Difficulty in artificial word learning impacts targeted memory reactivation and its underlying neural signatures
Figures
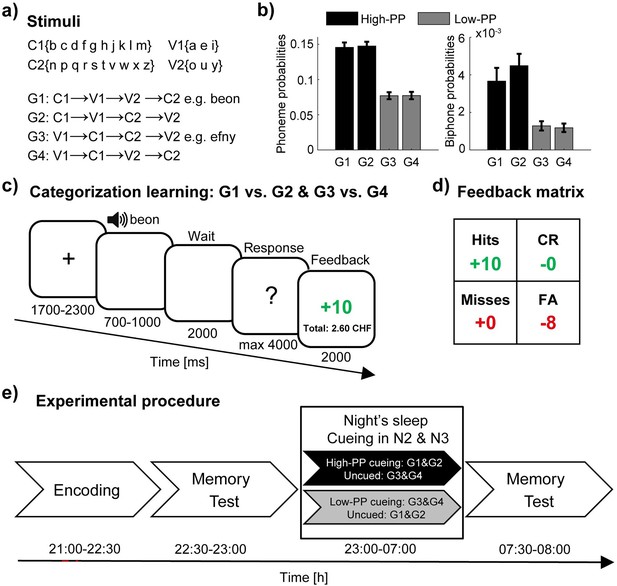
Experimental design.
(a) Artificial words consist of two vowels and two consonants according to four different sequences. (b) The phonotactic probabilities (PP) for single phonemes (left panel) and biphone probabilities (right panel) averaged values of the four sets (40 words per set) and pairing of the sets with respect to two distinct levels of PP in high- (black) and low-PP (gray). (c) Schematic trial structure of the learning task with screen images and the durations in milliseconds. As a two alternative forced-choice task, responses of left- and right-hand button presses were assigned to the rewarded and the unrewarded word category, respectively. The participants were instructed to respond to each word by left- or right-hand button presses, whereas one button means the word is rewarded (gain of money points) and the other button means the word is unrewarded (avoid the loss of money points). (d) Feedback matrix with the four answer types (hits: rewarded and correct; CR, correct rejections: unrewarded and correct; misses: rewarded and incorrect; FA, false alarms: unrewarded and incorrect) regarding to response and reward assignment of the word. Note, subjects could receive and lose money points dependent on correct and incorrect responses. (e) Experimental procedure with experimental tasks and phases in temporal order. TMR took place in the NREM sleep stages 2 and 3. Error bars reflect standard errors of the mean (SEM; n=40).
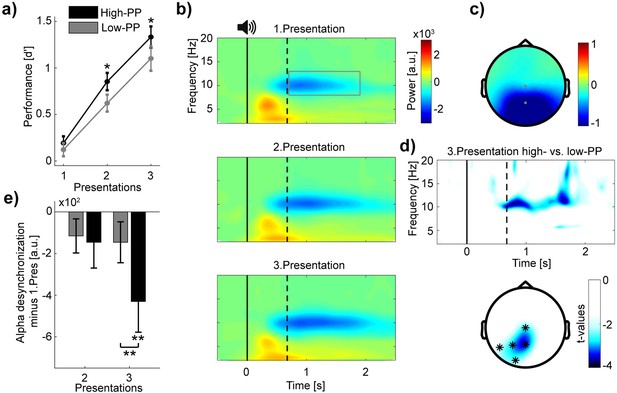
Distinct levels of encoding between high- and low-PP words.
(a) Learning curves showing encoding performance over presentations of high- (black) and low-PP (gray) words. Note significant greater performance of high- in comparison to low-PP over the second and third presentations. (b) Grand average time-frequency plots time-locked to word presentations. The gray rectangle within the top panel borders time and frequency range of interest (0.7–1.9 s; 8–13 Hz). Three different panels from top to down regarding to the three presentations. Solid and dotted lines within plots representing stimulus onset and averaged offset, respectively. Note, increases of oscillatory desynchronization in the alpha range (8–13 Hz) over the three presentations. (c) Topographic map shows power values averaged over the time window and frequency range of interest. (d) (Top) Time-frequency representation of t-values (merged over Pz and P3 electrodes) shows significant greater changed desynchronization in alpha oscillations of high- in contrast to low-PP during the third presentation. Below, topographic map indicates significant cluster of electrodes of comparison between PP conditions of the third presentation (0.7–1.9 s; 8–13 Hz). (e) The bar chart shows mean changes across subjects in alpha power (merged over Pz and Cz electrodes) of the second and third presentation by subtracting the first presentation in high- (black) vs. low-PP (gray). Statistical analyses revealed significant higher desynchronization of high- compared to low-PP and a significant decrease in alpha power under 0 of high-PP at the third presentation. Error bars reflect standard errors of the Mean (SEM; n=33); *p<0.05, **p<0.01.
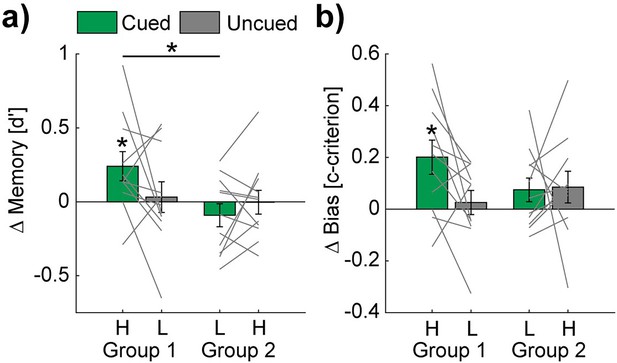
TMR affects the memory performance of the easy to learn words.
Bar charts show mean overnight changes of d' (a) and c-criterion (b) values of high (H) - and low (L) - PP and cued (green) vs. uncued (gray) conditions. Note, statistical analyses revealed significant overnight increases only in the high-PP cued condition. Gray lines represent individual data points. Error bars reflect SEM (n=11); *p<0.05.
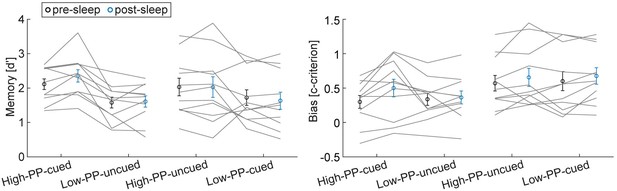
Pre- and post-sleep memory test data.
Pre (black) and post (blue) sleep mean group memory test data of d' (left panel) and c-criterion (right panel) divided into the conditions of high- and low-PP and cued and uncued. Gray lines represent individual data points. Error bars reflect SEM (n=11).
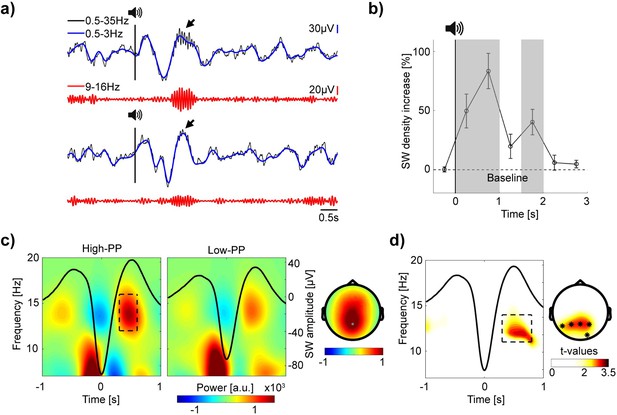
Increased spindle power during SW up-states in TMR of the easy to learn words.
(a) Top and bottom panel, two example EEG traces of auditory cueing during sleep (–2 until 6 s to stimulus onset). Top rows, in blue, signal filtered in the SW range (0.5–3 Hz) superimposed upon the broadband (0.5–35 Hz) signal in black. Vertical black lines with speaker symbols on top mark onsets of auditory presentations. Black arrows point to spindle activity during SW up-states. Bottom rows, in red, the same signal, but filtered in the spindle range (9–16 Hz). Note, elevated SW following cueing presentations with various spindle band activity nested during SW up-states. (b) Grand average baseline corrected curve of increased SW density after TMR in percentage. Shaded gray areas mark time windows (0–0.5 s, 0.5–1 s and 1.5–2 s) of significant increased SW density. Error bars reflect SEM (n=22). (c) Grand average time-frequency plots time-locked to the troughs of SW with averaged signals plotted as black lines. Two different panels (left and right) according to high- vs. low-PP cueing conditions. The rectangle within the left panel borders time (0.3 until 0.6 s to SW troughs) and frequency range of up-state fast spindle band activity (12–16 Hz). Corresponding topographic map at right shows elevated fast spindle power over mid-parietal electrodes. (d) Time-frequency representation of t-values time-locked to SW shows significant greater spindle band power during SW up-states for high- vs. low-PP (merged over Pz, P3, and P4 electrodes). Right, topographic map of t-values shows corresponding significant cluster of electrodes (0.3–0.8 s; 11–14 Hz), *p<0.05.
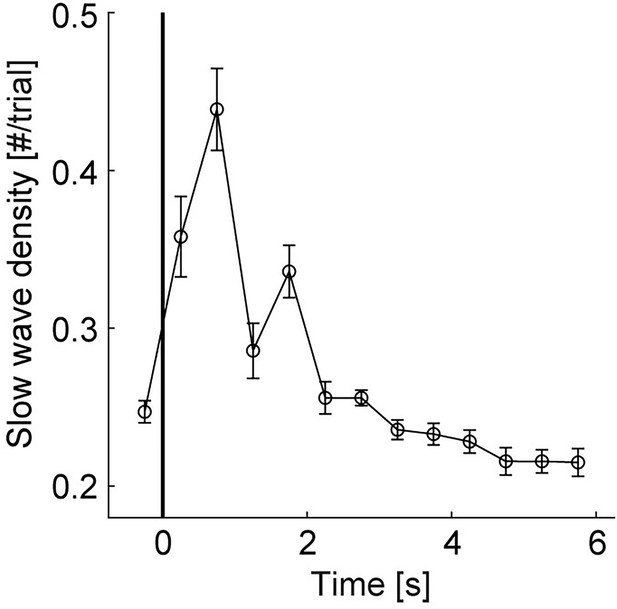
Slow wave density distribution.
Grand average curve of slow wave density time-locked to stimulus onset in number per trial with a bin size of 0.5 s from –0.5–6 s. Note, slow wave density peaks during the time window of 0–2 s and lower density values from 3 to 6 s after stimulus onset. Error bars reflect SEM (n=22).
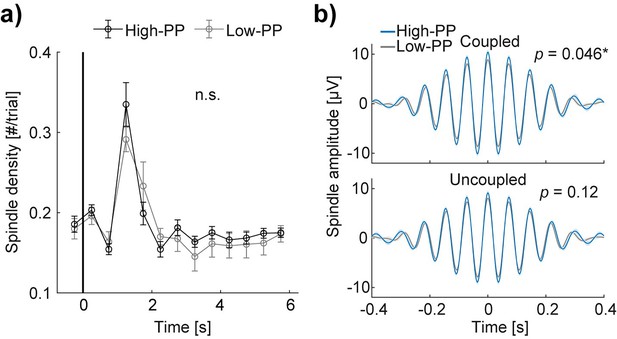
Detected sleep spindle analyses.
(a) Grand average curves of sleep spindle density time-locked to stimulus onset in number per trial with a bin size of 0.5 s from –0.5–6 s of the cueing condition of high (black) - and low (gray) - PP, respectively. Note, sleep spindle density peaks during the time window of 1–1.5 s after stimulus onset with no significant (n.s., p>0.05) difference between conditions. (b) Grand average signal filtered (12–16 Hz) of the Pz electrode time-locked to the sleep spindle amplitude peak of the high (blue) - and low (gray) - PP cueing conditions; shaded areas represent SEM (n=11). Two different panels according to sleep spindles coupled (top) and uncoupled (bottom) with detected slow waves during the time window of 0–6 s after stimulus onset. Note, significant higher amplitude sleep spindles coupled with slow waves in the cueing condition of high- compared to low-PP and no significant difference between the cueing conditions of the uncoupled sleep spindles. Error bars reflect SEM (n=11); *p<0.05.
Tables
| Respond: Reward Present | Respond: Reward Absent | |
|---|---|---|
| Reward Present | Hit | Miss |
| Reward Absent | False Alarm | Correct Rejection |
Additional files
-
Supplementary file 1
List of the high-PP words and their phoneme and biphone probabilities.
- https://cdn.elifesciences.org/articles/90930/elife-90930-supp1-v1.docx
-
Supplementary file 2
List of the low-PP words and their phoneme and biphone probabilities.
- https://cdn.elifesciences.org/articles/90930/elife-90930-supp2-v1.docx
-
Supplementary file 3
Gender, age and performance rates.
Data are means ± SEM. PP, phonotactic probability. P-values of statistical comparisons between groups by using unpaired t-tests. Note, no significant group differences, but a trend of significance for pre-sleep memory test of c-criterion values of the high-PP condition.
- https://cdn.elifesciences.org/articles/90930/elife-90930-supp3-v1.docx
-
Supplementary file 4
Statistics of SW density time-bin analyses from –0.5–3 s.
Data are means ± SEM. P-values (uncorrected for multiple comparisons) of statistical comparisons between groups by using paired t-tests against 0.
- https://cdn.elifesciences.org/articles/90930/elife-90930-supp4-v1.docx
-
Supplementary file 5
Sleep and reactivation parameter.
Data are means ± SEM. N1, N2: Non-REM sleep stages N1, N2 and N3; REM, rapid-eye movement sleep; WASO, wake after sleep onset; SW, slow wave; PP, phonotactic probability. P-values of statistical comparisons between groups by using unpaired t-tests. Note, no significant group differences, but a trend of significance for REM sleep parameters.
- https://cdn.elifesciences.org/articles/90930/elife-90930-supp5-v1.docx
-
Supplementary file 6
Density parameters of detected sleep spindles.
Data are means ± SEM. PP, phonotactic probability; SW, slow wave. General density: sleep spindle density during the time window of 0–6 s after stimulus onset in number per trial. SW-coupled: sleep spindles coupled to SW up-states divided by the total number of detected sleep spindles during the time window of interest (0–6 s after stimulus onset) in percent. P-values of statistical comparisons between groups by using unpaired t-tests.
- https://cdn.elifesciences.org/articles/90930/elife-90930-supp6-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/90930/elife-90930-mdarchecklist1-v1.pdf





