mice. ns indicates the difference is not significant. Two-way ANOVA with Šidák’s multiple comparisons test. ****p<0.0001. (F) Fat mass of brown adipocytes (BAT), subcutaneous inguinal (S.C.ing) and epididymal white adipose tissue (eWAT) in Ctrl and cKO mice. ns indicates the difference is not significant. Two-way ANOVA with Šidák’s multiple comparisons test. **p<0.01, ****p<0.0001. (G) Representative images showing eWAT adipocyte hypertrophy of the cKO mice (left). Scale bar = 500 µm. Frequency distribution of adipocytes at each cellular size (right) (Ctrl: n = 4, cKO: n = 5). Two-way ANOVA with Šidák’s multiple comparisons test. ***p<0.001, ****p<0.0001. (H) Female cKO mice showed significantly increased blood leptin levels. unpaired t-test, two-tailed, **p<0.01. (I) Female cKO mice showed reduced energy expenditure during the dark phase. ns indicates not significantly different. Two-way ANOVA with Šidák’s multiple comparisons test. *p<0.05. (J) Female cKO mice showed reduced locomotor activity during the dark phase. ns indicates the difference is not significant. Two-way ANOVA with Šidák’s multiple comparisons test. *p<0.05. (K) Female cKO mice showed similar food intake as control mice. ns indicates not significant. unpaired t-test, two-tailed. (L) Glucose tolerance test showing that female cKO mice became hyperglycaemic 20 min after intraperitoneal glucose administration, suggesting glucose intolerance (Ctrl: n = 9, cKO: n = 7). Two-way ANOVA with Šidák’s multiple comparisons test. *p<0.05, ***p<0.001. Data are shown as mean ± SEM.
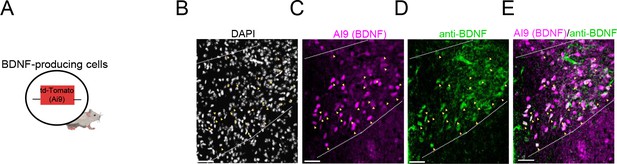
The BdnfCre allele labels brain-derived neurotrophic factor (BDNF)-producing neurons in the paraventricular nucleus of the hypothalamus.
(A) Schematic showing a transgenic mouse expressing BdnfCre-dependent td-Tomato (Ai9) signals in BDNF-expressing cells. (B–E) Colocalization of BdnfCre-dependent td-Tomato signals with endogenous BDNF protein. Black: DAPI, Magenta: BDNF-producing cells; Green: endogenous BDNF. Scale bar: 50 µm, yellow arrowheads indicate Ai9 marked BDNF cells coexpressing endogenous BDNF.
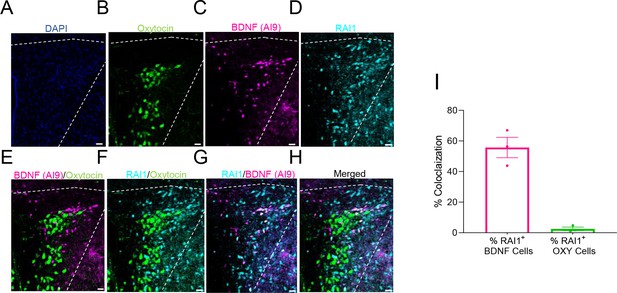
RAI1 expression in brain-derived neurotrophic factor (BDNF)-expressing but not oxytocin-expressing magnocellular paraventricular nucleus of the hypothalamus (PVH) neurons.
(A–H) Representative images of DAPI expression (blue, A), oxytocin (green, B), BDNF-producing cells (Ai9 reporter, magenta, C), and RAI1 protein (cyan, D) in the PVH. Scale bar: 25μm. Note that BDNF-producing neurons are distinct from the oxytocin-expressing neurons (E) and that RAI1 is more selectively expressed in the BDNF-expressing neurons (F–H). (I) Quantification showing the percentage of RAI1+ cells that co-express either BDNF (left bar, magenta) or oxytocin (right bar, green) in the PVH. n = 3 mice/group. Data are shown as mean ± SEM.
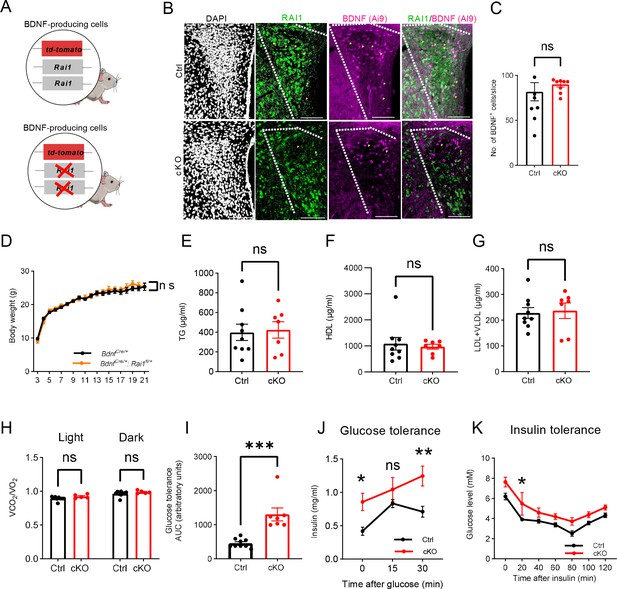
Ablating Rai1 from the brain-derived neurotrophic factor (BDNF)-producing cells induces body weight gain and defective energy homeostasis.
(A) Schematic representation of the conditional knockout mice carrying either normal Rai1 alleles and the Ai9 reporter in BDNF-producing cells (top) or homozygous Rai1 deletion and the Ai9 reporter in the BDNF-producing cells. (B) Representative images showing the expression of DAPI (white), RAI1-expressing cells (green), and BDNF-expressing cells (Ai9 reporter, magenta) in the paraventricular nucleus of the hypothalamus (PVH). The top panel shows the Ctrl group (n = 3 mice) with RAI1-Ai9 co-expression (yellow arrowheads), and the bottom panel shows reduced RAI1 and Ai9 colocalization (yellow arrowheads) (n = 3 mice). Note that RAI1 expression was still detected in non-BDNF neurons in the PVH. (Scale bar: 100μm ). (C) Quantification showing the percentage of BDNF-producing cells was not altered by Rai1 deletion, indicating that RAI1 loss did not impair the generation or survival of PVHBDNF neurons. ns indicates not significantly different, unpaired t-test, two-tailed. (D) The BdnfCre/+ (n=10) and BdnfCre/+; Rai1fl/+ (n=7) female mice had similar body weights. ns indicates not significantly different. Two-way ANOVA with Šidák’s multiple comparisons test. (E–G) Blood parameter analysis of triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein and very low-density lipoprotein (LDL + VLDL) did not differ between Ctrl (n = 9) and conditional knockout (cKO) (n = 7) mice. ns indicates not significantly different, unpaired t-test, two-tailed. (H) The respiratory exchange rate did not differ between Ctrl and cKO mice. ns indicates the difference is not significant. Two-way ANOVA with Šidák’s multiple comparisons test. (I) In the glucose tolerance test, the cKO mice showed an increased area under the curve (AUC). ***p<0.001, unpaired t-test, two-tailed. (J) In the glucose tolerance test, cKO mice showed increased insulin levels at the beginning of the test and 30 min after glucose administration. ns indicates the difference is not significant (Ctrl: n = 9, cKO: n = 7). Two-way ANOVA with Šidák’s multiple comparisons tests, *p < 0.05, **p<0.01. (K) In the insulin tolerance test, blood glucose level is measured after insulin injection (Ctrl: n = 9, cKO: n = 7). ns indicates the difference is not significant. Two-way ANOVA with Šidák’s multiple comparisons test, *p < 0.05. Data are shown as mean ± SEM.
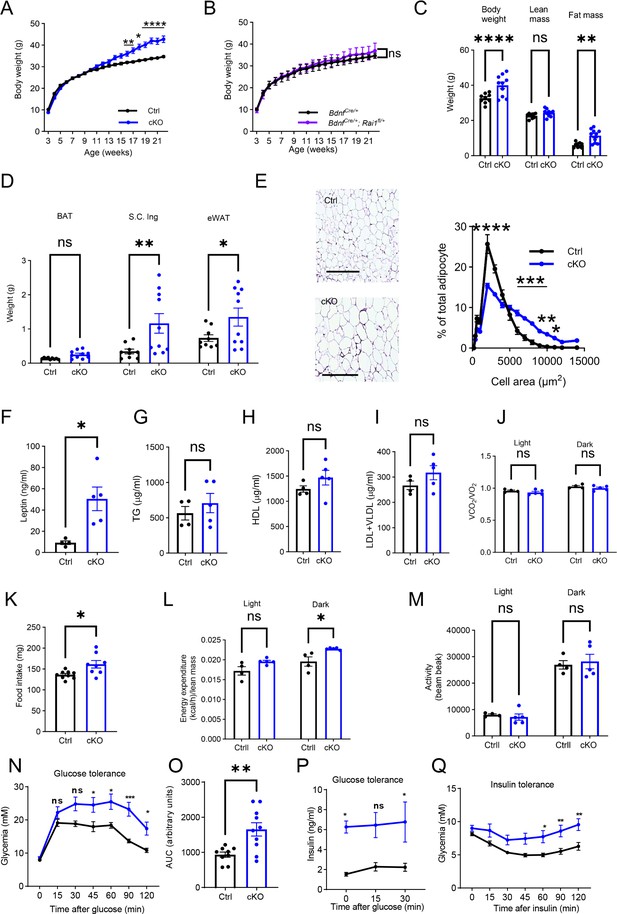
Metabolic profiles of male conditional knockout (cKO) mice lacking RAI1 expression in the brain-derived neurotrophic factor (BDNF)-producing cells.
(A) Male cKO mice gained significantly more weight than Ctrl mice beginning at 15 wk of age. Two-way ANOVA with Šidák’s multiple comparisons test, *p < 0.05, **p<0.01, ****p < 0.0001. Ctrl n = 9, cKO: n = 10. (B) Male BdnfCre/+ (n = 9) and BdnfCre/+; Rai1fl/+ (n = 9) mice had similar body weights. ns indicates the difference is not significant. Two-way ANOVA with Šidák’s multiple comparisons test. (C) Echo-MRI analysis showed that 26-week-old male cKO mice had a significantly increased body weight due to increased fat but not lean mass. ns indicates the difference is not significant. unpaired t-test, two-tailed, **p<0.01, ****p<0.0001. (D) Fat disposition analysis showing the weight of brown adipocytes (BAT), subcutaneous inguinal (S.C.ing), and epididymal white adipose tissues (eWAT). ns indicates the difference is not significant. unpaired t-test, two-tailed, *p<0.05, **p<0.01. (E) Male cKO mice showed eWAT cell hypertrophy. Top: representative images of the eWAT tissues in Ctrl (n = 4) and cKO (n = 5) mice. Scale bar = 500 µm. Bottom: frequency distribution of cellular sizes. Two-way ANOVA with Šidák’s multiple comparisons test, *p < 0.05, **p<0.01, ***p<0.001, ****p < 0.0001. (F–I) Blood parameter analysis showing that male cKO mice showed significantly increased leptin levels (F) without alterations in triglycerides (TG) (G), high-density lipoprotein (HDL) (H), and low-density lipoprotein and very low-density lipoprotein (LDL + VLDL) (I). ns indicates the difference is not significant. unpaired t-test, two-tailed. *p<0.05. (J) No differences were found in the respiratory exchange rate in male Ctrl and cKO mice. ns indicates the difference is not significant. Two-way ANOVA with Šidák’s multiple comparisons test. (K) Male cKO mice showed a significantly increased food intake compared to Ctrl littermates., unpaired t-test, two-tailed, *p<0.05. (L) Male cKO mice showed increased energy expenditure at the dark phase. ns indicates the difference is not significant. Two-way ANOVA with Šidák’s multiple comparisons test, *p<0.05. (M) Male Ctrl and cKO mice showed similar locomotor activities. ns indicates the difference is not significant. Two-way ANOVA with Šidák’s multiple comparisons test. (N–P) Glucose tolerance test shows that cKO male mice are glycaemic 45 min post intraperitoneal glucose administration (N) and had increased area under the curve (AUC) (unpaired t-test, two-tailed) (O), despite significantly higher insulin levels before and 30 min after glucose administration (P), suggesting potential insulin insensitivity. ns indicates the difference is not significant (Ctrl: n = 9, cKO: n = 10). Two-way ANOVA with Šidák’s multiple comparisons tests, *p<0.05, **p < 0.01, ***p<0.001. (Q) Insulin tolerance test showing that male cKO mice showed significantly higher blood glucose levels 60 min after intraperitoneal insulin administration (Ctrl: n = 9, cKO: n = 10). Two-way ANOVA with Šidák’s multiple comparisons test, *p<0.05, **p < 0.01. Data are shown as mean ± SEM.
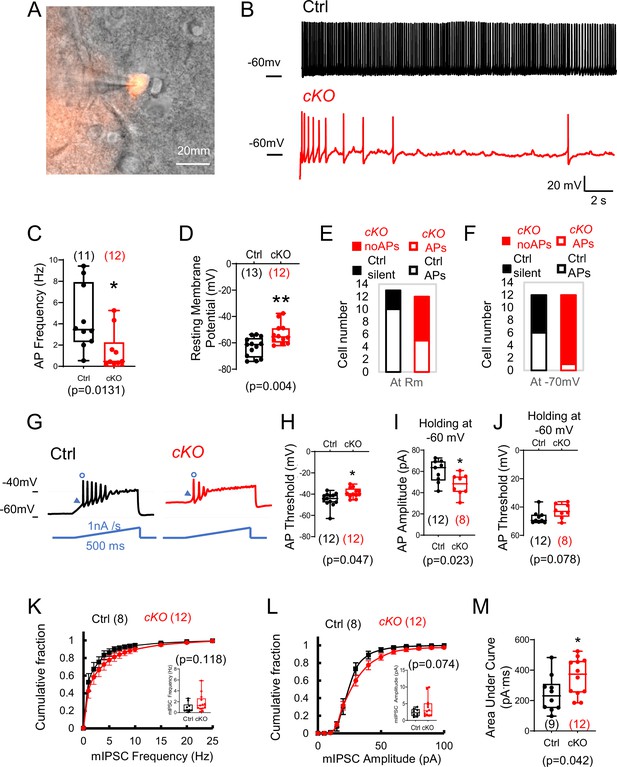
Rai1 loss reduces intrinsic neuronal excitability and enhances inhibitory synaptic transmission of PVHBDNF neurons.
(A) A representative image showing a patched PVHBDNF neuron labelled by BdnfCre-dependent tdTomato fluorescence signals. (B, C) Representative traces of spontaneous firing of control (black) and conditional knockout (cKO) (red) neurons at a holding voltage of –60 mV (B). The average spontaneous action potential (AP) firing frequencies are shown in (C). *p<0.05, unpaired Student’s t-tests. (D) The resting membrane potentials of control (black) and cKO (red) PVHBDNF neurons. **p<0.01, unpaired Student’s t-tests. (E, F) The number of control (black) and cKO (red) PVHBDNF neurons that showed spontaneous firing (open) or are silent (solid) at resting membrane potentials (E) and a holding voltage of –70 mV (F). (G) Representative traces of elicited APs in control (black) and cKO (red) PVHBDNF neurons responding to an inject current (bottom, blue), which ramps up from the resting membrane potentials at 1 nA/s for 500 ms. (H) The threshold of the first AP initiated by a current ramp in control (black) and cKO (red) PVHBDNF neurons. *p<0.05, unpaired Student’s t-tests. (I, J) The amplitude (I) and threshold (J) for the first AP firing at the holding voltage of –60 mV in control (black) and cKO (red) PVHBDNF neurons. *p<0.05, unpaired Student’s t-tests. (K) The cumulative fractions of miniature inhibitory postsynaptic current (mIPSC) frequency were measured in control (black) and cKO (red) PVHBDNF neurons. The average frequency of events from a 2 min stable recording of each neuron is shown in the inset, unpaired Student’s t-tests. (L) The cumulative fractions of mIPSC amplitude of control (black) and cKO (red) PVHBDNF neurons. The average amplitude of events from a 2 min stable recording of each neuron is normalized to its capacitance and shown in the inset, unpaired Student’s t-tests. (M) The area under the curve of each mIPSC event from a 2 min recording is averaged. Controls are shown in black, and cKOs are shown in red. unpaired t-tests, two-tailed. *p<0.05. Data are shown as mean ± SEM. Statistics: neuron numbers are in brackets (more than three mice/genotype).
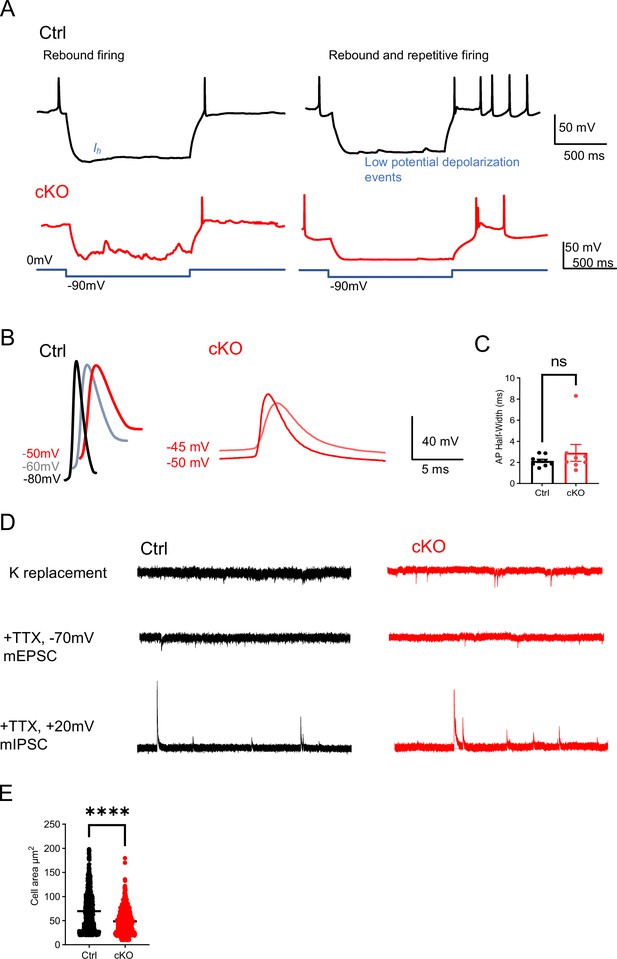
The cellular and synaptic properties of control and Rai1-deficient PVHBDNF neurons.
(A) Representative traces of rebound and rebound repetitive firing of PVHBDNF neurons in control (black) and conditional knockout (cKO) (red). (B) Representative AP waveforms at holding voltages of –80 mV, –60 mV, and –50 mV in control (left) and –50 mV and –45 mV in cKO (right). (C) The half-width of action potential (AP) initiated at a holding voltage of –60 mV (neuronal numbers in brackets, ns indicates the difference is not significant, unpaired t-test, two-tailed). (D) Representative traces of miniature recordings in control (black) and cKO (red) PVHBDNF neurons. The traces were recorded with a Cs-based internal at a holding voltage of –70 mV in artificial cerebrospinal fluid (aCSF) (top), spontaneous miniature excitatory postsynaptic currents (mEPSCs) were recorded in aCSF containing 1 uM TTX at a holding voltage of –70 mV (middle), and miniature inhibitory postsynaptic currents (mIPSCs) were recorded at holding voltage of +10 mV (bottom). (E) Quantification showing that the cellular size of cKO PVHBDNF neurons was significantly smaller than Ctrl neurons (Ctrl: n = 3 mice, cKO: n = 3 mice). unpaired t-test, two-tailed. ****p<0.0001.
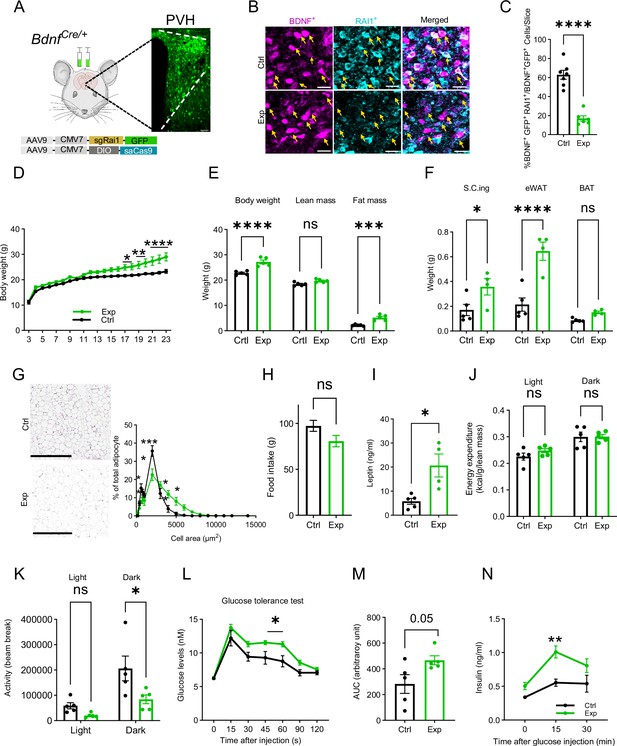
Selective Rai1 ablation in PVHBDNF neurons induces obesity.
(A) A schematic showing stereotaxic injection of adeno-associated viruses (AAVs) expressing Cas9 protein and single-guide RNAs targeting Rai1 (sgRai1) to delete Rai1 from the PVHBDNF neurons of BdnfCre/+ female mice. Scale bar = 50 µm. (B) Representative images showing that RAI1 immunoreactivity (cyan) was dramatically reduced in BdnfCre/+; Ai9 mice injected with both sgRai1 and DIO-saCas9 viruses (Exp group n = 3, bottom row) into the paraventricular nucleus of the hypothalamus (PVH). In contrast, many PVHBDNF neurons in BdnfCre/+; Ai9 mice injected only with the sgRai1 virus showed RAI1 expression (Ctrl group n = 3, top row). (C) The percentage of PVHBDNF neurons co-expressing RAI1 and GFP (virus) was significantly reduced in the Exp group, indicating a successful Rai1 deletion. unpaired t-test, two-tailed, ****p<0.0001 (D) PVHBDNF neuron-specific Rai1 deletion induced body weight gain in Exp mice (Ctrl group: n = 5, Exp group: n = 5). Two-way ANOVA with Šidák’s multiple comparisons test. *p<0.05, **p < 0.01, ****p < 0.0001. (E) Body composition was measured with Echo-MRI, showing an increased fat mass deposition in 26-week-old Exp mice. ns indicates the difference is not significant. Two-way ANOVA with Šidák’s multiple comparisons test. ***p<0.001, ****p < 0.0001. (F) Fat deposition analysis shows that the Exp group has significantly more subcutaneous inguinal (S.C.ing) and epididymal white adipose tissue (eWAT) mass (grams). ns indicates the difference is not significant. Two-way ANOVA with Šidák’s multiple comparisons test. *p<0.05, ****p < 0.0001. (G) Representative images showing eWAT adipocyte hypertrophy of the Exp group (left). Scale bar = 500 µm. Frequency distribution of adipocytes at each cellular size (right). ns indicates not significantly different. Two-way ANOVA with Šidák’s multiple comparisons test. *p<0.05, ***p<0.001. (H) Exp and control mice showed similar food intake. ns indicates not significantly different, unpaired t-test, two-tailed. (I) Blood leptin levels were significantly increased in the Exp mice. unpaired t-test, two-tailed, *p<0.05. (J) Exp and control mice showed similar energy expenditure. ns indicates the difference is not significant. Two-way ANOVA with Šidák’s multiple comparisons test. (K) Exp mice showed reduced locomotor activity in the dark phase. ns indicates the difference is not significant. Two-way ANOVA with Šidák’s multiple comparisons test. *p<0.05. (L) Exp mice became hyperglycaemic during the glucose tolerance test. ns indicates the difference is not significant. Two-way ANOVA with Šidák’s multiple comparisons test. *p<0.05. (M) Measurement of the area under the curve (AUC) in Exp and Ctrl mice during the glucose tolerance test. unpaired t-test, two-tailed, p=0.05. (N) Exp mice showed increased plasma insulin levels during the glucose tolerance test. Two-way ANOVA with Šidák’s multiple comparisons test, **p<0.01. Data are shown as mean ± SEM.
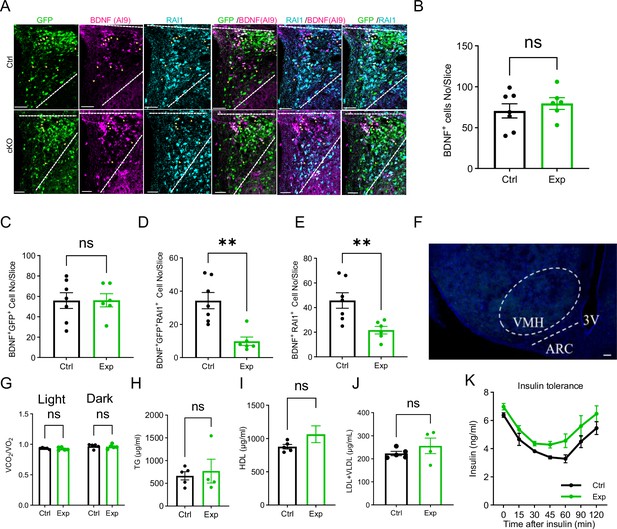
Metabolic profile of mice lacking Rai1 in PVHBDNF neurons.
(A) Representative images showing co-expression of virus-infected cells (GFP/green), BdnfCre-dependent Ai9 signals (brain-derived neurotrophic factor [BDNF] cells, magenta), and endogenous RAI1 (cyan). Yellow arrowheads indicate BDNF neurons infected with adeno-associated viruses (AAV) and in these cells, RAI1 signals were diminished in Exp but not Ctrl mice. Scale bar = 50 µm. (B, C) The total number of PVHBDNF neurons (B) and total number of PVHBDNF neurons infected with AAVs (C) per slice show that Rai1 deletion or virus infection did not alter the total number of PVHBDNF neurons. ns indicates the difference is not significant. unpaired t-test, two-tailed. (D) Rai1 expression was significantly reduced in PVHBDNF neurons infected with both AAVs compared to PVHBDNF neurons infected with sgRai1-GFP AAV alone. unpaired t-test, two-tailed, **p<0.01. (E) The number of PVHBDNF neurons that co-express RAI1 was significantly reduced in the Exp group. unpaired t-test, unpaired, **p<0.01. (F) Representative image showing lack of viral expression in the PVH neighbouring nuclei. ARC: arcuate nucleus. VMH: ventromedial nucleus of the hypothalamus. 3V: third ventricle. Scale bar = 50 µm. (G) The respiratory exchange rate remained similar in Ctrl and Exp mice (Ctrl: n = 5, Exp n = 4). ns indicates the difference is not significant. Two-way ANOVA, Šídák’s multiple comparisons test.(H–J) Blood parameter analysis showing triglycerides (TG), high-density lipoprotein (HDL), and low-density lipoprotein and very low-density lipoprotein (LDL + VLDL) levels were similar in Ctrl and Exp mice (Ctrl: n = 5, Exp n = 4). ns indicates the difference is not significant. unpaired t-test, two-tailed. (K) In the insulin tolerance test, the Exp group showed a trend towards increased blood glucose levels after insulin injection. (Ctrl: n = 5, Exp n = 4). Two-way ANOVA, Šídák’s multiple comparisons test. Data are shown as mean ± SEM.

LM22A-4 treatment partially alleviates obesity and stereotypic behaviour in adult Smith–Magenis syndrome (SMS) mice.
(A) Representative western blot showing p-AKT, total AKT, histone 3 (H3, a loading control) levels in the hypothalamus of saline-treated Ctrl, saline-treated SMS, and LM22A-4-treated SMS mice. (B) Quantification showing deficits in p-AKT levels of the hypothalamus were reversed by LM22A-4 treatment in SMS mice. Unpaired t-test, two-tailed, *p<0.05. (C–E) Body weight of saline-injected control (n = 8), saline-injected SMS (n = 8), and LM22A-4-injected SMS mice (n = 10). Saline or LM22A-4 treatment periods are highlighted in orange. ns indicates not significantly different. Two-way ANOVA with Šidák’s multiple comparisons test. *p<0.05, **p < 0.01, ****p < 0.0001. (F) Food intake (in grams) of saline-injected control, saline-injected SMS, and LM22A-4-injected SMS mice. ns indicates the difference is not significant. unpaired t-test, two-tailed, *p<0.05, **p < 0.01. (G) Locomotor activity (number of beam breaks) for saline-injected control, saline-injected SMS, and LM22A-4 injected SMS mice. ns indicates the difference is not significant. unpaired t-test, two-tailed, *p<0.05. (H) Fat deposition analysis shows that the saline-injected SMS group has significantly more subcutaneous inguinal (S.C.ing) and epididymal white adipose tissue (eWAT) mass (grams) than saline-injected Ctrl mice. S.C.ing and eWAT mass in LM22A-4-injected mice is comparable to the saline-injected mice. Two-way ANOVA with Šidák’s multiple comparisons test. **p < 0.01, ***p<0.001, ****p < 0.0001. (I) High-density lipoprotein (HDL) levels were restored to normal in LM22A-4-injected SMS mice. ns indicates the difference is not significant. unpaired t-test, two-tailed, **p < 0.01, ***p<0.001. (J) Insulin levels in saline-injected control, saline-injected SMS, and LM22A-4-injected SMS mice right before the start of the insulin tolerance test (time point 0). ns indicates the difference is not significant. unpaired t-test, two-tailed, *p<0.05. (K) Insulin tolerance test performed on saline-injected control (n = 8), saline-injected SMS ( n = 7), and LM22A-4-injected SMS mice (n = 7), 2 wk post daily administration of LM22A-4 or saline, shows reductions in the blood glucose levels of LM22A-4 treated SMS mice. * shows significance between saline-injected Ctrl and saline-injected SMS group. ns indicates the difference is not significant. Two-way ANOVA with Šidák’s multiple comparisons test. **p<0.01. (L) Stereotypic rearing behaviour was fully rescued in LM22A-4-treated SMS mice. ns indicates the difference is not significant. unpaired t-test, two-tailed. *p<0.05, **p < 0.01. Data are shown as mean ± SEM.
-
Figure 5—source data 1
Original uncropped western blot images for Figure 5.
- https://cdn.elifesciences.org/articles/90333/elife-90333-fig5-data1-v1.zip
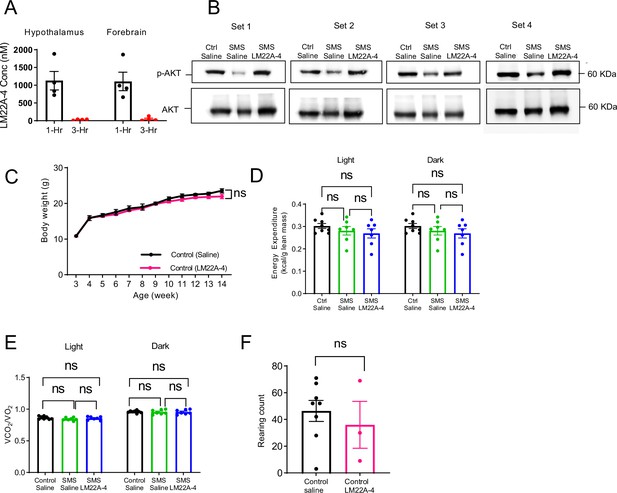
LM22A-4 treatment does not disrupt energy homeostasis and repetitive rearing in the Ctrl mice.
(A) LM22A-4 concentration in the hypothalamus and forebrain of Smith–Magenis syndrome (SMS) mice 1 hr (black dots) and 3 hr (red dots) after simultaneous intranasal and IP injections. (B) Four independent sets of western blotting analyses showing reduced p-AKT levels in the hypothalamus of saline-treated SMS mice, which were increased by LM22A-4 treatment. Total AKT protein was used as a control. (C) Similar body weight of Ctrl mice treated with either saline or LM22A-4 (n = 8). ns indicates the difference is not significant. Two-way ANOVA, Šídák’s multiple comparisons test. (D) Energy expenditure was not different between groups in the light vs. dark phase. ns indicates the difference is not significant. Two-way ANOVA, Šídák’s multiple comparisons test. (E) Respiratory exchange rates were not different between groups in the light vs. dark phase. ns indicates the difference is not significant. Two-way ANOVA, Šídák’s multiple comparisons test. (F) Repetitive rearing behaviour in Ctrl mice was not altered by LM22A-4 treatment. ns indicates the difference is not significant. unpaired t-test, two-tailed. Data are shown as mean ± SEM.
-
Figure 5—figure supplement 1—source data 1
Original uncropped western blot images for Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/90333/elife-90333-fig5-figsupp1-data1-v1.zip
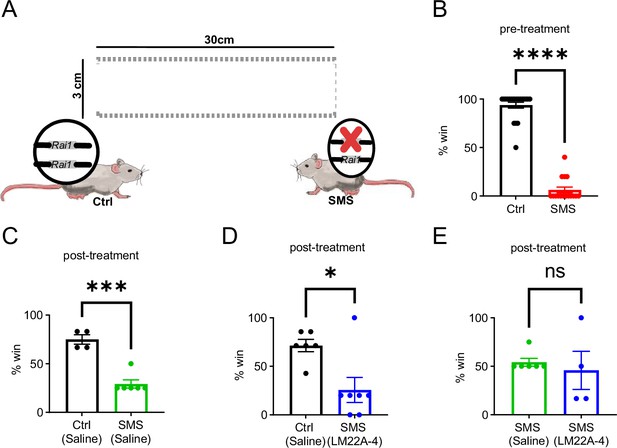
LM22A-4 treatment in adult Smith–Magenis syndrome (SMS) mice is insufficient to improve social interaction deficit.
(A) Schematic showing the tube test that assesses social interaction between stranger mice of different genotypes. (B) 7-week-old Ctrl mice won significantly more than SMS mice before the LM22A-4 treatment. unpaired t-test, two-tailed. ****p<0.0001. (C) 10-week-old saline-injected Ctrl mice won significantly more than the saline-injected SMS mice (2 wk post-LM22A-4 treatment). unpaired t-test, two-tailed, ***p<0.001. (D) 10-week-old saline-injected Ctrl mice won significantly more than LM22A-4 injected SMS mice. unpaired t-test, two-tailed, *p<0.05. (E) 10-week-old saline-injected SMS mice showed a similar winning rate to LM22A-4-injected SMS mice. ns indicates the difference is not significant. unpaired t-test, two-tailed. Data are shown as mean ± SEM.





