Targeting host deoxycytidine kinase mitigates Staphylococcus aureus abscess formation
Figures
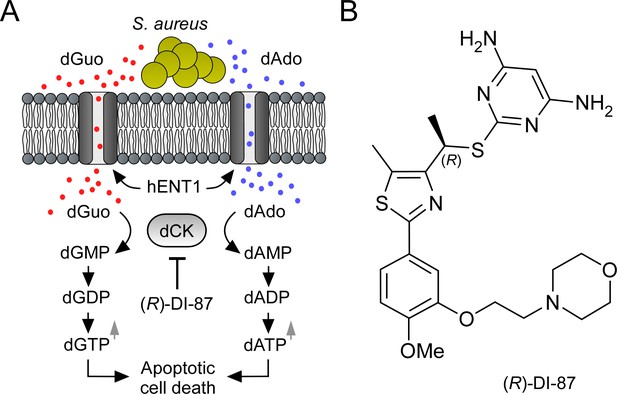
Mode of action of the dCK-specific inhibitor (R)-DI-87.
(A) Scheme illustrating the mode of action of the dCK-specific inhibitor (R)-DI-87. S. aureus-derived dAdo and dGuo are pumped into phagocytes via human equilibrative transporter 1 (hENT1). Deoxycytidine kinase (dCK) converts dAdo and dGuo into appropriate deoxyribonucleoside monophosphates thereby triggering an accumulation of apoptosis-stimulating deoxyribonucleoside di- and triphosphates. (R)-DI-87 interferes with this pathway by inhibiting dCK, thus preventing host cell death. (B) Structure of (R)-DI-87.
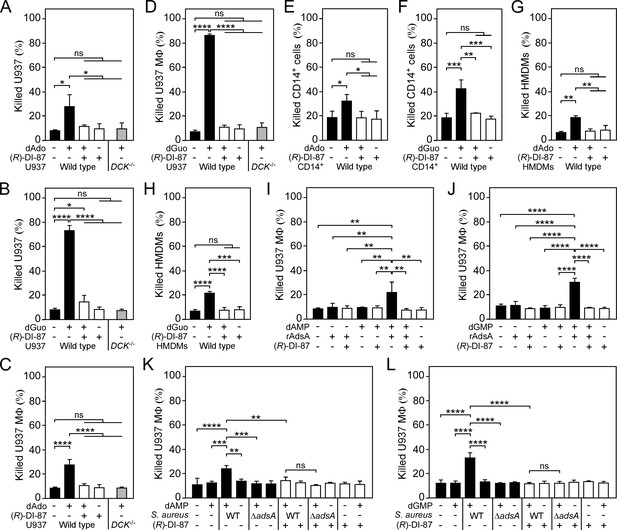
(R)-DI-87 protects phagocytes from death-effector deoxyribonucleoside-mediated cytotoxicity.
(A–D) Survival rates of human U937 monocyte-like cells (U937) (A, B) or U937-derived macrophages (U937 MФ) (C, D) exposed to dAdo or dGuo in the presence (+) or absence (-) of 1 µM (R)-DI-87. Cells were also exposed to the inhibitor or vehicle only. U937 DCK-/- were included as a control. (E–H) Survival rates of human CD14+ monocytes (E, F) or human monocyte-derived macrophages (HMDMs) (G, H) exposed to dAdo or dGuo in the presence (+) or absence (-) of 1 µM (R)-DI-87. Cells were also exposed to the inhibitor or vehicle only. (I–J) Survival rates of U937 MΦ exposed to rAdsA-derived dAdo (I) or dGuo (J). rAdsA was incubated with dAMP or dGMP and reaction products containing dAdo or dGuo were used to treat phagocytes in the presence (+) or absence (-) of 1 µM (R)-DI-87. Controls lacked rAdsA or deoxyribonucleoside monophosphates, or included reaction buffer only as indicated with + and − symbols. (K, L) Survival of vehicle- (-) or (R)-DI-87-exposed (+) U937 MΦ after treatment with culture medium (RPMI) that had been conditioned by incubation with either wild-type S. aureus Newman (WT) or its adsA mutant (ΔadsA) in the presence or absence of dAMP (K) or dGMP (L) as indicated with + and – symbols. Controls are indicated. 100 µM (A–B; E–F) or 200 µM (C-D; G–H) of dAdo or dGuo were used to treat the cells. Cell survival rates were analyzed 48 hr (A–J) or 24 h (K, L) post-treatment. Data are the mean (± standard deviation [SD]) values from at least three independent determinations. Primary cell experiments include at least three independent donors. Statistically significant differences were analyzed by two-way (A–D) or one-way (E–L) analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test; ns, not significant (P≥0.05); *, p<0.05; **, p < 0.01; ***, p<0.001; ****, p<0.0001.
-
Figure 2—source data 1
Data used to generate Figure 2.
- https://cdn.elifesciences.org/articles/91157/elife-91157-fig2-data1-v1.zip
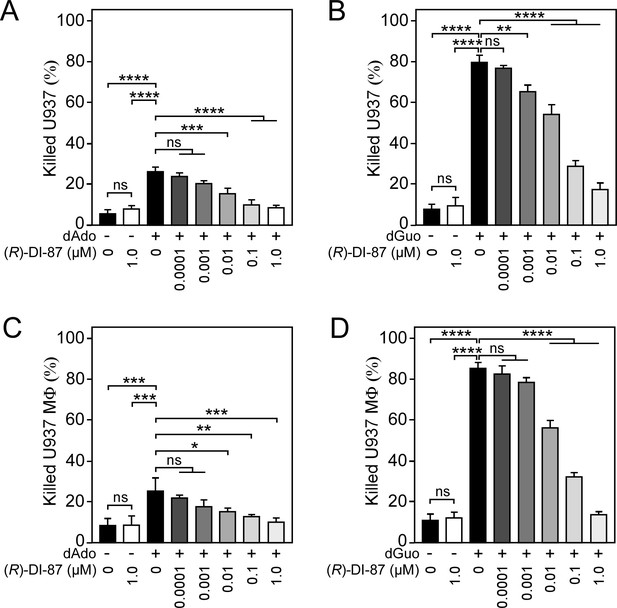
(R)-DI-87 prevents death-effector deoxyribonucleoside-triggered immune cell death in a dose-dependent manner.
(A–D) Survival rates of human U937 monocyte-like cells (U937) (A, B) or U937-derived macrophages (U937 MФ) (C, D) exposed to dAdo or dGuo in the presence (+) or absence (-) of various concentrations of (R)-DI-87. Cells were also exposed to the inhibitor or vehicle only. 100 µM (A, B) or 200 µM (C, D) of dAdo or dGuo were used to treat the cells. Cell survival rates were analyzed 48 hr post-treatment. Data are the mean (± standard deviation [SD]) values from three independent determinations. Statistically significant differences were analyzed with one-way analysis of variance (ANOVA) and Tukey’s multiple-comparison test; ns, not significant (p≥0.05); *, p<0.05; **, p < 0.01; ***, p<0.001; ****, p<0.0001.
-
Figure 2—figure supplement 1—source data 1
Data used to generate Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/91157/elife-91157-fig2-figsupp1-data1-v1.zip
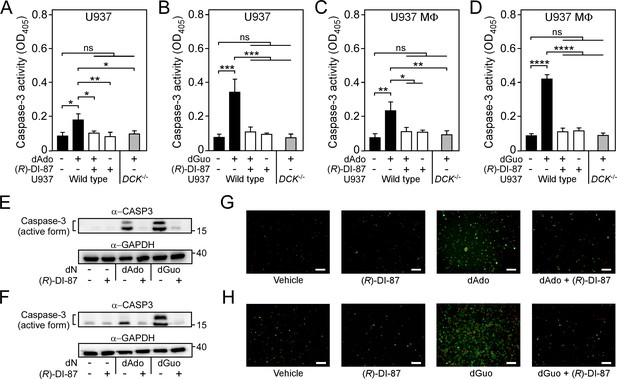
Selective inhibition of dCK prevents death-effector deoxyribonucleoside-mediated induction of immune cell apoptosis.
(A–D) Analysis of caspase-3 activity in human U937 monocyte-like cells (U937) (A, B) or U937-derived macrophages (U937 MФ) (C, D) exposed to dAdo or dGuo in the presence (+) or absence (-) of 1 µM (R)-DI-87. Cells were also exposed to the inhibitor or vehicle only. U937 DCK-/- were included as a control. Caspase-3 activity was analyzed using a colorimetric assay. (E–F) Immunoblotting of lysates obtained from U937 (E) or U937 MФ (F) exposed to dAdo or dGuo in the presence (+) or absence (-) of 1 µM (R)-DI-87. Controls are indicated (+/– symbols). A specific antibody was used that can also detect the cleaved (active) form of caspase-3 (α-CASP3). GAPDH was used as a loading control (α-GAPDH). Numbers to the right of blots indicate the migration of molecular weight markers in kilodaltons. (G–H) Analysis of (R)-DI-87-dependent prevention of host cell apoptosis via immunofluorescence microscopy. U937 MФ were exposed to dAdo (G) or dGuo (H) in the presence or absence of 1 µM (R)-DI-87 and stained using FITC-annexin-V/PI. Controls are indicated. Scale bars depict a length of 100 μm. Representative blots and images are shown. 100 µM (A–B; E) or 200 µM (C-D; F–H) of dAdo or dGuo were used to treat the cells. Apoptosis rates were analyzed 24 hr post-treatment. Data are the mean (± standard deviation [SD]) values from three independent determinations. Statistically significant differences were analyzed by two-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test; ns, not significant (p≥0.05); *, p<0.05; **, p < 0.01; ***, p<0.001; ****, p<0.0001.
-
Figure 3—source data 1
Original and unedited western blot scans used to generate Figure 3E–F.
- https://cdn.elifesciences.org/articles/91157/elife-91157-fig3-data1-v1.zip
-
Figure 3—source data 2
PDF containing uncropped and labeled western blot scans used to generate Figure 3E–F.
- https://cdn.elifesciences.org/articles/91157/elife-91157-fig3-data2-v1.zip
-
Figure 3—source data 3
Data used to generate Figure 3.
- https://cdn.elifesciences.org/articles/91157/elife-91157-fig3-data3-v1.zip
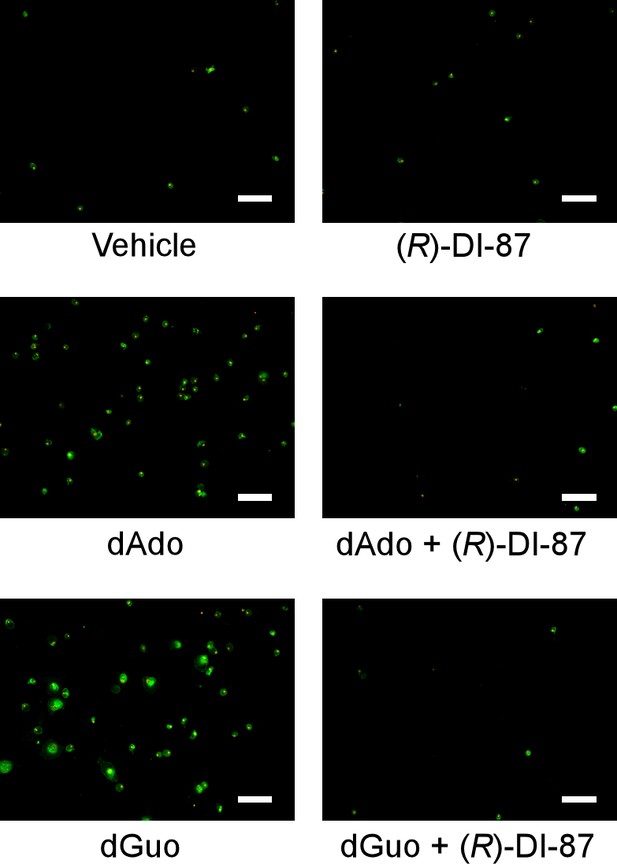
Inhibition of dCK prevents death-effector deoxyribonucleoside-mediated induction of immune cell apoptosis in primary human macrophages.
Analysis of (R)-DI-87-dependent prevention of host cell apoptosis via immunofluorescence microscopy. Primary human monocyte-derived macrophages (HMDMs) were exposed to dAdo or dGuo in the presence or absence of 1 µM (R)-DI-87 and stained using FITC-annexin-V/PI. Controls are indicated. Scale bars depict a length of 100 μm. Representative images are shown. 200 µM of dAdo or dGuo were used to treat the cells. Apoptosis rates were analyzed 24 hr post-treatment.
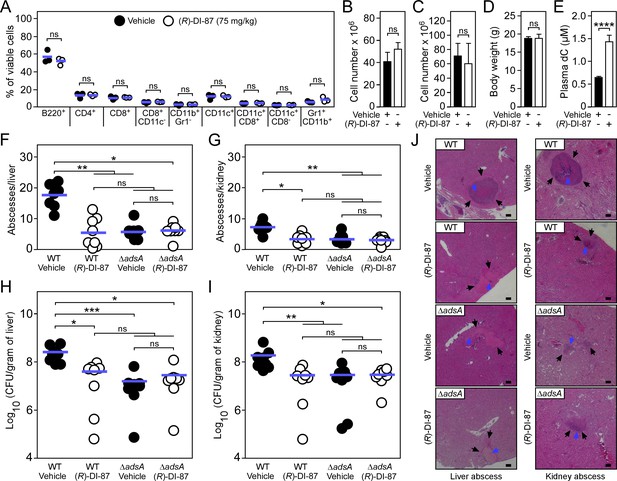
(R)-DI-87 protects against S.aureus invasive disease.
(A–D) Safety assessment of (R)-DI-87 in mice. Cohorts of female C57BL/6 mice were treated with (R)-DI-87 (75 mg/kg) or vehicle (40% Captisol) via oral gavage in 12 hr intervals for 23 days. On day 16, peripheral blood was collected and subjected to a FACS-based immuno-phenotyping approach (A). Subsequent panels indicate the cellularity of spleen (B) and thymus (C) tissues along with the body weight of mice (D) on day 23. (E) Analysis of deoxycytidine (dC) content in mouse plasma following continuous dCK inhibitor treatment on day 23. (F–I) Enumeration of visible surface abscesses and staphylococcal loads in organs of S. aureus-challenged C57BL/6 mice treated with (R)-DI-87 (75 mg/kg) or vehicle (40% Captisol). Mice received (R)-DI-87 (75 mg/kg) or vehicle (40% Captisol) via oral gavage every 12 hr and were challenged with 107 CFU of wild-type S. aureus Newman (WT) or its adsA mutant (ΔadsA). Data for female C57BL/6 mice are displayed (n=8). Bacterial burden was enumerated as log10 CFU per gram of tissue at 5 days post-infection. Horizontal blue bars represent the mean values of visible abscesses per organ (F–G) or indicate the mean CFU count in each cohort (H–I). (J) Microscopic images of H&E–stained liver or renal tissues obtained after necropsy of S. aureus-challenged C57BL/6 mice treated with (R)-DI-87 (75 mg/kg) or vehicle (40% Captisol). Mice received (R)-DI-87 (75 mg/kg) or vehicle (40% Captisol) via oral gavage every 12 hr and were challenged with 107 CFU of wild-type S. aureus Newman (WT) or its adsA mutant (ΔadsA). Arrows point to immune cell infiltrates (black) or replicating staphylococci (blue). Scale bars depict a length of 100 μm. Representative images are shown. Statistically significant differences were analyzed by a two-tailed Student’s t-test (A–E) or with the Kruskal–Wallis test corrected with Dunn’s multiple comparison (F–I). ns, not significant (p≥0.05); *, p<0.05; **, p < 0.01; ***, p<0.001; ****, p<0.0001.
-
Figure 4—source data 1
Data used to generate Figure 4.
- https://cdn.elifesciences.org/articles/91157/elife-91157-fig4-data1-v1.zip
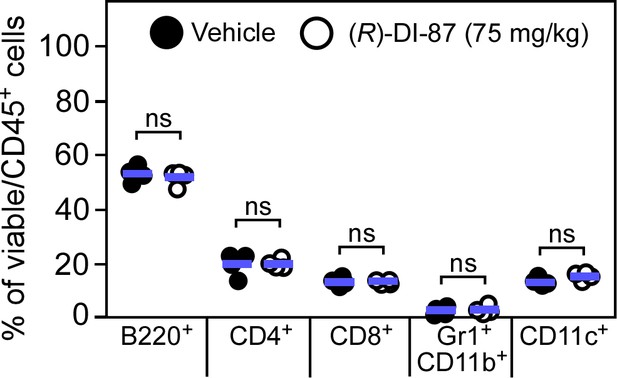
Immuno-phenotypic assessment of murine spleen tissues following continuous dCK inhibitor treatment.
Cohorts of female C57BL/6 mice were treated with (R)-DI-87 (75 mg/kg) or vehicle (40% Captisol) via oral gavage in 12 hr intervals for 23 days. On day 23, spleen tissues were collected and subjected to a FACS-based immuno-phenotyping approach. Statistically significant differences were analyzed by a two-tailed Student’s t-test. ns, not significant (p > 0.05).
-
Figure 4—figure supplement 1—source data 1
Data used to generate Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/91157/elife-91157-fig4-figsupp1-data1-v1.zip
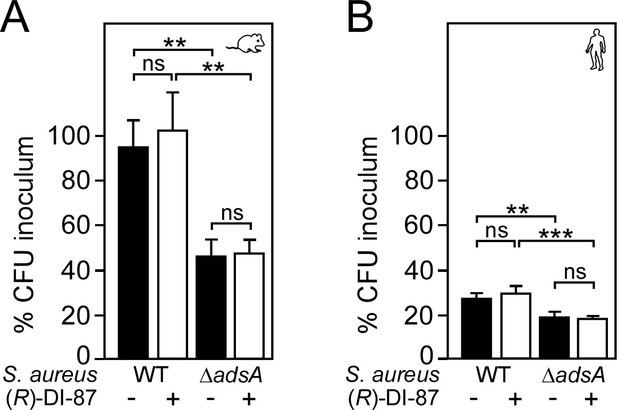
(R)-DI-87 does not interfere with staphylococcal survival in blood.
(A, B) Survival of wild-type S. aureus Newman (WT) or its adsA mutant (ΔadsA) in mouse (A) or human blood (B) in the presence (+) or absence (-) of (R)-DI-87 after 1 hr of incubation. Data were recorded as percent inoculum. For experiments with human blood, three independent donors have been used. Statistically significant differences were analyzed by two-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test; ns, not significant (p≥0.05); **, p < 0.01; ***, p<0.001.
-
Figure 4—figure supplement 2—source data 1
Data used to generate Figure 4—figure supplement 2.
- https://cdn.elifesciences.org/articles/91157/elife-91157-fig4-figsupp2-data1-v1.zip
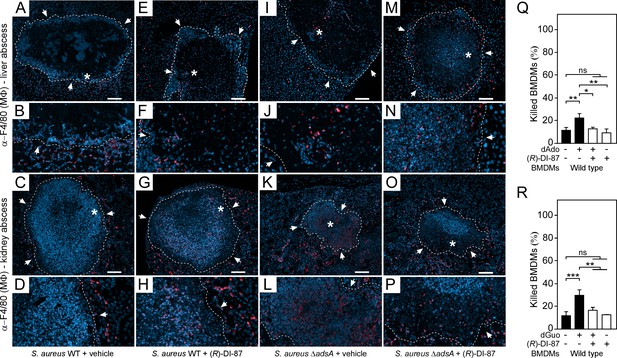
(R)-DI-87-mediated inhibition of dCK enhances macrophage infiltration into staphylococcal abscesses.
(A–P) Immunofluorescence microscopy-based detection of macrophages in liver or renal tissues isolated 5 days after intravenous injection of 107 CFU of wild-type S. aureus Newman (WT) (A–H) or its adsA mutant (ΔadsA) (I–P) into female C57BL/6 mice treated with (R)-DI-87 (75 mg/kg) or vehicle (40% Captisol). Mice received (R)-DI-87 (75 mg/kg) or vehicle (40% Captisol) via oral gavage every 12 hr. White arrows point at the periphery of infectious foci (dashed lines). Magnifications of lesions from upper panels are indicated. Asterisk symbols define the region enlarged in the magnification counterpart images. Thin sections were stained with α-F4/80 antibodies (macrophages; red). Nuclei were labeled with DAPI (blue). Scale bars shown in the upper panels depict 100 μm length. Representative images are shown. (Q, R) Survival rates of female mice-derived bone marrow-derived macrophages (BMDMs) exposed to dAdo (Q) or dGuo (R) in the presence (+) or absence (-) of 1 µM (R)-DI-87. Cells were also exposed to the inhibitor or vehicle only. 200 µM of dAdo or dGuo were used to treat the cells. Cell survival rates were analyzed 48 hr post-treatment. Data are the mean (± standard deviation [SD]) values from three independent determinations. Statistically significant differences were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test; ns, not significant (p≥0.05); *, p<0.05; **, p < 0.01; ***, p<0.001.
-
Figure 5—source data 1
Data used to generate Figure 5.
- https://cdn.elifesciences.org/articles/91157/elife-91157-fig5-data1-v1.zip
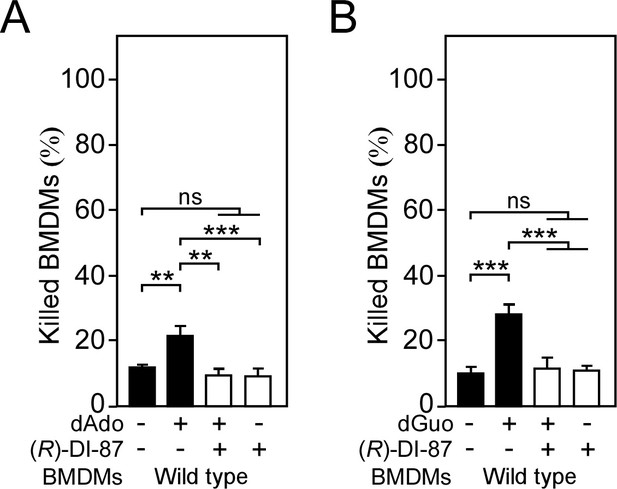
(R)-DI-87-mediated inhibition of dCK shields male mice-derived phagocytes from death-effector deoxyribonucleosides.
(A, B) Survival rates of male mice-derived bone-marrow-derived macrophages (BMDMs) exposed to dAdo (A) or dGuo (B) in the presence (+) or absence (-) of 1 µM (R)-DI-87. Cells were also exposed to the inhibitor or vehicle only. 200 µM of dAdo or dGuo were used to treat the cells. Cell survival rates were analyzed 48 hr post-treatment. Data are the mean (± standard deviation [SD]) values from three independent determinations. Statistically significant differences were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test; ns, not significant (p≥0.05); **, p < 0.01; ***, p<0.001.
-
Figure 5—figure supplement 1—source data 1
Data used to generate Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/91157/elife-91157-fig5-figsupp1-data1-v1.zip
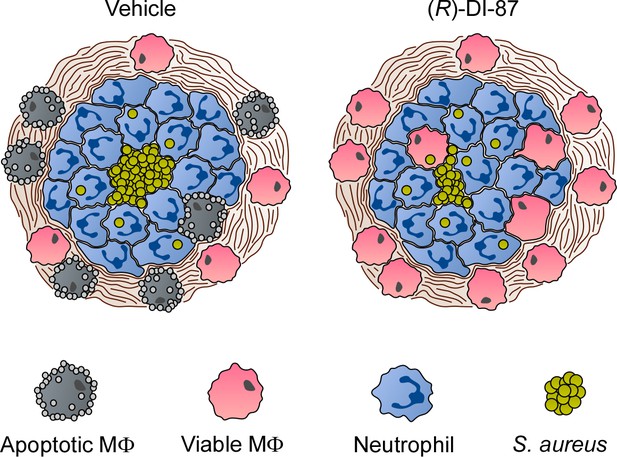
Proposed model of (R)-DI-87-mediated protection of phagocytes during S. aureus abscess formation.
Diagram illustrating the (R)-DI-87-mediated protection of macrophages during the development of staphylococcal abscesses. While phagocytes get killed by S. aureus-derived death-effector deoxyribonucleosides in vehicle-treated animals, (R)-DI-87 protects macrophages and boosts their infiltration into the deeper cavity of infectious foci thereby enhancing eradication of staphylococci.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Staphylococcus aureus Newman) | S. aureus Newman wild type | Duthie and Lorenz, 1952 | N/A | |
| Strain, strain background (S. aureus Newman ∆adsA) | S. aureus Newman ∆adsA | Tantawy et al., 2022 | N/A | |
| Strain, strain background (Escherichia coli BL21 (DE3) pGEX-2T-adsA) | E. coli BL21 (DE3) harboring pGEX-2T-adsA | Thammavongsa et al., 2009 | N/A | |
| Cell line (Homo sapiens) | U937 | ATCC | ATCC CRL-1593.2 | |
| Cell line (H. sapiens) | U937 DCK-/- | Winstel et al., 2018 | N/A | |
| Biological sample (human blood) | Blood samples from healthy donors | Hannover Medical School | N/A | |
| Antibody | α-CASP3, rabbit polyclonal | Cell Signaling | 9662 | 1:1000 |
| Antibody | α-GAPDH, rabbit monoclonal | Abcam | ab181602 | 1:10000 |
| Antibody | α-rabbit IgG, HRP-linked, goat polyclonal | Cell Signaling | 7074 | 1:10000 |
| Antibody | α-F4/80, rabbit monoclonal | Cell Signaling | 70076 | 1:150 |
| Antibody | α-rabbit IgG (H+L) Alexa Fluor 546, goat polyclonal | Invitrogen | A-11071 | 1:500 |
| Antibody | α-B220-PerCP/Cy5.5, rat monoclonal | BioLegend | 103236 | 1:100 |
| Antibody | α-CD4-BV711, rat monoclonal | BioLegend | 100550 | 1:100 |
| Antibody | α-CD8α-PE, rat monoclonal | BioLegend | 100708 | 1:100 |
| Antibody | α-CD11c-PE/Dazzle594, Armenian hamster monoclonal | BioLegend | 117347 | 1:100 |
| Antibody | α-CD11b-FITC, rat monoclonal | BioLegend | 101206 | 1:100 |
| Antibody | α-GR1-APC, rat monoclonal | BioLegend | 108412 | 1:100 |
| Antibody | α-CD16/32 (FC block), rat monoclonal | BioLegend | 101319 | 1:100 |
| Peptide, recombinant protein | Recombinant AdsA (rAdsA) | This study | N/A | |
| Commercial assay or kit | FITC Annexin V | BD | 556419 | |
| Commercial assay or kit | MojoSort Human CD14 Selection Kit | BioLegend | 480026 | |
| Commercial assay or kit | Caspase-3 Assay Kit | Sigma | CASP3C-1KT | |
| Chemical compound, drug | (R)-DI-87 | University of California, LA | N/A | |
| Chemical compound, drug | Captisol | CyDex Pharmaceuticals, Inc. | RC-0C7-020 | |
| Chemical compound, drug | Human macrophage colony stimulating factor | Genscript | Z02914 | |
| Chemical compound, drug | Mouse macrophage colony stimulating factor | Genscript | Z02930 | |
| Chemical compound, drug | Phorbol 12-myristate 13-acetate (PMA) | Sigma | P8139 |
Additional files
-
Supplementary file 1
Minimum inhibitory concentration of (R)-DI-87.
- https://cdn.elifesciences.org/articles/91157/elife-91157-supp1-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/91157/elife-91157-mdarchecklist1-v1.pdf





