Phasic locus coeruleus activity enhances trace fear conditioning by increasing dopamine release in the hippocampus
Figures
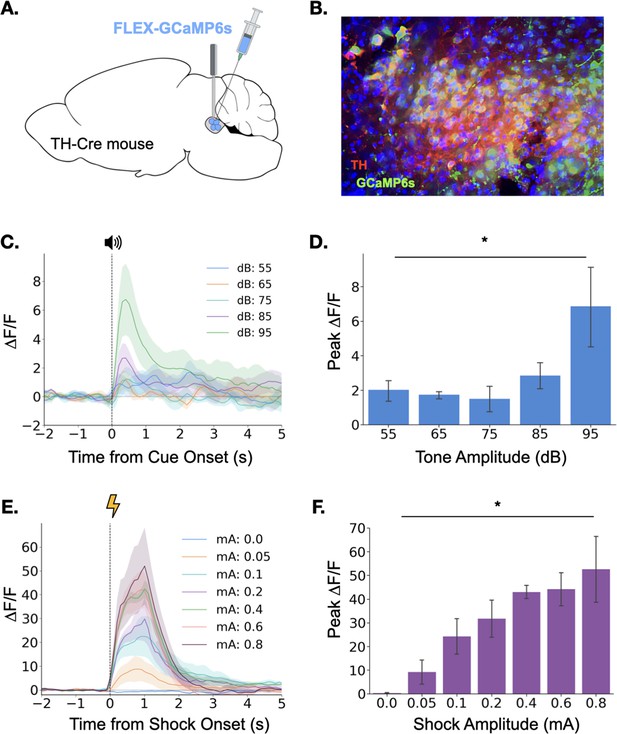
Locus coeruleus responses to neutral and aversive stimuli.
(A) Schematic of virus infusion and fiber implant in LC. FLEX-GCaMP6s was infused into the LC of TH-Cre mice (n=4) and an optical fiber was implanted just above the injection site. (B) GCaMP6s expression in a sagittal section of LC. Green = GCaMP, Red = Tyrosine Hydroxylase. (C) Fiber photometry traces of LC responses to tone onset at varying dB levels. Dashed line indicates tone onset. (D) Peak ΔF/F during tone onset differed across tone amplitudes (F(4,12) = 3.36, p<0.05). Mean +/- SEM. (E) Fiber photometry traces of LC responses to shock onset at varying mA levels. Dashed line indicates shock onset. (F) Peak ΔF/F at shock onset increased across shock amplitudes (F(6,18) = 6.46, p<0.001). Mean +/- SEM.
© 2024, BioRender Inc. Panel A was created using BioRender, and is published under a CC B-NC-ND license. Further reproductions must adhere to the terms of this license.
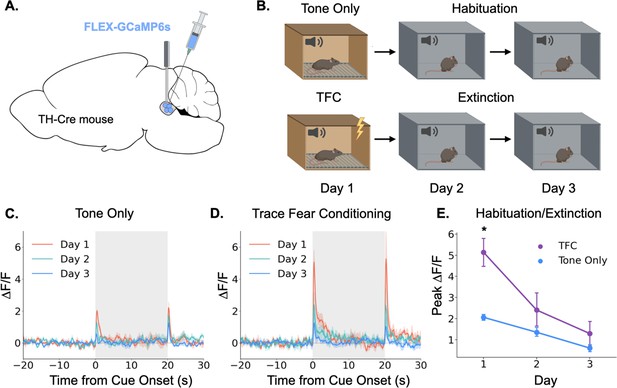
Locus coeruleus responses are modified by learning.
(A) Cre-dependent GCaMP6s was infused into the LC of Th-Cre mice (TFC, n=4; tone only, n=5) and a fiber optic was implanted above the injection site. (B) Schematic of behavioral conditions. On Day 1, mice received either 10 tone presentations or 10 tone-shock pairings (trace fear conditioning) in Context A. On Day 2, all mice received 10 tone presentations in Context B. On Day 3, all mice received 20 tone presentations in Context B. (C) Fiber photometry traces of LC responses to tone onset and termination in tone only animals across the 3 experimental days. The response to tone onset decreased significantly across days (F(2,8) = 18.3, p<0.01). The response to tone termination did not change across days (F(2,8) = 3.36, p>0.05).(D) Fiber photometry traces of LC responses to tone onset and termination in trace fear conditioned animals across the 3 experimental days. The responses to both tone onset and tone termination decreased significantly across days (Onset: F(2,6) = 13.8, p<0.01; Termination: F(2,6) = 9.84, p<0.05). (E) Peak ΔF/F during tone onset for trace fear conditioned and tone only mice across the 3 experimental days differed significantly on day 1, but not days 2 or 3 (Day x Group interaction: F(2,14) = 6.45, p<0.05; Day 1, TFC vs tone only: t(7) = 3.74, p<0.05; Day 2: t(7) = 1.24, p>0.05; Day 3: t(7) = 1.09, p>0.05).
© 2024, BioRender Inc. Panels A and B were created using BioRender, and are published under a CC B-NC-ND license. Further reproductions must adhere to the terms of this license.
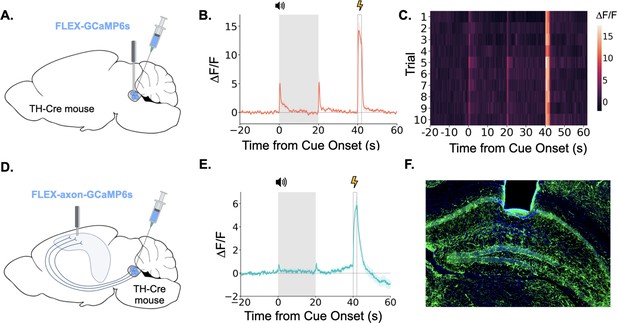
LC neurons and LC-dHPC projections respond to trace conditioning.
(A) Cre-dependent GCaMP6s was infused into the LC of TH-Cre mice (n=4) and an optical fiber implanted above the infusion site. (B) Fiber photometry trace showing LC Ca2+ activity during trace fear conditioning. Ca2+ activity increased significantly at tone onset (t(3) = 6.30, p<0.01) and shock (t(3) = 9.82, p<0.01). Activity increased numerically at tone termination, but did not reach significance (t(3) = 2.91, p=0.062) However, as seen in Figure 2, this response did significantly decrease with extinction. (C) Heatmap of trial by trial Ca2+ activity in LC across 10 trace fear conditioning trials. Activity was consistent across trials. (D) Cre-dependent axon-GCaMP6s was infused into the LC of TH-Cre mice (n=11) and an optical fiber was implanted in the dHPC. (E) Fiber photometry traces showing axon-GCaMP responses to trace conditioning. Ca2+ activity significantly increased at tone onset tone onset (t(10) = 2.77, p<0.05), tone termination (t(10) = 3.03, p<0.05), and shock (t(10) = 8.09, p<0.01). (F) Image showing axon-GCaMP expression (green) in dHPC. Blue = DAPI.
© 2024, BioRender Inc. Panels A and D were created using BioRender, and are published under a CC B-NC-ND license. Further reproductions must adhere to the terms of this license.

Phasic activation of the locus coeruleus enhances long-term memory formation.
(A) Cre-dependent ChR2 was infused into the LC of TH-Cre+ (n=7) and TH-Cre- (n=7) mice and optical fibers were implanted above the infusion site. (B) Expression of ChR2 in LC. Green = ChR2 eYFP, Red = tyrosine hydroxylase, Blue = DAPI. (C) Schematic of behavioral procedures. Animals were trained in trace fear conditioning in Context A on Day 1 and tested in Context B on Day 2. During training, 20 Hz blue light was delivered to the locus coeruleus for two seconds at the beginning and end of the tone as well as during the shock. (D) Memory performance for the two groups during the tone test as indexed by freezing behavior. The groups did not differ at baseline, but the Cre +mice expressing ChR2 froze significantly more during the tone and the post-tone period corresponding to the trace interval on the training day (significant Group x Trial Phase interaction: F(2,24) = 5.28, p<0.05; tones (t(12) = 3.08, p<0.05);traces (t(12) = 3.52, p<0.01)).
© 2024, BioRender Inc. Panels A and C were created using BioRender, and are published under a CC B-NC-ND license. Further reproductions must adhere to the terms of this license.
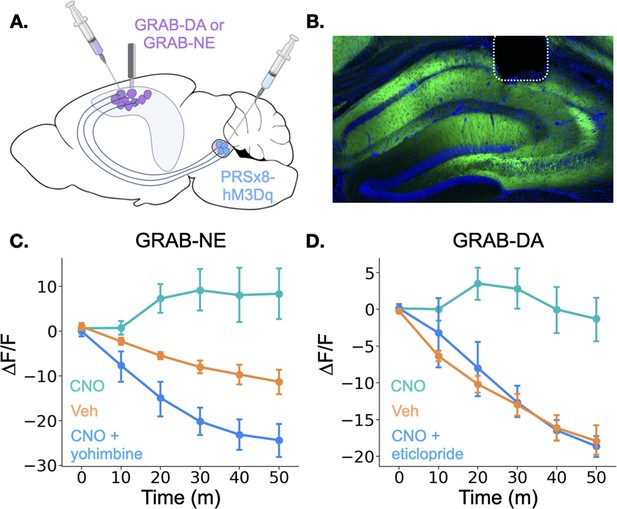
Locus coeruleus activation increases norepinephrine and dopamine in the dorsal hippocampus.
(A) GRAB-DA (n=6) or GRAB-NE (n=5) was infused into the dorsal hippocampus and an optical fiber was implanted in CA1. PRSx8-hM3Dq was infused into the locus coeruleus. (B) GRAB-NE expression and fiber placement in dHPC. (C) CNO injections increased dHPC norepinephrine relative to vehicle or combined CNO and yohimbine injections (Treatment x Time interaction: F(10,40) = 12.5, p<0.05). (D) CNO injections increased dHPC dopamine relative to vehicle or combined CNO and eticlopride injections (Treatment x Time interaction: F(10,50) = 6.45, p<0.05).
© 2024, BioRender Inc. Panel A was created using BioRender, and is published under a CC B-NC-ND license. Further reproductions must adhere to the terms of this license.
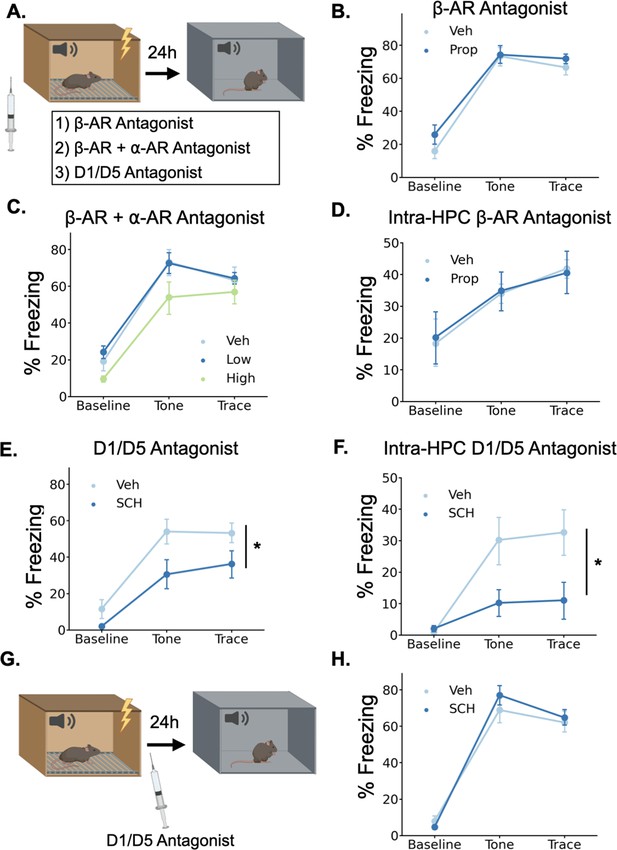
Dopamine, not norepinephrine, is required for trace fear memory formation.
(A) Mice were injected with either propranolol, propranolol and prazosin, or SCH23390 thirty minutes before undergoing trace fear conditioning. The next day, mice were tested for their memory of the tone-shock association in a novel context. (B) Freezing behavior during the tone test did not differ between animals that received propranolol injections (Prop, n=8) and vehicle controls (Veh, n=8) (Drug main effect F(1,14) = 1.15, p>0.05; Drug x Epoch interaction F(2, 28)=0.55, p>0.05). (C) Freezing behavior during the tone test did not differ between animals that received propranolol +prazosin (low (n=6) and high (n=6) doses) and in vehicle controls (n=6)(Drug main effect F(2,15) = 2.26, p>0.05; Drug x epoch interaction F(4, 30)=0.59, p>0.05). (D) Freezing behavior during the tone test did not differ between animals that received intra-hippocampal propranolol infusions (n=8) prior to training and vehicle controls (n=9) (drug main effect F(1,15) = 0.00, p>0.05; drug x epoch interaction F(2,30) = 0.11, p>0.05). (E) Animals that received SCH23390 (SCH, n=8) injections froze significantly less than vehicle controls (Veh, n=8) (Main effect of drug F(1,14) = 6.14, p<0.05). (F) Animals that received intra-hippocampal infusions of SCH23390 (n=5) froze significantly less than vehicle controls (n=5) (Treatment x Phase interaction F(2,16) = 4.62, p<0.05). (G) Mice were injected with SCH23390 immediately after training and tested the next day. (H) There was no significant difference between mice injected with SCH23390 (n=8) after training and vehicle controls (n=8) (Main effect of treatment: F(1, 14)=0.175, p>0.05; Treatment X Phase interaction: F(2,28) = 1.65, p>0.05).
© 2024, BioRender Inc. Panels A and G were created using BioRender, and are published under a CC B-NC-ND license. Further reproductions must adhere to the terms of this license.
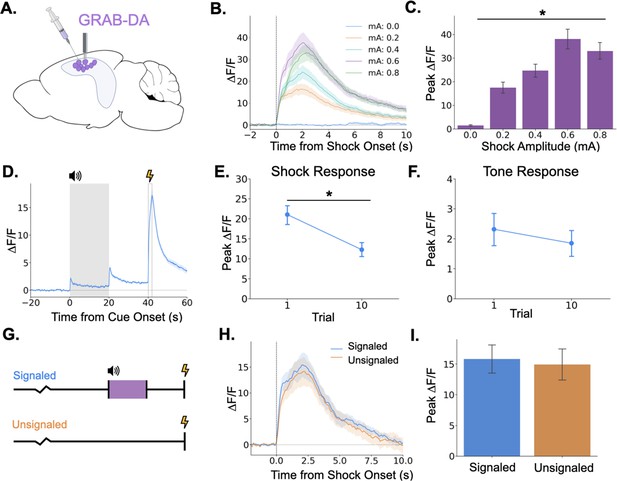
Dopamine release in the hippocampus is consistent with release from locus coeruleus axons.
(A) GRAB-DA3h was infused into the dorsal hippocampus and an optical fiber was implanted in CA1. (B) Fiber photometry traces of GRAB-DA responses to shock onset at varying mA levels. Dashed line indicates shock onset. (C) Peak ΔF/F during shock onset differed across shock amplitudes (n=10) (F(4,36) = 42.4, p<0.001). (D) GRAB-DA photometry traces during trace fear conditioning (n=10). Dopamine increased significantly at tone onset (t(9) = 6.35, p<0.001), tone termination (t(9) = 5.89, p<0.001), and shock (t(9) = 13.0, p<0.001). (E) The shock response decreased significantly between trials 1 and 10 (t(9) = 2.42, p<0.05). (F) The tone response did not change across trials (t(9) = 0.65, p>0.05). (G) Signaled trials were standard trace fear conditioning trials, a twenty-second tone was followed by foot shock after a twenty-second trace interval. Unsignaled trials were the same length, but no tone CS was present before shock. (H) Average photometry traces for the shock response in signaled vs unsignaled trials (n=10 animals; 5 trials signaled, 5 unsignaled). (I) There was no difference in dopamine response on signaled versus unsignaled trials (t(4) = 1.76, p>0.05).
© 2024, BioRender Inc. Panel A was created using BioRender, and is published under a CC B-NC-ND license. Further reproductions must adhere to the terms of this license.





