Increasing adult-born neurons protects mice from epilepsy
Figures
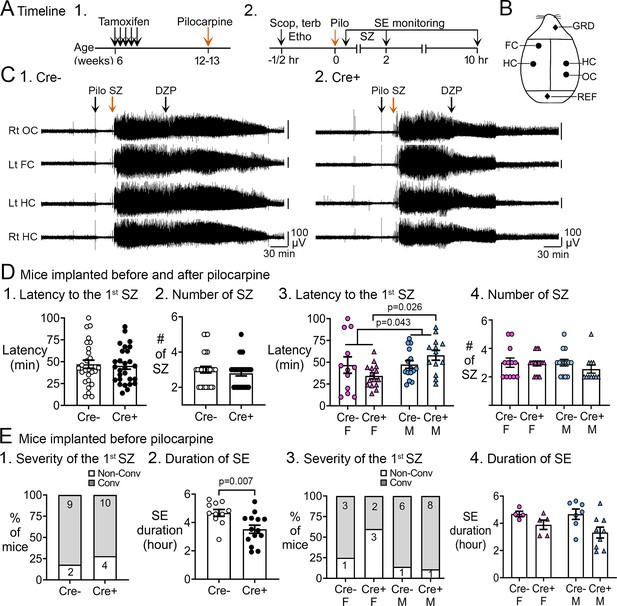
Pilocarpine-induced SE in Cre+ and Cre- mice.
(A) The experimental timeline is shown. (1) Tamoxifen was injected 1 /day for 5 days in 6-week-old Nestin-CreERT2Baxfl/flmice. Six weeks after the last tamoxifen injection, mice were injected with pilocarpine (Pilo) at a dose that induces SE. (2) On the day of the pilocarpine injection, one group of mice without electrodes for electroencephalographic (EEG) was monitored for behavioral seizures for 2 hr after the pilocarpine injection. Another group of mice were implanted with EEG electrodes 3 weeks prior to pilocarpine injection. In these mice, video and EEG was used to monitor SE for 10 hr after pilocarpine injection. (B) Locations to implant EEG electrodes are shown. Four circles represent recording sites: left frontal cortex (Lt FC), left hippocampus (Lt HC), right hippocampus (Rt HC), and right occipital cortex (Rt OC). Two diamonds represent ground (GRD), and reference (REF) electrodes. (D) Pooled data for mice that were implanted with EEG electrodes and unimplanted mice. These data showed no significant genotypic differences but there was a sex difference. (1) The latency to the onset of the first seizure was similar in both genotypes (t-test, p=0.761). The seizure was a behavioral seizure >stage 3 of the Racine scale (unilateral forelimb jerking). For this figure and all others, detailed statistics are in the Results. (2) The number of seizures in the first 2 hr after pilocarpine injection was similar in both genotypes (t-test, p=0.377). (3) After separating males and females, females showed a shorter latency to the onset of the first seizure compared to males (two-way ANOVA, p=0.043); Cre+ females had a shorter latency to the first seizure relative to Cre+ males (Tukey's post-hoc test, p=0.026). (4) The number of seizures in the first 2 hr after pilocarpine injection were similar in males and females (two-way ANOVA, p=0.436). (E) Implanted mice. These data showed a significant protection of Cre+ mice on SE duration. (1) The severity of the first seizure (non-convulsive or convulsive) was similar between genotypes (Fisher’s exact test, p=0.093). (2) Cre+ mice had a shorter duration of SE than Cre- mice (t-test, p=0.007). (3) After separating males and females, the first seizure was mostly non-convulsive in Cre+ females compared to Cre- females (60% vs 14%) but no groups were statistically different (Fisher’s exact tests, p>0.05). (4) Once the sexes were separated, there was no effect of sex by two-way ANOVA but there was a trend in Cre+ males to have a shorter SE duration than Cre- males (Tukey’s post-hoc test, p=0.078).
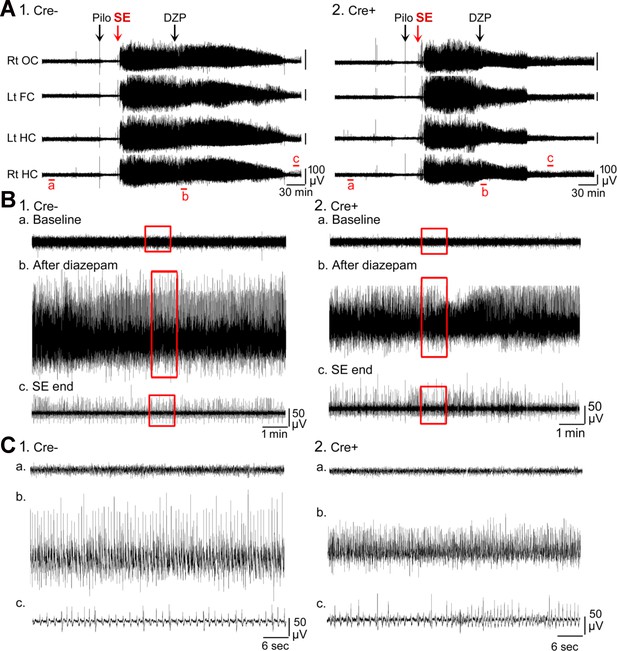
Examples of the electroencephalogram (EEG) during SE.
(A) Representative examples of a 10 hr-long EEG recording are shown for Cre- (1) and Cre+ (2) mice. These records are the same as in Figure 1. (B) 10 min-long EEG recording segments from the left hippocampus in A are shown with higher temporal gain. (1) Cre- mouse. (a) Part of the baseline is shown. The area surrounded by the red box is expanded in C1a. (b) The time when diazepam (DZP) was injected is shown. The area surrounded by the red box is expanded in C1b. (c) The time following the seizure is shown. The area surrounded by the red box is expanded in C1c. (2) Cre+ mouse. (a) Part of the baseline is shown. The area surrounded by the red box is expanded in C2a. Note the baselines are similar in the two mice, suggesting no effect of genotype. (b) The time when DZP was injected is shown. The area surrounded by the red box is expanded in C2b. Note there was a reduction in EEG amplitude in the Cre+ mouse. (c) The time at the end of SE is shown. The area surrounded by the red box is expanded in C2c. Note that these two mice were similar after SE ended. (C) The areas surrounded by the red boxes in B are expanded. The traces are 1 min-long.
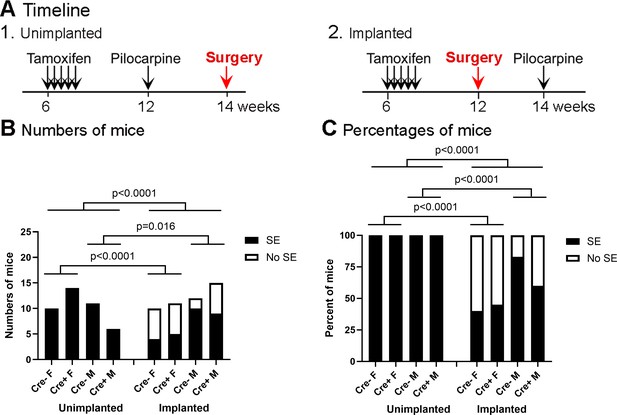
Incidence of SE in the unimplanted and implanted mice.
(A) The experimental timelines of pilocarpine injection and surgery. (1) Unimplanted mice. (2) Implanted mice. Only the timing of surgery with respect to pilocarpine injection was different. (B) The incidence of SE in unimplanted and implanted mice is shown based on the numbers of mice. The incidence of SE was significantly higher in the unimplanted mice when sexes and genotypes were pooled (Fisher’s exact test, p<0.0001). Genotype had no effect on the incidence of SE (Unimplanted, p>0.999; Implanted, p=0.565). (C) The incidence of SE is shown as percentages. Fisher’s exact tests showed significant differences between unimplanted and implanted mice, as for part B (all p<0.0001).
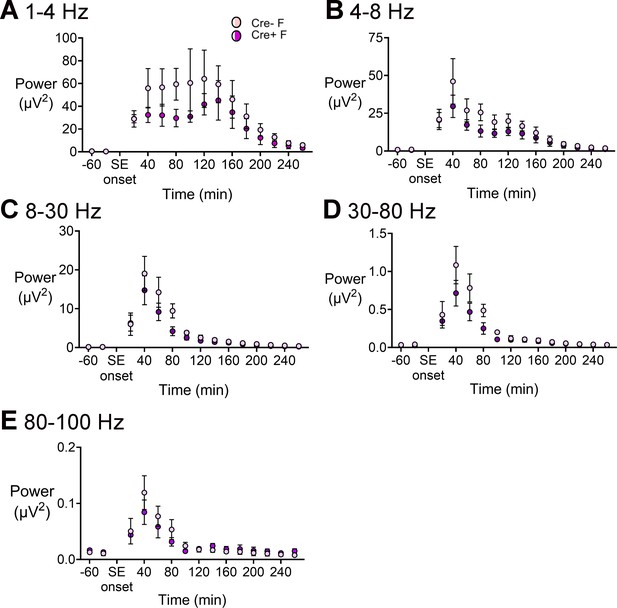
Power during SE in Cre+ female and Cre- female mice.
(A) Power was calculated for consecutive 20 min-long bins before and during SE. Power in the 1–4 Hz band was decreased during SE in female Cre+ mice (blue triangles) relative to Cre- mice (red circles) but it was not statistically significant. A two-way ANOVA showed no effect of genotype for any of the frequency bands (all p>0.1) but there were effects of time (all p<0.01). (B) Power in the 4–8 Hz band. (C) Power in 8–30 Hz range. (D) Power in 30–80 Hz range. (E) Power between 80 and 100 Hz.
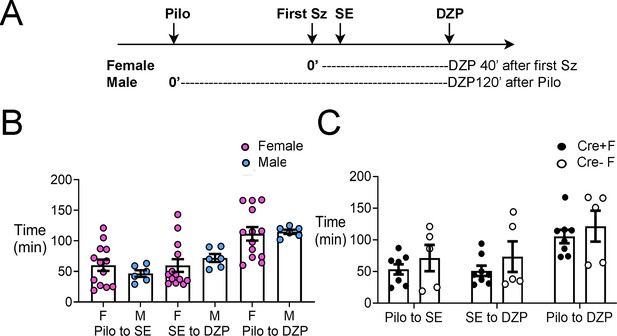
The latency to SE, interval between SE and diazepam administration, and interval between pilocarpine and diazepam injections were not significantly different in experimental groups.
(A) A timeline of experimental procedures is shown for the day of pilocarpine-induced SE. Pilocarpine was injected and the first seizure stage 3 or greater was noted. The onset of SE was noted also. For females, diazepam (DZP) was injected 45 min after the first seizure (Sz). Males were administered DZP 2 hrs after pilocarpine (Pilo). The reason for the difference is that it made the latencies to SE, interval between SE and DZP injection, and interval between pilocarpine and DZP injections similar. (B) The mean ± SEM is shown for the time from pilocarpine to SE, SE to DZP injection, and pilocarpine to DZP injection. There were no sex differences: a two-way ANOVA with sex and type of measurement as main factors showed no effect of sex (F(1,45)=0004, p=0.949). However, there were differences between the types of measurements (F(2,45)=11.89, p<0.0001). (C) When Cre+ and Cre- females were compared, there was no effect of genotype (F(1,33)=2.33, p=0.136) but there was a significant effect of the type of measurement (F(2,33)=7.66, p=0.002).
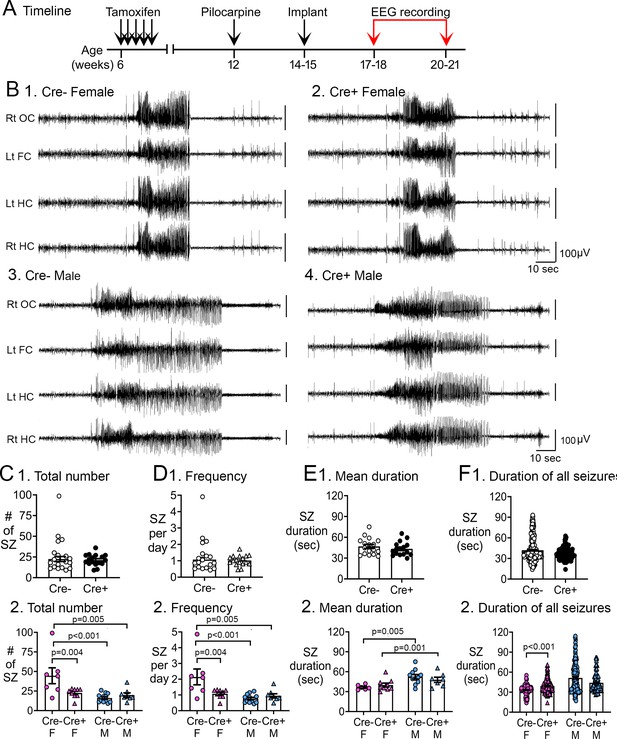
Reduced chronic seizures in Cre+ mice.
(A) The experimental timeline is shown. Six weeks after pilocarpine injection, continuous video-EEG was recorded for 3 weeks to capture chronic seizures. Mice that were unimplanted prior to SE were implanted at 2–3 weeks after pilocarpine injection. (B) Representative examples of 2 min-long EEG segments show a seizure in a Cre- (1, 3) and Cre+ (2, 4) mouse. (C) Numbers of chronic seizures. (1) Pooled data of females and males showed no significant effect of genotype on chronic seizure number. The total number of seizures during 3 weeks of recording were similar between genotypes (t-test, p=0. 882). (2) After separating data based on sex,Cre+ females had fewer seizures than Cre- females (two-way ANOVA followed by Tukey’s post-hoc test, p=0.004). There was a sex difference in control mice, with fewer seizures in Cre- males compared to Cre- females (p<0.001). Cre- females also had more seizures than Cre+ males (p=0.005). (D) Chronic seizure frequency. (1) Pooled data of females and males showed no significant effect of genotype on chronic seizure frequency. The frequency of chronic seizures (number of seizures per day) were similar (Welch’s t-test, p=0.717). (2) Seizure frequency was reduced in Cre+ females compared to Cre- females (Tukey’s post-hoc test, p=0.004). There was a sex difference in control mice, with higher seizure frequency in Cre- females compared to Cre- males (p<0.001) and Cre+ males (p=0.005). (E) Seizure duration per mouse. (1) Each data point is the mean seizure duration for a mouse. Pooled data of females and males showed no significant effect of genotype on seizure duration (t-test, p=0.379). (2) There was a sex difference in seizure duration, with Cre- males having longer seizures than Cre- females (Tukey’s post-hoc test, p=0.005). Because females exhibited more postictal depression (see Figure 3), corresponding to spreading depolarization (Ssentongo et al., 2017), the shorter female seizures may have been due to truncation of seizures by spreading depolarization. (F) Seizure durations for all seizures. (1) Every seizure is shown as a data point. The durations were similar for each genotype (Mann-Whitney U test, p=0.079). (2) Cre+ females showed longer seizures than Cre- females (two-way ANOVA followed by Tukey’s post-hoc test, p<0.001). Cre+ females may have had longer seizures because they were protected from spreading depolarization.
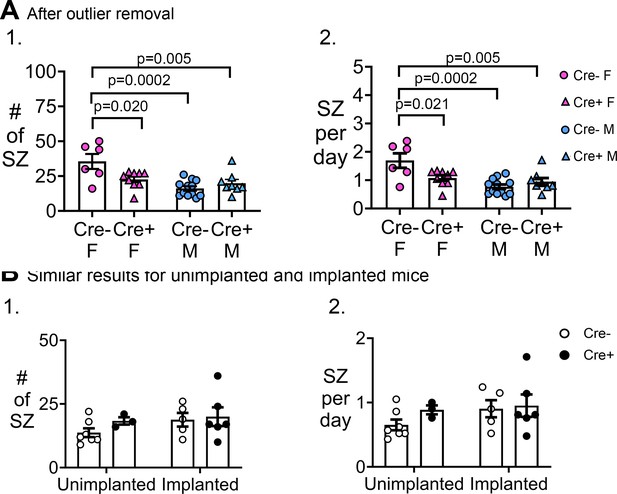
Additional analyses of chronic seizures.
(A) Similar results after outlier removal. (1) A two-way ANOVA with sex and genotype as factors showed a significant effect of sex on the # of seizures (F(1,31)=16.04, p=0.0004) and an interaction (F(1,31)=9.20, p=0.005) with no main effect of genotype (F(1,31)=1.75, p=0.107). Cre- females had significantly more seizures than all other groups (post-hoc tests, Cre- females vs. Cre+ females, p=0.020; vs. Cre- males, p=0.0002; vs. Cre+ males, p=0.005). Cre- males and Cre+ males were not different (p=0.722). (2) A two-way ANOVA with sex and genotype as factors showed a significant effect of sex (F(1,31)=15.84, p=0.0004) on seizure frequency and an interaction (F(1,31)=9.16, p=0.005) although no main effect of genotype (F(1,31)=2.72, p=0.109). Cre- females had significantly more seizures than all other groups (post-hoc tests, Cre- females vs. Cre+ females, p=0.021; vs. Cre- males, p=0.0002; vs. Cre+ males, 0.005). Cre- males were not different from Cre+ males (p=0.720). (B) Results were independent of the time when EEG electrodes were implanted. (1) The number of chronic seizures were similar between mice that were implanted before and after SE by two-way ANOVA with implant status and genotype as factors (implant status, F(1,17)=1.33, p=0.265; genotype, F(1,17)=0.88, p=0.334). Sexes were pooled. (2) The frequency of seizures was similar between mice implanted before or after SE (implant status, F(1,17)=1.27, p=0.276; genotype, F(1,17)=1.00, p=0.330). Sexes were pooled.
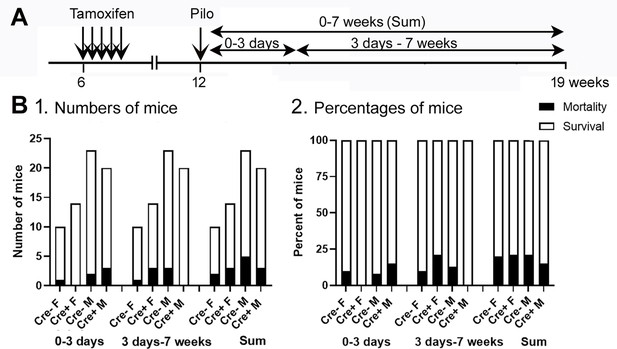
Mortality was not significantly affected by genotype or sex.
(A) The timeline of measurements of mortality is shown. Mice were categorized as dying during SE or within 3 days of SE (0–3 days), 3 days to 7 weeks, or both (Sum). Mice are included whether they were implanted with EEG electrodes before SE or implanted 3 weeks after SE. (B) (1) The numbers of mice that died were not significantly different between genotypes or sexes (Fisher’s Exact test, all p>0.05). (2) The percent of mice that died is shown.
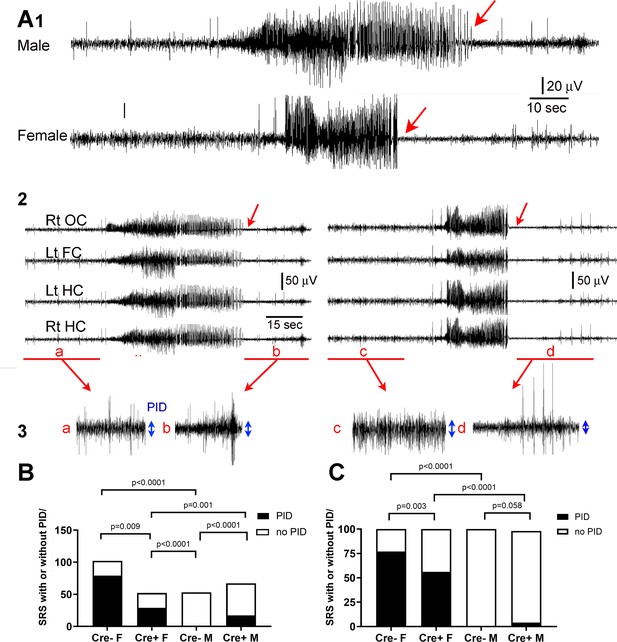
Reduced postictal depression in Cre+ female mice.
(A) (1) A seizure of a male mouse and a female mouse are shown to illustrate the end of the seizure (red arrow). (2) All four channels are shown for the male (left) and female mouse (right). Rt OC, right occipital cortex; Lt FC, left frontal cortex; Lt HC, left hippocampus; Rt HC, right hippocampus. The seizure is shown on a compressed scale. (3) The areas in A2 marked by the red bar are expanded. The blue double-sided arrows reflect the mean EEG amplitude before (a, c) and after the seizure (b, d). The female seizure shows postictal depression (PID). (B) For all spontaneous recurrent seizures (SRS) in the 3 week-long recording period (n=5 mice/group), there were significant differences between groups, with the number of SRS with PID greater in females (Cre- females vs. Cre- males). PID was reduced in Cre+ females compared to Cre- females. All comparisons were Fisher’s exact tests. (C). The same data are plotted but the percentages are shown instead of the numbers of seizures. Fisher’s exact tests showed similar group differences except for Cre+ and Cre- males which only showed a trend. Together the data show females exhibited more PID than males and Cre+ mice were protected, especially females.
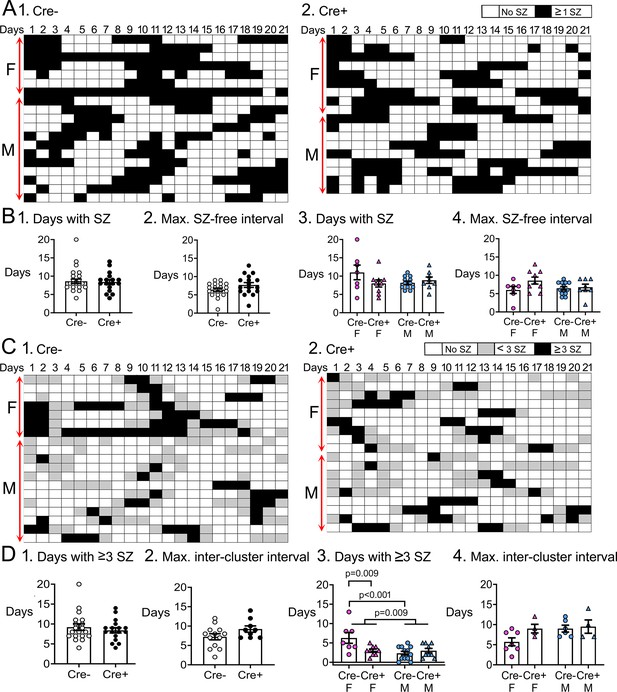
Temporal dynamics of chronic seizures.
(A) Each day of the 3-weeks-long EEG recording periods are shown. Each row is a different mouse. Days with seizures are coded as black boxes and days without seizures are white. (B) (1) The number of days with seizures were similar between genotypes (t-test, p=0.822). (2) The maximum seizure-free interval was similar between genotypes (t-test, p=0.107). After separating females and males, two-way ANOVA showed no effect of genotype or sex on days with seizures. (4) Two-way ANOVA showed no effect of genotype or sex on the maximum seizure-free interval. (C) Then same data are shown but days with >3 seizures are black, days with <3 seizures as gray, and are white. Clusters of seizures are reflected by the consecutive black boxes. (D) The cluster durations were similar between genotypes (Mann-Whitney’s U test, p=0.723). (1) The maximum inter-cluster interval was similar between genotypes (t-test, p=0.104). (2) Cre+ females had significantly fewer clusters than Cre- females (two-way ANOVA followed by Tukey’s post-hoc test, p=0.009). There was a sex difference, with females having more clusters than males (p=0.009). Cre- females had more days with >3 seizures than control males (Cre- females: 6.3±1.4 days; Cre- males: 2.3±0.5 days; Tukey’s post-hoc test, p<0.001). (3) There was no significant effect of genotype or sex on the maximum inter-cluster interval. However, there was a trend for the inter-cluster interval to be longer in Cre+ females relative to Cre- females.
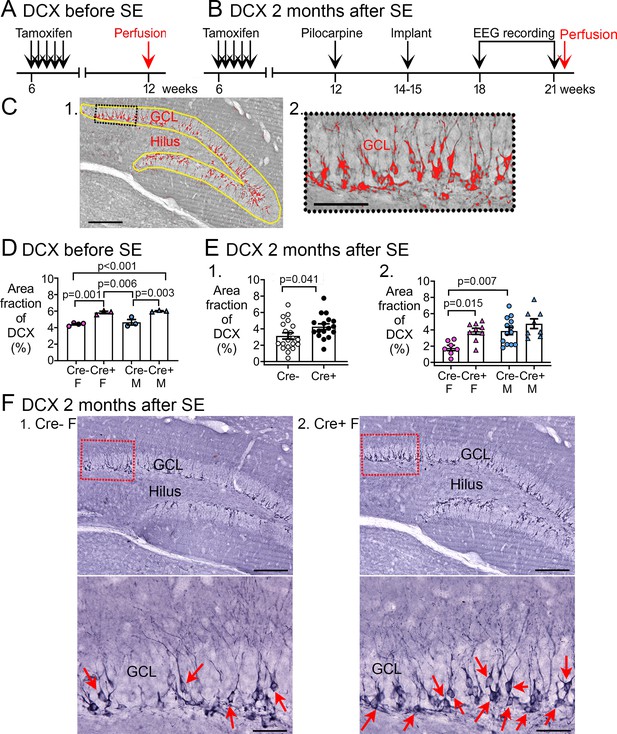
Increased Doublecortin (DCX) in Cre+ mice.
(A–B) The experimental timelines are shown. (A) Mice were perfusion-fixed 6 weeks after tamoxifen injection, just before SE. Sections were then stained for DCX. (B) Mice were tested 2 months after SE, after EEG recording. Then mice were perfused and staining was conducted for DCX. (C) (1) DCX quantification. DCX-ir within a region of interest (ROI; yellow lines) including the subgranular zone (SGZ) and GCL was thresholded. DCX-ir above the threshold is shown in red. Calibration, 100 μm. (2) The inset is expanded to the right. Calibration, 50 μm. (D) The area of DCX-ir relative to the area of the ROI (referred to as area fraction) was greater in Cre+ mice compared to Cre- mice. Two-way ANOVA followed by Tukey’s post-hoc tests, all p<0.05. (E) Cre+ mice had increased DCX-ir relative to Cre- mice 2 months after SE. (1) Sexes were pooled. The area fraction of DCX-ir was greater in Cre+ than Cre- mice (t-test, p=0.041). (2) When sexes were separated, Cre+ females showed greater DCX-ir than Cre- females (two-way ANOVA followed by Tukey’s post-hoc test, p=0.015). There was a sex difference, with Cre- males showing more DCX-ir than Cre- females (p=0.007). DCX-ir was similar in Cre- and Cre+ males (p=0.498). (F) Representative examples of DCX-ir 2 months after SE. (1) Cre- female mouse. (2) Cre+ female mouse. The red boxes in a are expanded in b. Arrows point to DCX-ir cells. Calibration, 100 μm (a); 50 μm (b).
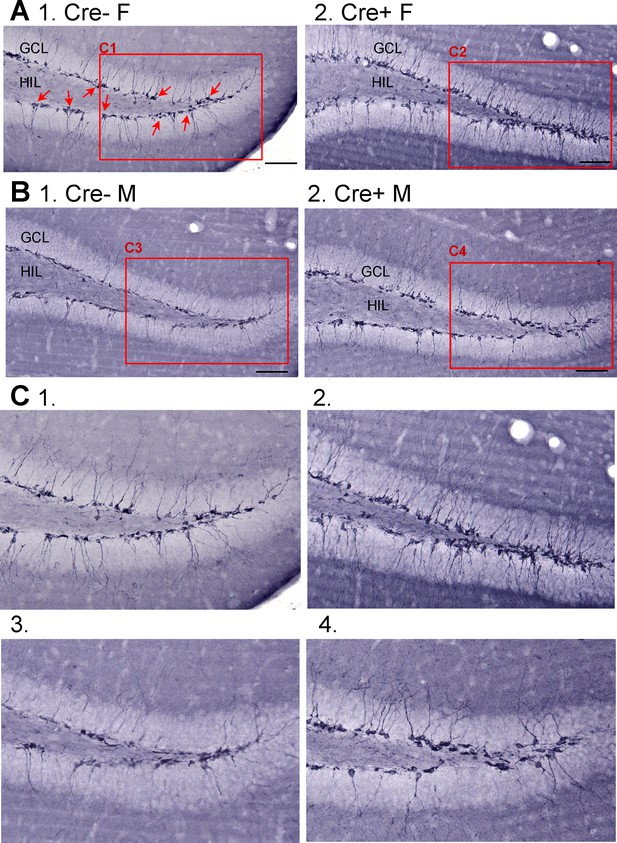
Doublecortin (DCX) in Cre- and Cre+ mice before SE.
(A) Mice were administered tamoxifen for 5 days at 6 weeks of age as for other experiments. Six weeks after tamoxifen, mice were perfusion-fixed and staining was conducted using an antibody to DCX. (1) Representative image from a Cre- female (F) of dorsal dentate gyrus in coronal section shows many DCX-ir cells in the subgranular zone (SGZ) and granule cell layer (GCL) (red arrows). The area surrounded by the red box is expanded in C1. Calibration, 100 µm. (2) Example of DCX-ir from a Cre+ female mouse shows more DCX-ir, reflecting more immature neurons. The area surrounded by the red box is expanded in C2. Calibration, 100 µm. (B) (1) Example from a Cre- male (M). The area surrounded by the red box is expanded in C3. (2) Example of a Cre+ M showing more DCX-ir than the Cre- M. The area surrounded by the red box is expanded in C4. Calibration, 100 µm. (C) Expanded insets from A-B. 1. Cre- F, Cre- female; 2. Cre+ F, Cre+ female; 3. Cre- M, Cre- male; 4. Cre+ M, Cre+ male.
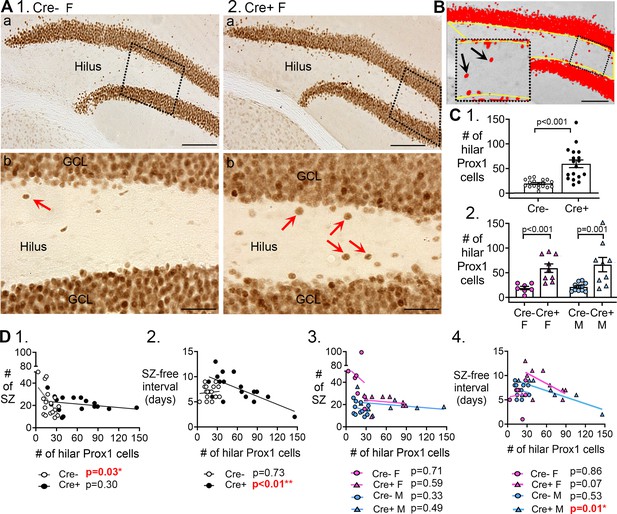
Hilar Prox1-ir cells increased in Cre+ mice.
(A) Representative examples of hilar Prox1-ir in Cre- (1) and Cre+ (2) mice are shown. The boxes in a are expanded in b. Arrows point to hilar Prox1-ir cells, corresponding to hilar ectopic GCs. Calibration, 100 μm (a); 50 μm (b). (B) Prox1-ir is shown, within a hilar region of interest (ROI). The area of the ROI above the threshold, relative to the area of the ROI, is red. This area is called the area fraction, and was used to quantify hilar Prox1-ir. Calibration, 100 μm. (C) (1) Cre+mice had more hilar Prox1-ir cells than Cre- mice (t-test, p<0.001). (2) When sexes were divided, Cre+ mice had more hilar Prox1-ir cells than Cre- mice in both female (two-way ANOVA followed by Tukey’s post-hoc test, p<0.001) and male mice (p=0.001). (D) Correlations between hilar Prox1-ir cells and measurements of chronic seizures. (1) All Cre- and Cre- mice were compared regardless of sex. For the Cre- mice there was a significant inverse correlation between the # of Prox1-ir cells and # of chronic seizures (R2=0.296). Thus, the more Prox1-ir cells there were, the fewer chronic seizures there were. However, that was not true for Cre+mice (R2=0.072). (2) There was an inverse correlation between the number of hilar Prox1-ir cells and the seizure-free interval for Cre+ mice (R2=0.467) but not Cre- mice (R2=0.008). Thus, the more hilar Prox1-ir cells there were, the shorter the seizure-free periods were. However, this was not true for Cre- mice. (3) When data were divided by genotype and sex there was no significant correlation between hilar Prox1-ir cells and # of seizures (Cre- F, R2=0.0035; Cre+ F, R2=0.043; Cre- M, R2=0.104; Cre+ M, R2=0.083). (4) When data were divided by genotype and sex, there was a significant inverse correlation for the # of hilar Prox1-ir cells and seizure-free interval, but only for male Cre+ mice (R2=0.704). Cre+ females showed a trend (R2=0.395) and Cre- mice did not (Cre- F, R2=0.007, Cre- M, R2=0.046).
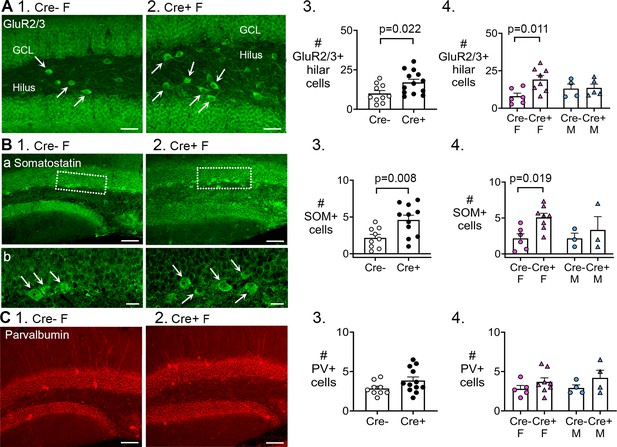
Preserved mossy cells and hilar somatostatin (SOM) cells in Cre+ female mice but not parvalbumin interneurons.
(A, 1–2) Representative examples of GluR2/3 labeling of Cre- (1) and Cre+ mice (2). Calibration, 50 μm. (3) Cre+ mice had more hilar GluR2/3-immunofluorescent (positive; +) cells than Cre- mice (t-test, p=0.022). Sexes were pooled. (4) After separating females and males, Cre+ females showed more hilar. GluR2/3 + cells than Cre- females (two-way ANOVA followed by Tukey’s post-hoc test, p=0.011). Hilar GluR2/3+ cells were similar between genotypes in males (p=0.915). (B, 1–2) Representative examples of SOM labeing in Cre- and Cre+ mice are shown. Calibration, 100 μm (a); 20 μm (b). (3) In pooled data, Cre+ mice had more hilar SOM cells than Cre- mice (t-test, p=0.008). (4) After separating females and males, Cre+ females showed more hilar SOM cells than Cre- females (p=0.019). Hilar SOM cells were similar between genotypes in males (p=0.897). (C, 1–2) Representative examples of parvalbumin labeling in Cre- and Cre+ mice are shown. Calibration, 100 μm. (3) The number of parvalbumin+ cells in the dentate gyrus (DG) were similar in Cre- and Cre+ mice in pooled data (t-test, p=0.095). (4) There was no effect of genotype (p=0.096) or sex (p=0.616) on the number of DG parvalbumin+ cells.
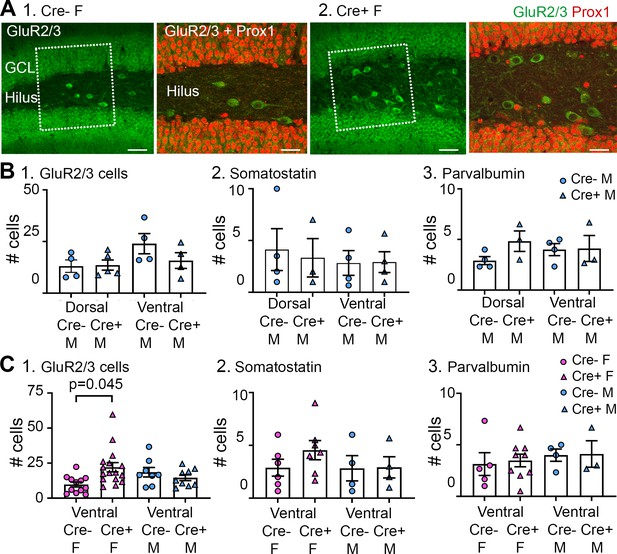
Additional analyses of GluR2/3, somatostatin (SOM), and parvalbumin-expressing cells.
(A) GluR2/3 hilar cells lacked Prox1 expression. (1) Cre- female mouse. Left: several GluR2/3+ cells (green) are located in the hilus within the box (marked by dotted white lines). Calibration, 70 µm. Right: The area within the box in the left panel is expanded. The merged image of GluR2/3+ (green) and Prox1+ (red) cells shows no double labeling. Calibration, 40 µm. (2) Cre+ female mouse. Similar results are shown for the Cre- mouse. White arrows mark ectopic GCs. Calibrations are the same as for the Cre- mouse. (B) A comparison of dorsal and ventral measurements for Cre- and Cre+ male mice shows no significant genotype effects. (1) GluR2/3. A two-way ANOVA showed no effect of dorsal or ventral location (F(1,13)=3.38; p=0.089) or genotype (F(1,13)=1.158; p=0.302). (2) SOM. A two-way ANOVA showed no effect of dorsal or ventral location (F(1,10)=0.172; p=0.687) or genotype (F(1,10)=0.014; p=0.908). (3) Parvalbumin. A two-way ANOVA showed no effect of dorsal or ventral location (F(1,13)=0.358; p=0.560) or genotype (F(1,13)=1.068 p=0.320). (C) A comparison of ventral measurements for both Cre- and Cre+ female and male mice. (1) There were significantly more GluR2/3+hilar cells in Cre+ female mice compared to Cre- female mice, like the dorsal hippocampus (Figure 7). Thus, GluR2/3+hilar cells were spared in Cre+ females in dorsal and ventral hippocampus. A two-way ANOVA showed o effect of sex (F(1,18)=0.744; p=0.400) or genotype (F(1,18)=0.386; p=0.542) but there was a significant interaction F(1,18)=5.433; p=0.0316, and post-hoc tests showed that Cre+ females had significantly more GluR2/3+ cells than Cre- females (p=0.045). (2) There were no significant differences among groups for SOM+ cells. Thus, there was an effect in dorsal (Figure 7) but not ventral hippocampus. Also, SOM cells were spared in Cre+ females dorsally but not ventrally. A two-way ANOVA showed no effect of sex (F(1,17)=0.718; p=0.408) or genotype (F (1, 17)=0.769; p=0.393). (3) There were no significant differences in numbers of parvalbumin+ cells, like dorsal hippocampus (Figure 7). Thus, parvalbumin cells were similar regardless of genotype in dorsal and ventral hippocampus. A two-way ANOVA showed no significant effect of sex (F(1,16)=0.401; p=0.536) or genotype (F(1,16)=0.221; p=0.645).
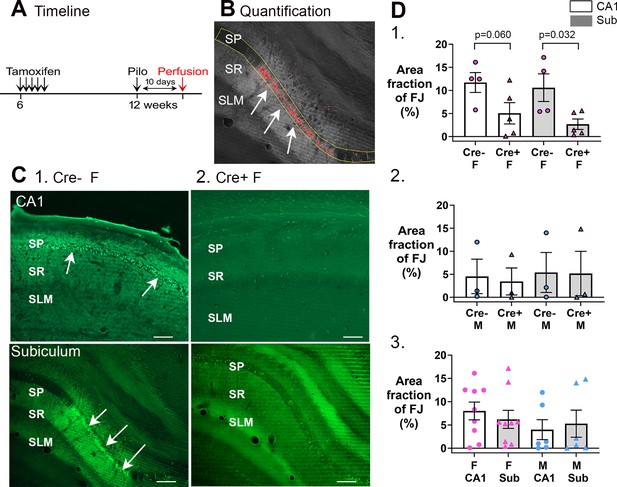
Cre+ female mice had less neuronal loss in hippocampus after SE.
(A) A timeline is shown to illustrate when mice were perfused to examine Fluorojade C staining. All mice were perfused 10 days after SE, a time when delayed cell death occurs after SE, mainly in area CA1 and subiculum. Note that prior studies showed hilar and CA3 neurons, which exhibit more rapid cell death after SE, are protected from cell loss in Cre+ mice examined 3 days after SE (Jain et al., 2019). Also, there was protection of CA1 for 3 days (Jain et al., 2019). (B) Quantification. Fluorojade C was thresholded using ImageJ and the pyramidal cell layer outlined in yellow. The fraction above threshold relative to the entire region of interest (ROI) (area fraction) was calculated (see Methods). (C) Examples of Fluorojade C staining in CA1 (top) and subiculum (bottom) of Cre+ female (1) and Cre- female (2) mice. SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum; SLM, stratum lacunosum-moleculare. Arrows point to numerous Fluorojade C-stained neurons in Cre- mice but not Cre+ mice. Calibration, 200 μm. (D) (1) Comparisons of female mice by two-way ANOVA showed less Fluorojade C in Cre+ mice for subiculum (p=0.032). (2) Comparisons of male mice showed no significant effect of genotype on Fluorojade C (F(1,8)=0.16, p=0.698) in either subfield (F(1,8)=0.02, p=0.872). (3) When genotypes were pooled, female mice did not have significantly more damage than males (two-way ANOVA, F(1,32)=2.28, p=0.149) and there was no effect of subfield (F(1,32)<0.001, p=0.997).
Tables
Key resources.
| Primary antibodies | Secondary antibodies | ||||
|---|---|---|---|---|---|
| Name | Dilution | Source, Identifier | Name | Dilution | Source, Identifier |
| anti-doublecortin (rabbit monoclonal) | 1:1000 | Cell Signaling Technology Cat# 4604 S, RRID:AB_10693771 | Biotinylated goat anti-rabbit IgG | 1:500 | Vector Laboratories Cat# BA-1000, RRID:AB_2313606 |
| anti-human Prox1 (goat polyclonal) | 1:2,000 | R and D systems Cat# AF2727, RRID:AB_2170716 | Biotinylated horse anti-goat IgG | 1:500 | Vector Laboratories Cat# BA-9500, RRID:AB_2336123 |
| anti-GluR2/3 (rabbit polyclonal) | 1:300 | Millipore Cat# AB1506, RRID:AB_90710 | Donkey anti-rabbit, Alexa Fluor 488 | 1:500 | Thermo Fisher Scientific Cat# A-21206, RRID:AB_2535792 |
| anti-Prox1 (goat polyclonal) | 1:2000 | R and D Systems Cat# AF2727, RRID:AB_2170716 | Donkey anti-goat, Alexa Fluor 546 | 1:500 | Thermo Fisher Scientific Cat# A-11056, RRID:AB_2534103 |
| anti-somatostatin (rabbit polyclonal) | 1:750 | Peninsula Laboratories Cat# T-4103.0050, RRID:AB_518614 | Goat anti-rabbit, Alexa Fluor 488 | 1:500 | Thermo Fisher Scientific Cat# A-11034, RRID:AB_2576217 |
| anti-parvalbumin (mouse monoclonal) | 1:1000 | Millipore Cat# MAB1572, RRID:AB_2174013 | Goat anti-mouse, Alexa Fluor 568 | 1:500 | Thermo Fisher Scientific Cat# A-11004, RRID:AB_2534072 |





