Genetic inactivation of zinc transporter SLC39A5 improves liver function and hyperglycemia in obesogenic settings
Figures
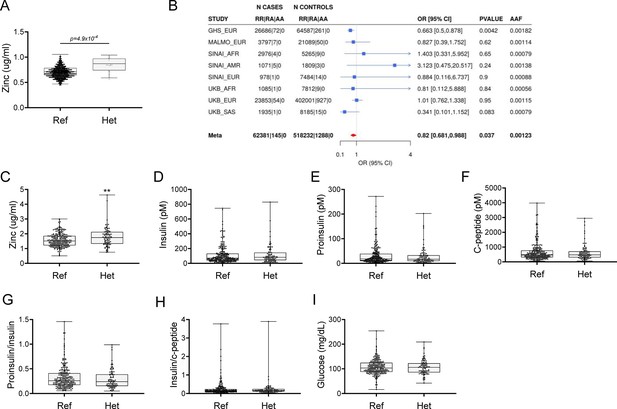
Rare putative LOF (pLOF) variants in SLC39A5 are associated with elevated serum zinc and nominal protection against type II diabetes (T2D).
(A) Serum zinc in carriers of SLC39A5 pLOF variants in the discovery cohort. Controls (Ref; SLC39A5+/+) and heterozygous carriers of pLOF variant alleles in SLC39A5 (Het; SLC39A5+/-). Subject numbers: Ref and Het, respectively: n=5317 and n=15. (B) Trans-ancestry meta-analysis of the association of SLC39A5 pLOF variants with T2D. (C–I) Serum zinc and insulin profile of age, sex and BMI-matched controls in serum call back study. Subject numbers: Ref and Het, respectively: n=246–253 and n=86–91, **p<0.01, unpaired t-test. Numeric data is summarized in Supplementary file 1.
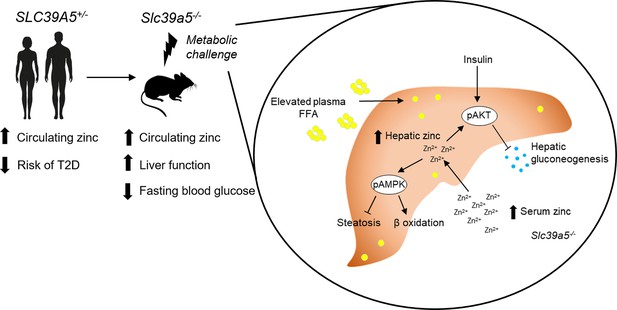
Graphic abstract.
Heterozygous loss-of-function mutations in SLC39A5 are associated with elevated circulating zinc levels and nominal reduction in type II diabetes risk in humans. Loss of Slc39a5 results in elevated circulating and hepatic zinc levels in mice. Mice lacking Slc39a5 function are protected against hepatic steatosis and hyperglycemia resulting from diet-induced obesity or leptin-receptor deficiency and display reduced hepatic inflammation and fibrosis resulting from diet-induced non-alcoholic steatohepatitis (NASH). Loss of Slc39a5 function results in hepatic AMPK and AKT activation.
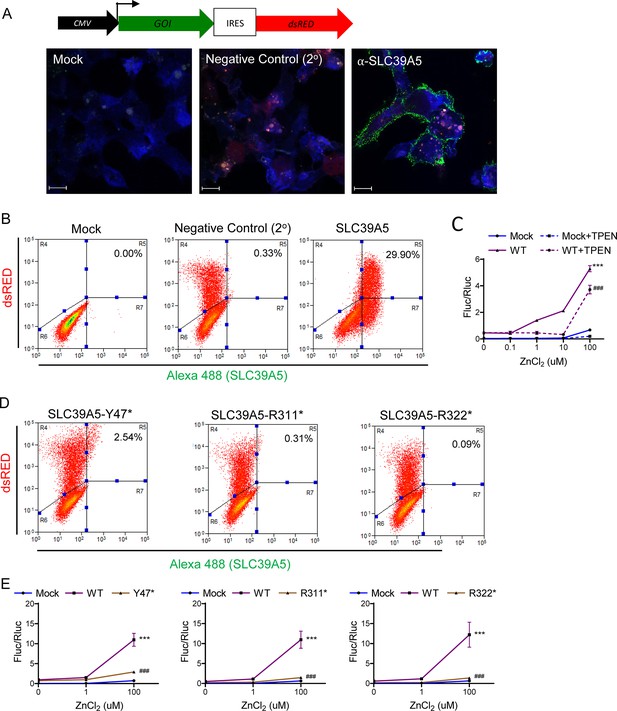
SLC39A5 putative LOF (pLOF) variants p.Y47*(c.141C>G), p.R311*(c.931C>T), and p.R322*(c.964C>T) encode for non-functional proteins.
HEK293 cell transfected with expression constructs encoding SLC39A5 wild-type (WT), Y47*, R311*, and R322* variants. (A, B) Immunostaining and FACS analysis demonstrating WT SLC39A5 localization to the cell surface. Scale bar, 10 um. (C) Overexpression of WT SLC39A5 results in Zn2 + mediated MRE activation in a dose-dependent manner, n=4. (D) FACS analyses demonstrate that cell surface expression of SLC39A5 Y47*, R311*, and R322* muteins is markedly reduced. (E) Variants Y47*, R311*, and R322* did not mediate Zn2 + induction of MRE, n=8, Statistical comparison to Mock and WT, respectively: ***p<0.001, ###p<0.001, two-way ANOVA with post hoc Tukey’s test. Metal regulatory element (MRE), firefly luciferase (Fluc), renilla luciferase (Rluc), cytomegalovirus (CMV), gene of interest (GOI), internal ribosome entry site (IRES).
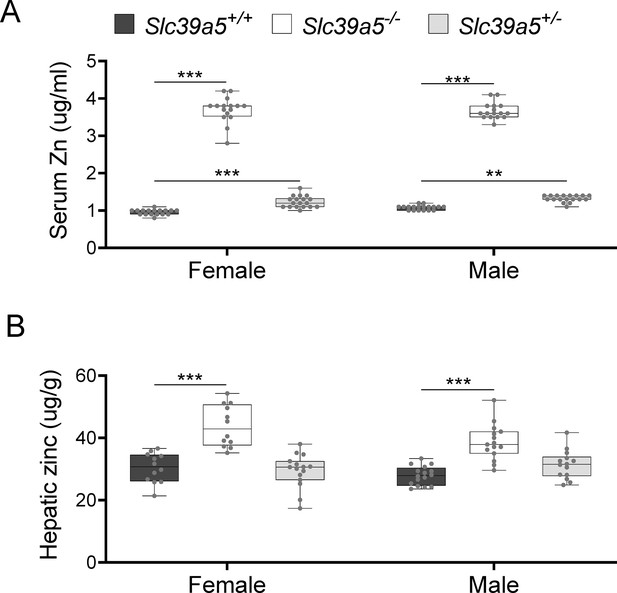
Loss of Slc39a5 results in elevated circulating and hepatic zinc levels in mice.
Serum zinc (A) and hepatic zinc (B) in Slc39a5+/+, Slc39a5-/-, and Slc39a5+/-mice at 40 wk of age, n=16–18. **p<0.01, ***p<0.001, two-way ANOVA with post hoc Tukey’s test.
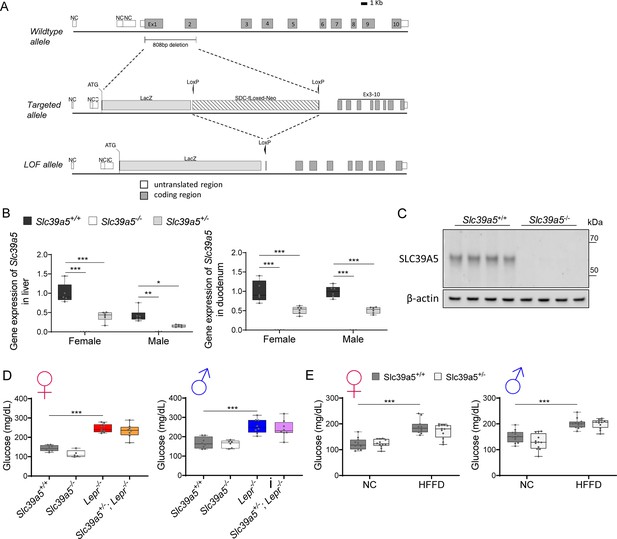
Generation and characterization of the Slc39a5-/- mice.
(A) Schematic representation of the Slc39a5 null allele. (B) Slc39a5 gene expression in liver and duodenum of Slc39a5-/- mice at 20 wk of age, n=3–6. (C) Immunoblotting analyses demonstrating an absence of SLC39A5 protein in the liver of Slc39a5-/- mice at 34 wk of age. (D–E) Heterozygous loss of Slc39a5 does not reduce fasting blood glucose in (D) Lepr-/- mice (at 20 wk of age) and in (E) mice challenged with high-fat high fructose diet (HFFD) for 18 wk. *p<0.05, **p<0.01, ***p<0.001, two-way ANOVA with post hoc Tukey’s test.
-
Figure 2—figure supplement 1—source data 1
Original files of the full raw uncropped, unedited blots.
- https://cdn.elifesciences.org/articles/90419/elife-90419-fig2-figsupp1-data1-v1.zip
-
Figure 2—figure supplement 1—source data 2
Figures with the uncropped blots with the relevant bands clearly labelled.
- https://cdn.elifesciences.org/articles/90419/elife-90419-fig2-figsupp1-data2-v1.zip
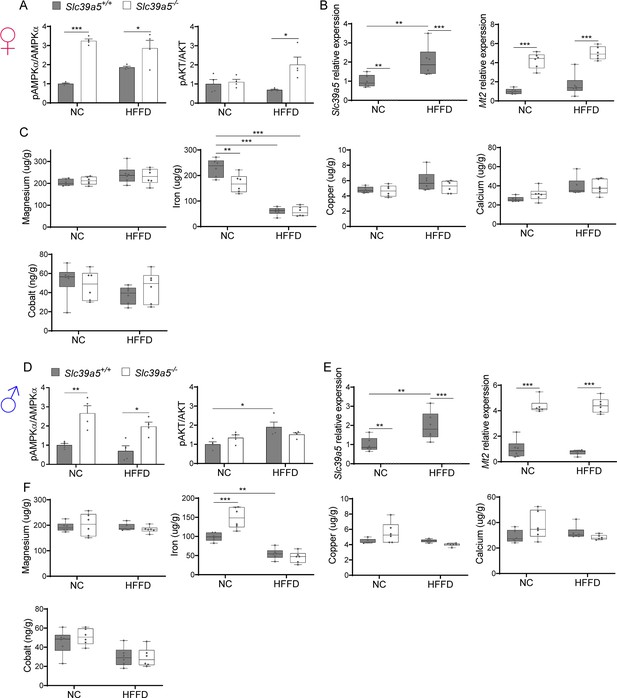
Loss of Slc39a5 does not alter hepatic magnesium, iron, copper, calcium, and cobalt levels in mice challenged with high-fat high fructose diet (HFFD).
Female (A–C) and male (D–F) mice were fed HFFD or NC for 30 wk. (A, D) Densitometric analysis of hepatic AMPK and AKT. (B, E) Hepatic gene expression of Slc39a5 and Mt2. (C, F) Hepatic ion quantification by flame atomic absorption spectrometry. *p<0.05, **p<0.01, ***p<0.001, two-way ANOVA with post hoc Tukey’s test.
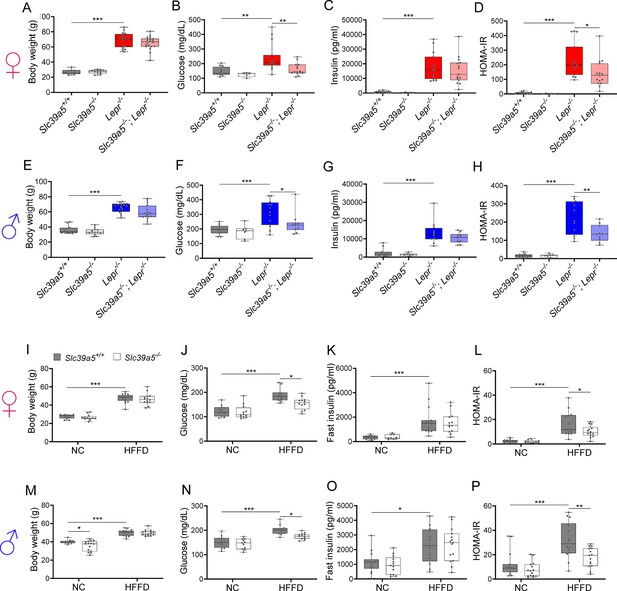
Loss of Slc39a5 improves glycemic traits in leptin-receptor deficient mice and in mice challenged with high-fat high fructose diet (HFFD).
Female (A-D, I-L; ♀) and Male (E-H, M-P; ♂) mice. (A–H) Slc39a5-/-;Lepr-/- and corresponding control mice. (A, E) Body weight at 34 wk. (B, F) Fasting blood glucose at 34 wk. (C, G) Fasting insulin at 34 wk. (D, H) Homeostatic model assessment for insulin resistance (HOMA-IR) at 34 wk. Slc39a5+/+ and Slc39a5-/- (n=5–12), Lepr -/- and Slc39a5 -/-; Lepr -/- (n=10–15). *p<0.05, **p<0.01, ***p<0.001, one-way ANOVA with post hoc Tukey’s test. (I–P) Slc39a5-/- and Slc39a5+/+ mice were fed HFFD or NC for 30 wk. (I, M) Body weight at 30 wk. (J, N) Fasting blood glucose at 30 wk. (K, O) Fasting insulin at 30 wk. (L, P) HOMA-IR at 30 wk, n=11–15. *p<0.05, **p<0.01, ***p<0.001, two-way ANOVA with post hoc Tukey’s test. Numeric data is summarized in Supplementary file 4 and Supplementary file 5.
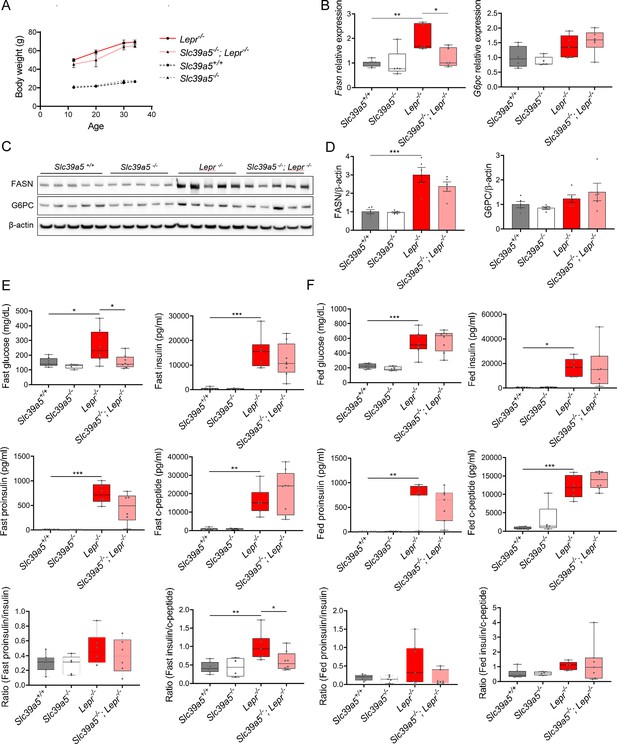
Metabolic profiling of female Slc39a5-/-; Lepr-/- mice.
(A) Longitudinal body weight. (B–D) Analyses were done on explanted liver samples collected after 16 hr of fasting at 34 wk of age. (B) Hepatic expression of fatty acid synthase (Fasn) and glucose-6-phosphatase (G6c). (C) Hepatic FASN and G6PC protein Serum insulin profile upon fasting at 34 wk of levels. (D) Densitometric analysis of hepatic FASN and G6PC. (F) Serum insulin profile in fed state at 32 wk of age. *p<0.05, **p<0.01, ***p<0.001, ANOVA with post hoc Tukey’s test.
-
Figure 3—figure supplement 1—source data 1
Original files of the full raw uncropped, unedited blots.
- https://cdn.elifesciences.org/articles/90419/elife-90419-fig3-figsupp1-data1-v1.zip
-
Figure 3—figure supplement 1—source data 2
Figures with the uncropped blots with the relevant bands clearly labelled.
- https://cdn.elifesciences.org/articles/90419/elife-90419-fig3-figsupp1-data2-v1.zip
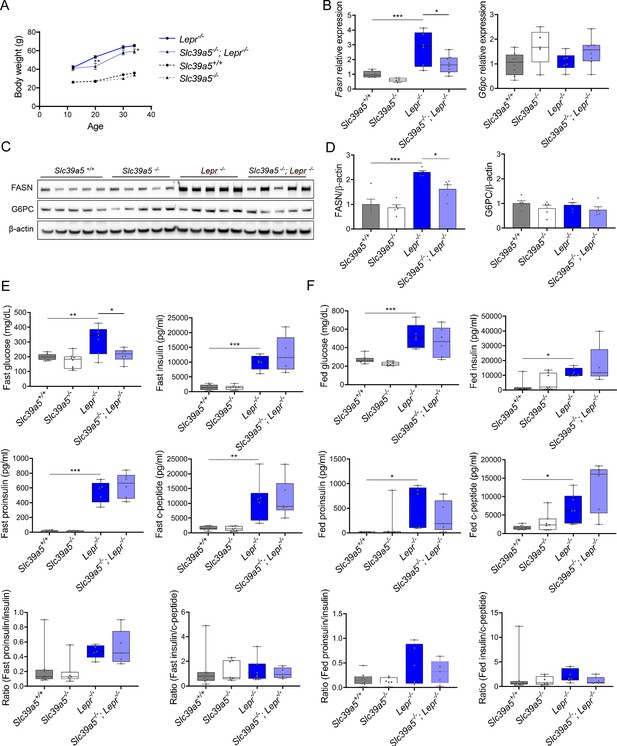
Loss of Slc39a5 reduces hepatic fatty acid synthase expression but does not change the insulin profile of male Lepr-/- mice.
(A) Longitudinal body weight. (B–D) Analyses were done on explanted liver samples collected after 16 hr of fasting at 34 wk of age. (B) Hepatic expression of fatty acid synthase (Fasn) and glucose-6-phosphatase (G6pc). (C) Hepatic FASN and G6PC protein levels. (D) Densitometric analysis of hepatic FASN and G6PC. (E) Serum insulin profile upon fasting at 34 wk of age. (F) Serum insulin profile in fed state at 32 wk of age. *p<0.05, **p<0.01, ***p<0.001, ANOVA with post hoc Tukey’s test.
-
Figure 3—figure supplement 2—source data 1
Original files of the full raw uncropped, unedited blots.
- https://cdn.elifesciences.org/articles/90419/elife-90419-fig3-figsupp2-data1-v1.zip
-
Figure 3—figure supplement 2—source data 2
Figures with the uncropped blots with the relevant bands clearly labelled.
- https://cdn.elifesciences.org/articles/90419/elife-90419-fig3-figsupp2-data2-v1.zip
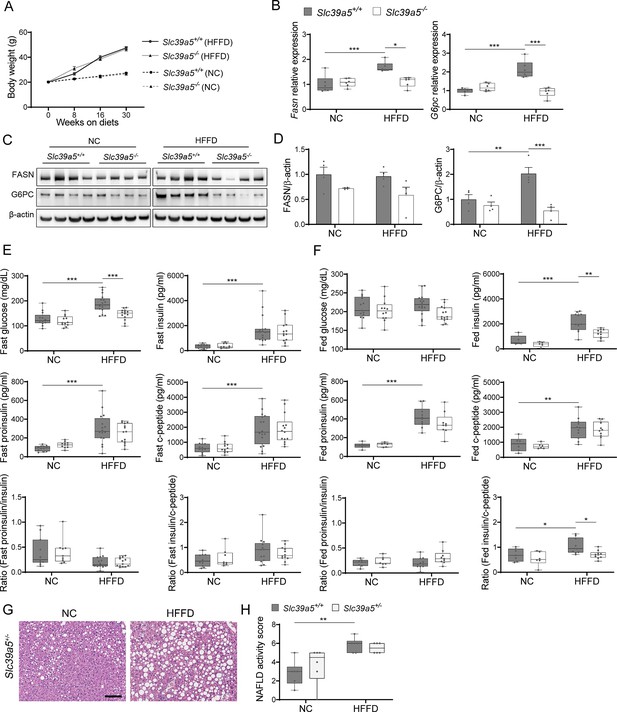
Loss of Slc39a5 reduces hepatic fatty acid synthase expression but does not change insulin profile in female mice challenged with high-fat high fructose diet (HFFD).
(A) Longitudinal body weight during dietary intervention. (B–D) Analyses were done on explanted liver samples collected after 16 hr of fasting in mice fed HFFD or NC for 30 wk. (B) Hepatic expression of fatty acid synthase (Fasn) and glucose-6-phosphatase (G6pc). (C) Hepatic FASN and G6PC protein levels. (D) Densitometric analysis of hepatic FASN and G6PC. (E) Fasting serum insulin profile. (F) Fed serum insulin profile. (G) Representative images of Slc39a5+/- livers stained with hematoxylin & eosin (H&E). Scale bar, 100 um. (H) Non-alcoholic fatty liver disease (NAFLD) activity score. *p<0.05, **p<0.01, ***p<0.001, two-way ANOVA with post hoc Tukey’s test.
-
Figure 3—figure supplement 3—source data 1
Original files of the full raw uncropped, unedited blots.
- https://cdn.elifesciences.org/articles/90419/elife-90419-fig3-figsupp3-data1-v1.zip
-
Figure 3—figure supplement 3—source data 2
Figures with the uncropped blots with the relevant bands clearly labelled.
- https://cdn.elifesciences.org/articles/90419/elife-90419-fig3-figsupp3-data2-v1.zip
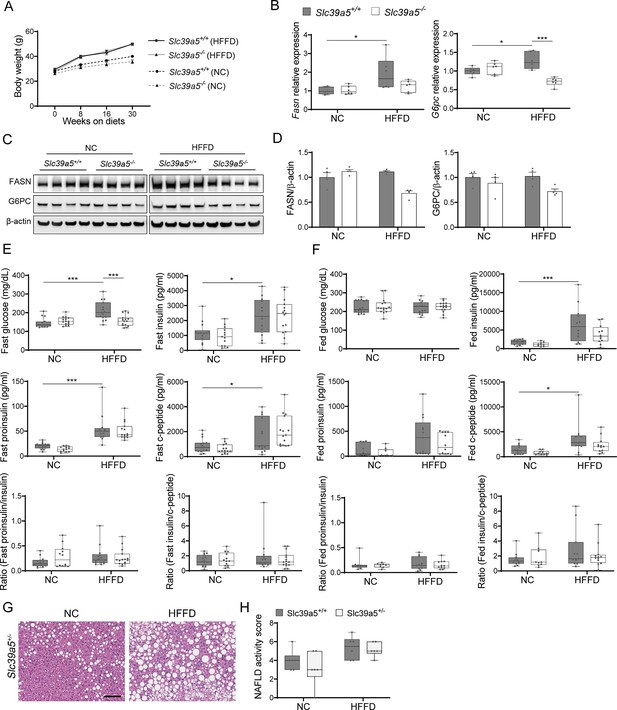
Loss of Slc39a5 reduces hepatic fatty acid synthase expression but does not change insulin profile in male mice challenged with high-fat high fructose diet (HFFD).
(A) Longitudinal body weight during dietary intervention. (B–D) Analyses were done on explanted liver samples collected after 16 hr of fasting in mice fed HFFD or NC for 30 wk. (B) Hepatic expression of fatty acid synthase (Fasn) and glucose-6-phosphatase (G6pc). (C) Hepatic FASN and G6PC protein levels. (D) Densitometric analysis of hepatic FASN and G6PC. (E) Fasting serum insulin profile. (F) Fed serum insulin profile. (G) Representative images of Slc39a5+/- livers stained with H&E. Scale bar, 100 μm. (H) Non-alcoholic fatty liver disease (NAFLD) activity score. *p<0.05, **p<0.01, ***p<0.001, two-way ANOVA with post hoc Tukey’s test.
-
Figure 3—figure supplement 4—source data 1
Original files of the full raw uncropped, unedited blots.
- https://cdn.elifesciences.org/articles/90419/elife-90419-fig3-figsupp4-data1-v1.zip
-
Figure 3—figure supplement 4—source data 2
Figures with the uncropped blots with the relevant bands clearly labeled.
- https://cdn.elifesciences.org/articles/90419/elife-90419-fig3-figsupp4-data2-v1.zip
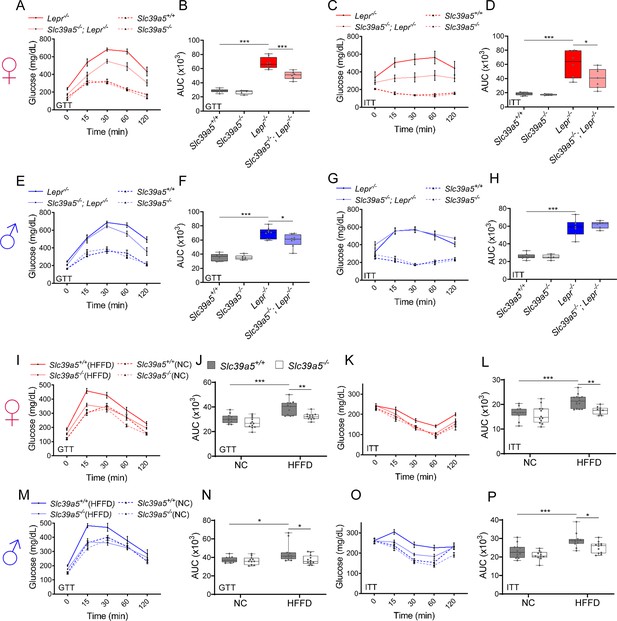
Loss of Slc39a5 improves glycemic traits in Lepr-/- mice and in mice challenged with high-fat high fructose diet (HFFD).
Female (A-D, I-L; ♀) and Male (E-H, M-P; ♂) mice. (A–H) Slc39a5-/-; Lepr-/- and corresponding control mice. (A–B, E–F) Oral glucose tolerance test (GTT) after 16 hr of fasting, at 20 wk. (C–D, G–H) Intraperitoneal insulin tolerance test (ITT), at 33 wk. n=5–8. *p<0.05, **p<0.01, ***p<0.001, one-way ANOVA with post hoc Tukey’s test. (I–P) HFFD mice. (I–J, M–N) GTT after 16 hr of fasting, at 18 wk. (K-L, O–P) ITT after 4 hr fasting, at 27 wk. n=11–12. *p<0.05, **p<0.01, ***p<0.001, two-way ANOVA with post hoc Tukey’s test. Area under the curve (AUC). Numeric data is summarized in Supplementary file 4 and Supplementary file 5.
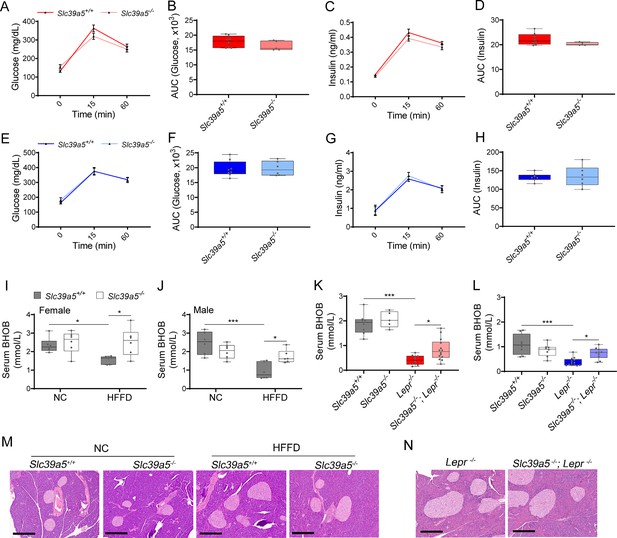
Additional data of glucose-stimulated insulin secretion, serum hepatic beta-hydroxybutyrate (BHOB), and pancreas histology in mouse models.
(A–H) Oral glucose tolerance test (GTT) was performed in Slc39a5-/- female (A–D) and male (E–H) mice after 16- hr of fasting, at 15 wk. Glucose (A–B, E–F) and insulin (C–D, G–H) levels were measured at 0, 15, and 60 mins. n=6–8. (I–J) Serum BHOB levels in female (I) and male (J) mice challenged with highfat high fructose diet (HFFD). *p<0.05, ***p<0.001, two-way ANOVA with post hoc Tukey’s test. (K–L) Serum BHOB levels in Slc39a5-/-; Lepr-/- female (K) and male (L) mice. *p<0.05, ***p<0.001, one-way ANOVA with post hoc Tukey’s test. (M–N) No overt morphological deficits in the pancreas resulting from Slc39a5 deficiency. Scale bar, 300 µm.
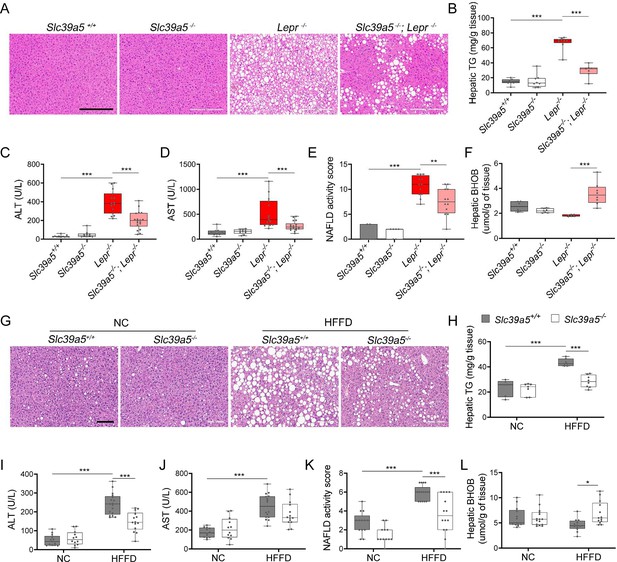
Loss of Slc39a5 improves liver function and steatosis in leptin-receptor deficient female mice and in female mice challenged with high-fat high fructose diet (HFFD).
Slc39a5-/-;Lepr-/- and corresponding control mice (A–F) were sacrificed after 16 hr fasting at 34 wk of age. (G–L) Slc39a5-/- and Slc39a5+/+ mice were fed HFFD or NC for 30 wk and sacrificed after 16 hr of fasting. (A, G) Representative images of livers stained with H&E. Scale bar, 200 µm. (B, H) Hepatic triglyceride (TG) content in explanted liver samples at an endpoint. (C, I) Serum ALT. (D, J) Serum AST. (E, K) Non-alcoholic fatty liver disease (NAFLD) activity score, (F, L) Hepatic beta-hydroxybutyrate (BHOB). *p<0.05, **p<0.01, ***p<0.001, Slc39a5-/-;Lepr-/- and corresponding control mice: one-way ANOVA with post hoc Tukey’s test, HFFD or NC: two-way ANOVA with post hoc Tukey’s test. Numeric data is summarized in Supplementary file 4 and Supplementary file 5.
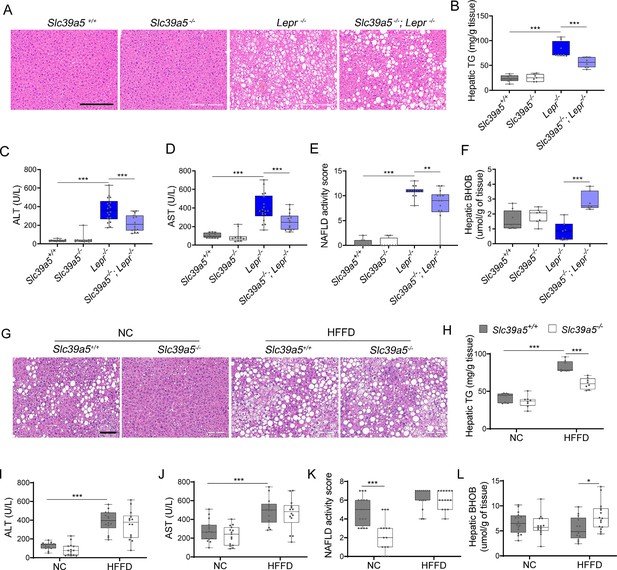
Loss of Slc39a5 improves liver function and steatosis in Lepr-/- male mice and reduces hepatic triglyceride in male mice challenged with high-fat high fructose diet (HFFD).
Slc39a5-/-; Lepr-/- and corresponding control mice (A–F) were sacrificed after 16 hr fasting at 34 wk of age. (G–L) Slc39a5-/- and corresponding control mice were fed HFFD or NC for 30 wk and sacrificed after 16 hr of fasting. (A, G) Representative images of livers stained with H&E. Scale bar, 200 µm. (B, H) Hepatic triglyceride (TG) content in explanted liver samples at an endpoint. (C, I) Serum ALT. (D, J) Serum AST. (E, K) NAFLD activity score, (F, L) Hepatic beta-hydroxybutyrate (BHOB). *p<0.05, **p<0.01, ***p<0.001, Slc39a5-/-; Lepr-/- mice: one-way ANOVA with post hoc Tukey’s test, HFFD: two-way ANOVA with post hoc Tukey’s test. Numeric data is summarized in Supplementary file 4 and Supplementary file 5.
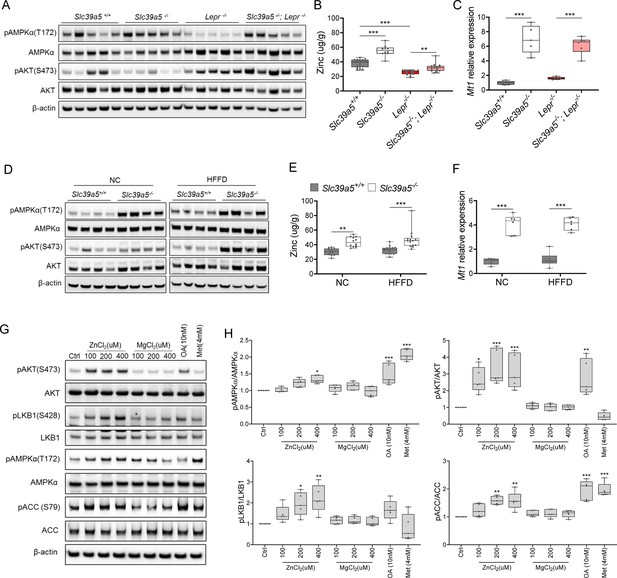
Loss of Slc39a5 results in elevated hepatic zinc and activation of hepatic AMPK signaling in leptin-receptor deficient female mice and female mice challenged with high-fat high fructose diet (HFFD).
Analyses were done on explanted liver samples collected after 16 hr of fasting at an endpoint in Lepr-/- (A–C) and HFFD mice (D–F). (A, D) Immunoblot analysis of hepatic AMPK and AKT activation. AMPK and AKT signaling is activated in Lepr-/-; Slc39a5-/- mice and HFFD Slc39a5-/- mice (compared to their Scl39a5+/+ counterparts). (B, E) Hepatic zinc is elevated in Lepr-/-; Slc39a5-/- mice and HFFD Slc39a5-/- mice (n=10–21). (C, F) Elevated hepatic zinc results in increased Mt1 (zinc responsive gene) expression in both models. (G) Immunoblot analysis of primary human hepatocytes treated with zinc chloride (ZnCl2), and magnesium chloride (MgCl2), okadaic acid (OA), metformin (Met) for 4 hr. Zinc-activated AMPK and AKT signaling in primary human hepatocytes. (H) Densitometric analysis of immunoblots (compared to control). *p<0.05, **p<0.01, ***p<0.001, ANOVA with post hoc Tukey’s test.
-
Figure 5—source data 1
Original files of the full raw uncropped, unedited blots.
- https://cdn.elifesciences.org/articles/90419/elife-90419-fig5-data1-v1.zip
-
Figure 5—source data 2
Figures with the uncropped blots with the relevant bands clearly labeled.
- https://cdn.elifesciences.org/articles/90419/elife-90419-fig5-data2-v1.zip
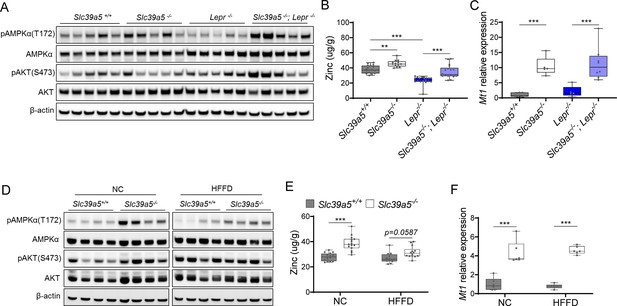
Loss of Slc39a5 results in elevated hepatic zinc and activation of hepatic AMPK signaling in congenital and diet-induced obesity models.
Analyses were done on explanted liver samples collected from male mice after 16 hr of fasting at an endpoint of congenital (A–C) and diet-induced obesity (D–F). (A, D) Immunoblot analysis of hepatic AMPK and AKT activation. (B, E) Hepatic zinc measurements (n=10–21). (C, F) Hepatic Mt1 gene expression. *p<0.05, **p<0.01, ***p<0.001, ANOVA with post hoc Tukey’s test.
-
Figure 5—figure supplement 1—source data 1
Original files of the full raw uncropped, unedited blots.
- https://cdn.elifesciences.org/articles/90419/elife-90419-fig5-figsupp1-data1-v1.zip
-
Figure 5—figure supplement 1—source data 2
Figures with the uncropped blots with the relevant bands clearly labeled.
- https://cdn.elifesciences.org/articles/90419/elife-90419-fig5-figsupp1-data2-v1.zip
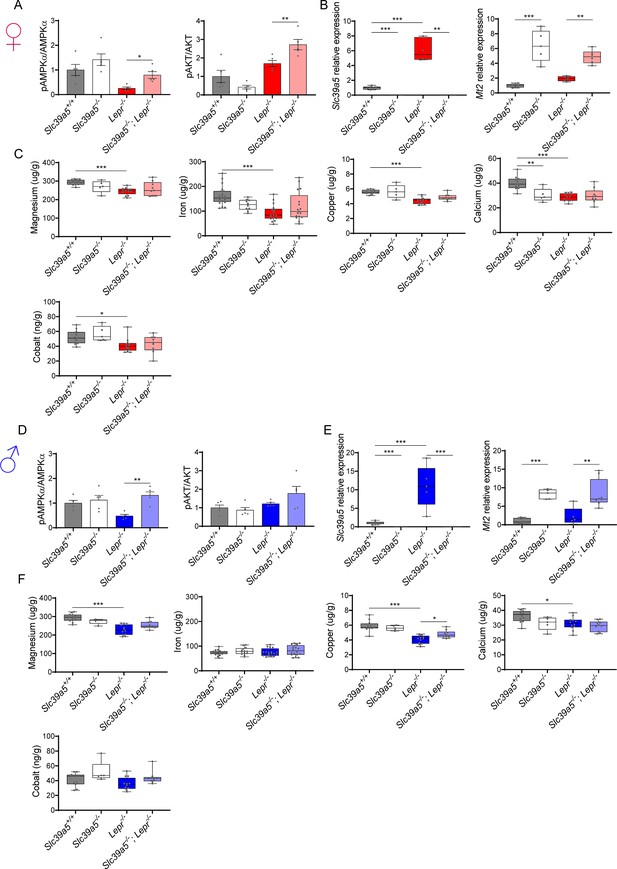
Loss of Slc39a5 does not alter hepatic magnesium, iron, copper, calcium, and cobalt levels in Lepr-/- mice.
Female (A–C) and male (D–F) mice were examined at 34 wk of age. (A, D) Densitometric analysis of hepatic AMPK and AKT signaling. (B, E) Hepatic expression of Slc39a5 and Mt2. (C, F) Hepatic ion quantification by flame atomic absorption spectrometry. *p<0.05, **p<0.01, ***p<0.001, ANOVA with post hoc Tukey’s test.
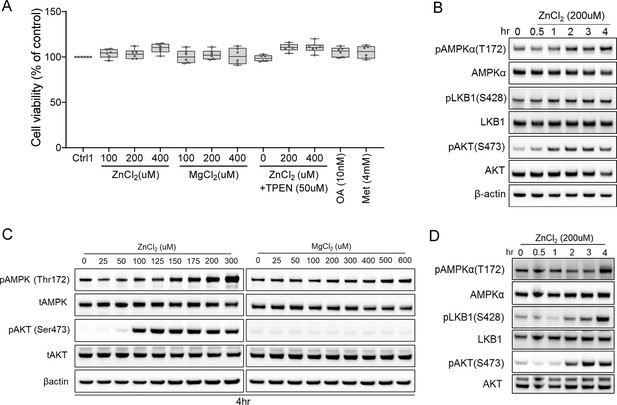
Zinc activates AMPK and AKT signaling in a time-dependent and dose-dependent manner.
(A) No differences in cell viability were observed in human primary hepatocytes (after 4 hr treatment) across different experimental groups. (B) Time-resolved (0–4 hr) immunoblotting analyses of primary human hepatocytes treated with zinc chloride. (C) Immunoblots of HepG2 were treated with zinc chloride (ZnCl2) and magnesium chloride (MgCl2). (D) Time-resolved (0–4 hr) immunoblotting analyses of HepG2 treated with zinc chloride. Okadaic acid (OA), metformin (Met), N,N,N',N'-Tetrakis(2-pyridylmethyl)ethylenediamine (TPEN).
-
Figure 5—figure supplement 3—source data 1
Original files of the full raw uncropped, unedited blots.
- https://cdn.elifesciences.org/articles/90419/elife-90419-fig5-figsupp3-data1-v1.zip
-
Figure 5—figure supplement 3—source data 2
Figures with the uncropped blots with the relevant bands clearly labeled.
- https://cdn.elifesciences.org/articles/90419/elife-90419-fig5-figsupp3-data2-v1.zip
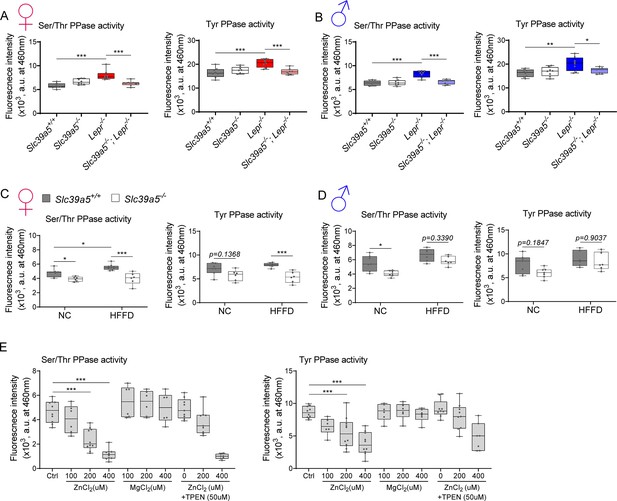
Elevated hepatic zinc results in reduced protein phosphatase activity.
Analyses were done on explanted liver samples collected after 16 hr fast at the endpoint of congenital obesity (A–B) and diet-induced obesity (C–D) challenges. Female (A, C) and Male (B, D) mice. (A–D) Ser/Thr and Tyr protein phosphatase activity. (E) Ser/Thr and Tyr protein phosphatase activity in primary human hepatocytes treated with zinc chloride (ZnCl2), magnesium chloride (MgCl2), and N,N,N',N'-Tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) for 4 hr. *p<0.05, **p<0.01, ***p<0.001, ANOVA with post hoc Tukey’s test.
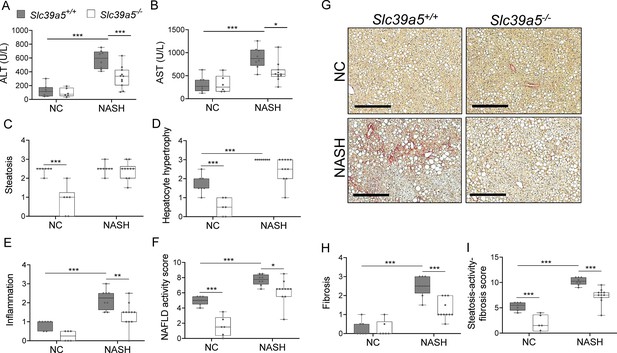
Loss of Slc39a5 improves hepatic inflammation and fibrosis in female mice challenged with diet-induced non-alcoholic steatohepatitis (NASH).
Slc39a5-/- and Slc39a5+/+ mice were placed on a NASH-inducing diet or NC for 40 wk and sacrificed after 16 hr of fasting. (A, B) NASH Slc39a5-/- mice display reduced serum ALT and AST levels. (C–E) Histology scores for steatosis, hepatocyte hypertrophy, and inflammation. (F) NAFLD activity score was reduced in NASH Slc39a5-/- mice. (G–I) NASH Slc39a5-/- mice display reduced fibrosis. (G) Representative images of explanted livers sample stained with picrosirius red indicative of collagen deposition. Scale bar, 300 µm. (H, I) Fibrosis and steatosis-activity-fibrosis scores. n=6–7 (NC) and 8–11 (NASH), *p<0.05, **p<0.01, ***p<0.001, two-way ANOVA with post hoc Tukey’s test. Numeric data is summarized in Supplementary file 6.
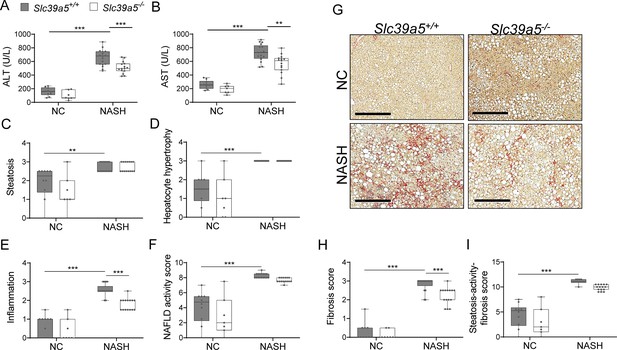
Loss of Slc39a5 reduces hepatic inflammation and fibrosis in male mice challenged with diet-induced non-alcoholic steatohepatitis (NASH).
Mice were fed NASH diet or NC for 40 wk and sacrificed after 16 hr fasting. (A–B) Loss of Slc39a5 reduces serum ALT and AST levels (biomarkers of liver damage). (C–E) Histology scores for steatosis, hepatocyte hypertrophy, inflammation. (F) Non-alcoholic fatty liver disease (NAFLD) activity score. (G–I) Loss of Slc39a5 improves fibrosis in mice upon NASH dietary challenge. (G) Representative images of explanted livers sample stained with picrosirius red indicative of collagen deposition. Scale bar, 300 µm. (H–I) Histology score for fibrosis and steatosis-activity-fibrosis score. n=7–10 (NC) and 15–17 (NASH), *p<0.05, **p<0.01, ***p<0.001, two-way ANOVA with post hoc Tukey’s test. Numeric data is summarized in Supplementary file 6.
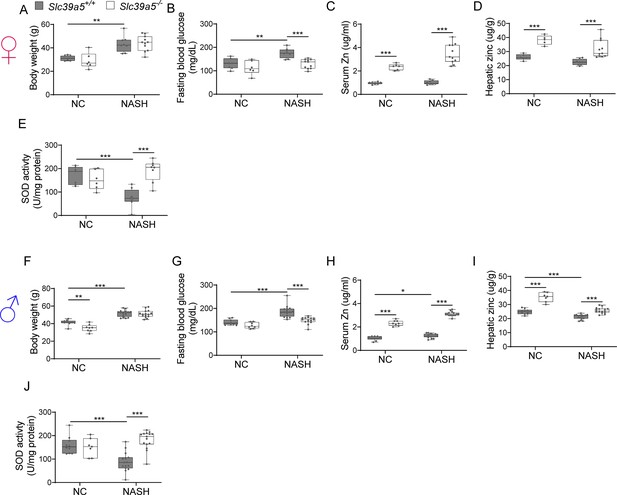
Loss of Slc39a5 improves liver function in mice challenged with diet-induced non-alcoholic steatohepatitis (NASH).
Female (A–E) and Male (F–J) mice. (A, F) Body weight. (B, G) Fasting blood glucose. (C, H) Serum zinc. (D, I) Hepatic zinc. (E, J) Total hepatic superoxide dismutase (SOD) activity. n=6–10 (NC) and 8–17 (NASH), *p<0.05, **p<0.01, ***p<0.001, two-way ANOVA with post hoc Tukey’s test.
Additional files
-
Supplementary file 1
Serum zinc and insulin profile assessment in the serum call back study.
Serum zinc levels in SLC39A5 heterozygous loss of function carriers are elevated by 12% as compared to age, sex, and BMI-matched reference controls. Analyses of insulin production (insulin/c-peptide ratio), insulin clearance (proinsulin/insulin), and blood glucose in these samples demonstrated no differences based on genotype. Data is represented in a graphical format in Figure 1.
- https://cdn.elifesciences.org/articles/90419/elife-90419-supp1-v1.xlsx
-
Supplementary file 2
Tissue zinc content in humans and mouse.
Human data adapted from Jackson et al., 1982 *p<0.05; **p<0.01, ***p<0.001, not significant (n.s.), unpaired t-test. Values represent mean ± SD.
- https://cdn.elifesciences.org/articles/90419/elife-90419-supp2-v1.xlsx
-
Supplementary file 3
No differences in the serum chemistry profile of Slc39a5+/+ and Slc39a5-/- mice.
Serum chemistry analysis in adult mice (40 wk of age, both sexes) demonstrated no differences in pancreatic amylase, renal function parameters (blood urea nitrogen, creatinine, total protein, and uric acid), and electrolytes (chloride, potassium, and sodium) or liver enzymes (alanine aminotransferase; ALT and aspartate aminotransferase; AST).
- https://cdn.elifesciences.org/articles/90419/elife-90419-supp3-v1.xlsx
-
Supplementary file 4
Summary statistics for the congenital obesity model.
Loss of Slc39a5 improves glycemic traits and liver function in leptin-receptor (Lepr) deficient mice. Loss of Slc39a5 does not change insulin production (proinsulin/insulin), or insulin clearance (insulin/c-peptide ratio).
- https://cdn.elifesciences.org/articles/90419/elife-90419-supp4-v1.xlsx
-
Supplementary file 5
Summary statistics for the diet-induced obesity model.
Loss of Slc39a5 improves glycemic traits and liver function in mice upon a high-fat high fructose diet (HFFD) dietary challenge. Moreover, loss of Slc39a5 does not change insulin production (proinsulin/insulin), or insulin clearance (insulin/c-peptide ratio).
- https://cdn.elifesciences.org/articles/90419/elife-90419-supp5-v1.xlsx
-
Supplementary file 6
Summary statistics for the diet-induced non-alcoholic steatohepatitis (NASH) model.
Loss of Slc39a5 improves hepatic inflammation and fibrosis in both female and male mice challenged with diet-induced NASH.
- https://cdn.elifesciences.org/articles/90419/elife-90419-supp6-v1.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/90419/elife-90419-mdarchecklist1-v1.docx





