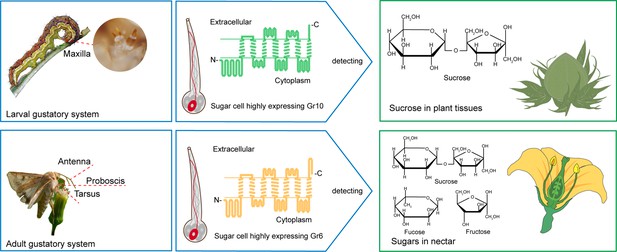
The larva and adult of Helicoverpa armigera use differential gustatory receptors to sense sucrose
Figures
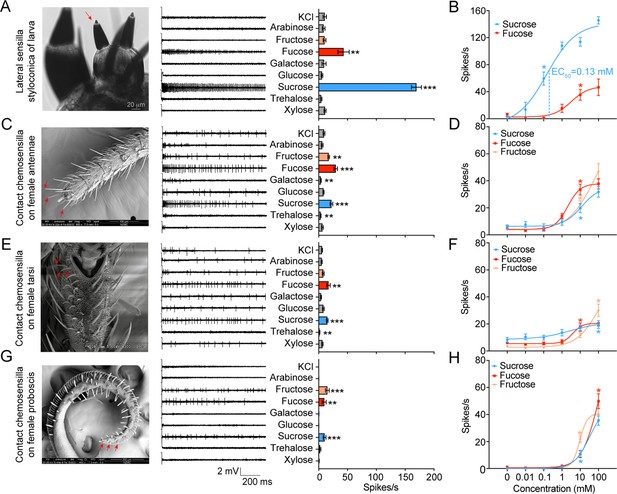
Electrophysiological responses of larval and adult contact chemosensilla in Helicoverpa armigera to sugars.
(A) The maxilla of the larva (left), the representative spike traces of the responses of lateral sensilla styloconica on larval maxillary galea to eight sugars at 10 mM (middle), and quantifications of the firing rates (right) (n=13). The red arrow marks the recorded lateral sensillum styloconicum. Scale bar represents 20 μm. (B) Dose–responses of the lateral sensilla styloconica on larval maxillary galea to sucrose and fucose (sucrose: n=8; fucose: n=6). (C) The antenna terminal of the female (left), the representative spike traces of the responses of the contact chemosensilla on female antennae to eight sugars at 10 mM (middle), and quantifications of the firing rates (right) (n=21). The red arrows mark the recorded contact chemosensilla. Scale bar represents 100 μm. (D) Dose–responses of the contact chemosensilla on female antennae to sucrose, fucose, and fructose (n=21). (E) The fifth tarsomere of the female (left) (Zhang et al., 2010), the representative spike traces of the responses of contact chemosensilla on female tarsi to eight sugars at 10 mM (middle), and quantifications of the firing rates (right) (n=15). The red arrows mark the recorded contact chemosensilla. Scale bar represents 100 μm. (F) Dose–responses of the contact chemosensilla on female tarsi to sucrose, fucose, and fructose (n=21). (G) The proboscis terminal of the female (left), the representative spike traces of the responses of contact chemosensilla on female proboscis to eight sugars at 10 mM (middle), and quantifications of the firing rates (right) (n=21). The red arrows mark the recorded contact chemosensilla. Scale bar represents 200 μm. (H) Dose–responses to sucrose (n=21), fucose (n=21), and fructose (fructose 0 mM, n=18; fructose 0.01 mM-100 mM, n=21) of the contact chemosensilla on the female proboscis. (A to H) Data are mean ± SEM; * p<0.05; ** p<0.01; *** p<0.001. (A, C, E, G) Data were analyzed by independent-samples t test (compared with control). (B, D, F, H) Data were analyzed by one-way ANOVA with Tukey’s HSD test.
© 2010, Journal of Experimental Biology. Figure 1E is taken from Figure 1A in Zhang et al., 2010, Journal of Experimental Biology. It is not covered by the CC-BY 4.0 license and further reproduction of this panel would need permission from the copyright holder.
-
Figure 1—source data 1
Electrophysiological responses of larval and adult contact chemosensilla in Helicoverpa armigera to sugars.
(Figure 1A) The electrophysiological responses of the lateral sensilla styloconica on the maxillary galea of H. armigera 5th instar larvae to test compounds. (Figure 1B) The electrophysiological responses of the lateral sensilla styloconica on the maxillary galea of H. armigera 5th instar larvae to sucrose and fucose at different concentrations. (Figure 1C) The electrophysiological responses of the contact chemosensilla on antennae of H. armigera female adults to test compounds. (Figure 1D) The electrophysiological responses of the contact chemosensilla on the antennae of H. armigera female adults to sucrose, fucose and fructose at different concentrations. (Figure 1E) The electrophysiological responses of the contact chemosensilla on the tarsi of H. armigera female adults to test compounds. (Figure 1F) The electrophysiological responses of the contact chmosensilla on the tarsi of H. armigera female adults to sucrose, fucose, and fructose at different concentrations. (Figure 1G) The electrophysiological responses of the contact chemosensilla on the proboscis of H. armigera female adults to test compounds. (Figure 1H) The electrophysiological responses of the contact chemosensilla on the proboscis of H. armigera female adults to sucrose, fucose, and fructose at different concentrations.
- https://cdn.elifesciences.org/articles/91711/elife-91711-fig1-data1-v2.xlsx
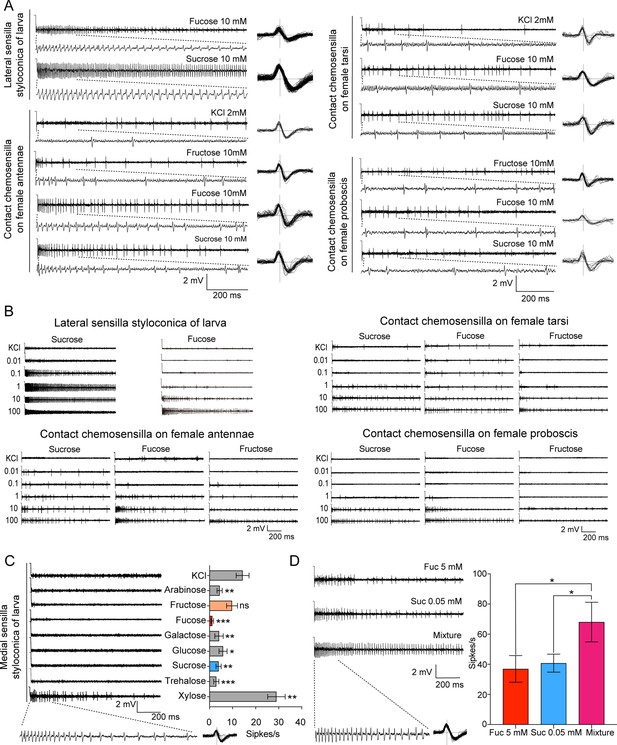
Electrophysiological responses of contact chemosensilla in Helicoverpa armigera to sugars.
(A) The representative spike traces and waveforms of lateral sensilla styloconica on larval maxillary galea and contact chemosensilla on female antennae, tarsi and proboscis responding to test compounds. (B) Representative dose–response spike traces of the lateral sensilla styloconica on larval maxillary galea and contact chemosensilla on female antennae, tarsi and proboscis to sucrose, fucose, and fructose. (C) The representative spike traces of the responses of medial sensilla styloconica on larval maxillary galea to eight sugars at 10 mM (left), and quantifications of the firing rates (right) (n=13). (D) The representative spike traces of the responses of lateral sensilla styloconica on larval maxillary galea (left), and quantifications of the firing rates to 0.05 mM sucrose, 5 mM fucose, and the mixture of those (right) (n=6). (A–B) The dotted lines indicate the spike trace of the first 200ms. (C–D) Data are mean ± SEM; * p<0.05; ** p<0.01; *** p<0.001; ns indicates no significance (independent-samples t test).
-
Figure 1—figure supplement 1—source data 1
Electrophysiological responses of contact chemosensilla in Helicoverpa armigera to sugars.
(Figure 1—figure supplement 1C) The electrophysiological responses of the medial sensilla styloconica on maxillary galea of H. armigera 5th instar larvae to test compounds. (Figure 1—figure supplement 1D) The electrophysiological responses of the lateral sensilla styloconica on maxillary galea of H. armigera 5th instar larvae to fucose, sucrose and their mixture.
- https://cdn.elifesciences.org/articles/91711/elife-91711-fig1-figsupp1-data1-v2.xlsx
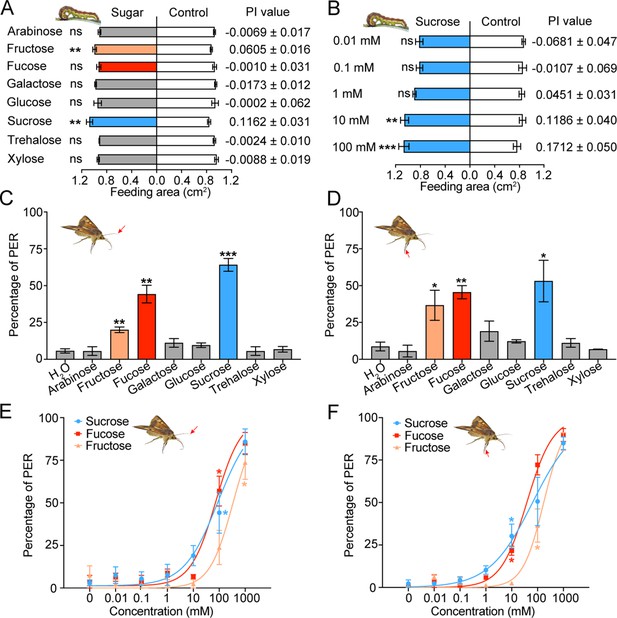
Behavioral responses of Helicoverpa armigera larvae and adults to sugars.
(A) Feeding responses and the preference index (PI) value of 5th instar larvae to eight sugars painted on the cabbage leaf discs at 10 mM in two-choice tests. ** p<0.01; ns indicates no significance, p≥0.05 (paired t test, n=20). (B) Feeding responses and the PI value of 5th instar larvae to different concentrations of sucrose painted on the cabbage leaf discs in two-choice tests. ** p<0.01, *** p<0.001; ns indicates no significance, p≥0.05 (paired t test, n=20). (C) Proboscis extension reflex (PER) in adult females upon antennal stimulation by eight sugars at 100 mM. ** p<0.01; *** p<0.001 (independent-samples t test compared with control, n=3). (D) PER in adult females upon tarsal stimulation by eight sugars at 100 mM. * p<0.05; ** p<0.01 (independent-samples t test, n=3). (E) PER in adult females upon antennal stimulation by different concentrations of sucrose, fucose, and fructose. * p<0.05 (one-way ANOVA with Tukey’s HSD test, n=3). (F) PER in adult females upon tarsal stimulation by different concentrations of sucrose, fucose, and fructose (n=3). * p<0.05 (one-way ANOVA with Tukey’s HSD test, n=3). (A to F) Data are mean ± SEM. The red arrow indicates the stimulating site.
-
Figure 2—source data 1
Behavioral responses of Helicoverpa armigera larvae and adults to sugars.
(Figure 2A) Feeding area of H. armigera 5th instar larvae in two-choice tests of eight sugars. (Figure 2B) Feeding area of H. armigera 5th instar larvae in two-choice tests of sucrose at different concentrations. (Figure 2C) Proboscis extension reflex (PER) in adult females upon antennal stimulation by eight sugars. (Figure 2D) PER in adult females upon tarsal stimulation by eight sugars. (Figure 2E) PER in adult females upon antennal stimulation by different concentrations of sucrose, fucose, and fructose. (Figure 2F) PER in adult females upon tarsal stimulation by different concentrations of sucrose, fucose, and fructose.
- https://cdn.elifesciences.org/articles/91711/elife-91711-fig2-data1-v2.xlsx
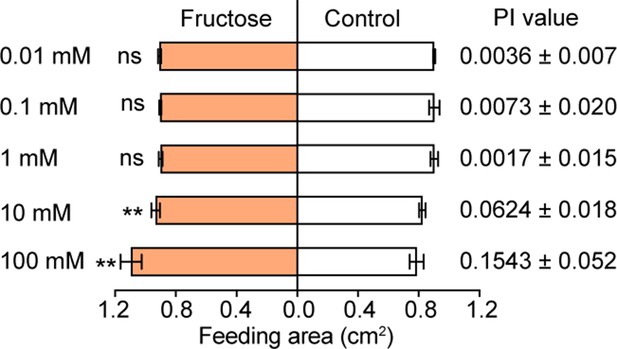
Feeding responses and the PI value of 5th instar larvae of Helicoverpa armigera to different concentrations of fructose painted on the cabbage leaf discs in two-choice tests.
Data are mean ± SEM; ** p<0.01; ns indicates no significance (paired t test, n=20).
-
Figure 2—figure supplement 1—source data 1
Feeding area of H. armigera 5th-instar larvae in two-choice tests to fructose at different concentrations.
- https://cdn.elifesciences.org/articles/91711/elife-91711-fig2-figsupp1-data1-v2.xlsx
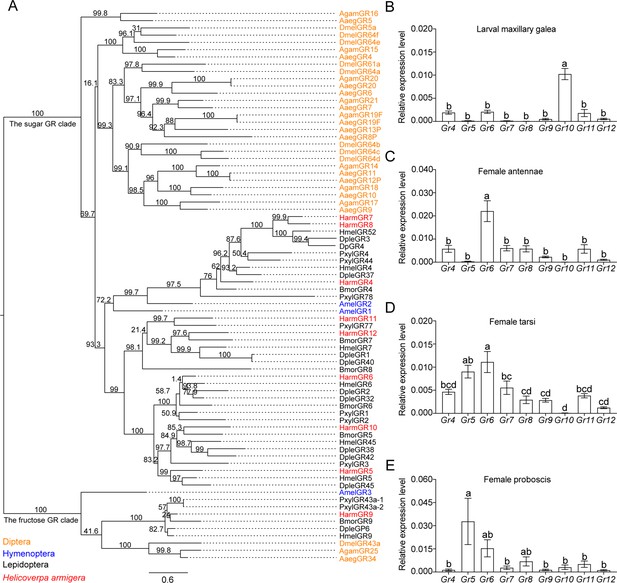
The phylogenetic relationship and the expression level of sugar GRs in larval maxilla and female adult antennae, tarsi and proboscis of Helicoverpa armigera.
(A) The phylogenetic tree of insect sugar GRs. Diptera (orange): Aaeg, Aedes aegypti; Agam, Anopheles gambiae; Dmel, Drosophila melanogaster. Hymenoptera (blue): Am, Apis mellifera. Lepidoptera (black): Bmor, Bombyx mori; Dple, Danaus plexippus; Hmel, Heliconius melpomene; Pxyl, Plutella xylostella. Harm (red), Helicoverpa armigera. Numbers above branches indicate ultrafast bootstrap approximation (UFBoot). (B) Relative expression levels of sugar GRs in the maxillary galea of 5th instar larvae of H. armigera determined by qRT-PCR. (C) Relative expression levels of sugar GRs in the female adult antennae. (D) Relative expression levels of sugar GRs in female adult tarsi. (E) Relative expression levels of sugar GRs in female adult proboscis. (B to E) Data are mean ± SEM. One-way ANOVA was used, and different letters labeled indicate significant difference (Tukey’s HSD test, p<0.05, n=3).
-
Figure 3—source data 1
The expression levels of sugar GRs in larval maxilla and female adult antennae, tarsi and proboscis of Helicoverpa armigera.
(Figure 3B) Expression levels of sugar GRs in the maxillary galea of H. armigera larvae. (Figure 3C) Expression levels of sugar GRs in the antennae of H. armigera female adults. (Figure 3D) Expression levels of sugar GRs in the tarsi of H. armigera female adults. (Figure 3E) Expression levels of sugar GRs in the proboscis of H. armigera female adults.
- https://cdn.elifesciences.org/articles/91711/elife-91711-fig3-data1-v2.xlsx
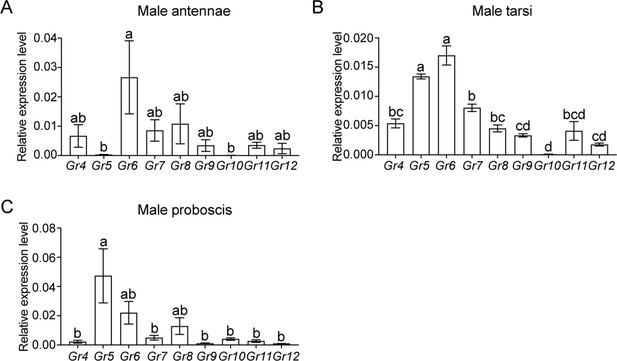
Expression patterns of sugar GRs in taste organs of Helicoverpa armigera male adults.
(A) Relative expression levels of sugar GRs in male antennae determined by qRT-PCR. (B) Relative expression levels of sugar GRs in male tarsi. (C) Relative expression levels of sugar GRs in male proboscis. (A–C) Data are mean ± SEM. One-way ANOVA was used, and different letters labeled indicate significant difference (Tukey’s HSD test, p<0.05, n=3).
-
Figure 3—figure supplement 1—source data 1
Expression patterns of sugar GRs in taste organs of Helicoverpa armigera male adults.
(Figure 3—figure supplement 1A) Expression levels of sugar GRs in the antennae of H. armigera male adults. (Figure 3—figure supplement 1B) Expression levels of sugar GRs in the tarsi of H. armigera male adults. (Figure 3—figure supplement 1C) Expression levels of sugar GRs in the proboscis of H. armigera male adults.
- https://cdn.elifesciences.org/articles/91711/elife-91711-fig3-figsupp1-data1-v2.xlsx
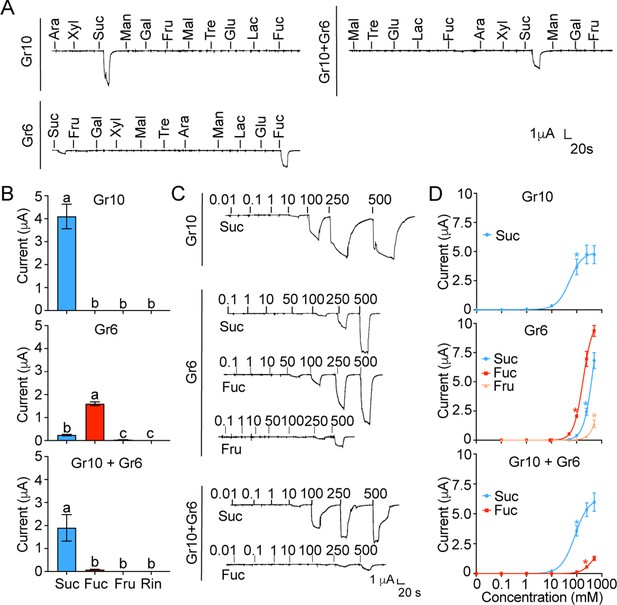
The inward current responses of the Xenopus oocytes expressing sugar GRs of Helicoverpa armigera to sugars.
(A) The representative traces of the oocytes expressing Gr10, Gr6, and Gr10 +Gr6 to 11 sugars at 100 mM. (B) The responses of the oocytes expressing Gr10, Gr6, and Gr10 +Gr6 to sugars at 100 mM (Gr10, n=14. Gr6, n=20. Gr10 +Gr6, n=7). (C) The representative trace of the oocytes expressing Gr10 to sucrose (the upper), the representative trace of the oocytes expressing Gr6 to sucrose, fucose, and fructose (the middle), the representative trace of the oocytes expressing Gr10 +Gr6 to sucrose and fucose (the lower). (D) The dose-responses of the oocytes expressing Gr10 to sucrose (the upper, n=13); the dose-responses of the oocytes expressing Gr6 to sucrose, fucose, and fructose (the middle, sucrose: n=6; fucose: n=6; fructose: n=3); the dose-responses of the oocytes expressing Gr10 +Gr6 to sucrose and fucose (the lower, sucrose: n=4; fucose: n=3). (B and D) Data are mean ± SEM, and were analyzed by one-way ANOVA with Tukey’s HSD test (p<0.05). Different letters labeled indicate significant differences, * p<0.05. (A to D) Ara, arabinose; Fru, fructose; Fuc, fucose; Gal, galactose; Glu, glucose; Lac, lactose; Mal, maltose; Man, mannose; Suc, sucrose; Tre, trehalose; Xyl, xylose; Rin, Ringer solution.
-
Figure 4—source data 1
The inward current responses of the Xenopus oocytes expressing sugar GRs of Helicoverpa armigera to sugars.
- https://cdn.elifesciences.org/articles/91711/elife-91711-fig4-data1-v2.xlsx
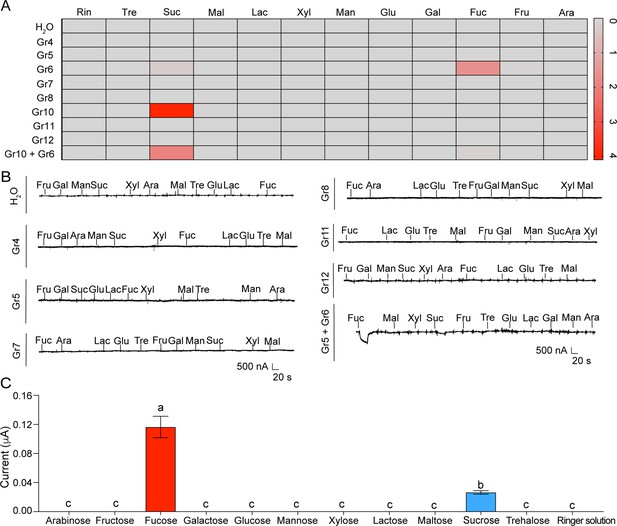
The inward current responses and representative traces of Xenopus oocytes expressing sugar GRs of Helicoverpa armigera.
(A) Heat-map signal indicates the mean of the responses to eleven sugars at 100 mM of the oocytes expressing H. armigera sugar GRs (H2O: mannose, n=5; other sugars and ringer solution: n=6. Gr4: arabinose, n=3; other sugars and ringer solution: n=7. Gr5: n=7. Gr6: mannose, n=15; other sugars and ringer solution: n=20. Gr7: n=5. Gr8: n=5. Gr10: maltose, n=13; other sugars and ringer solution: n=14. Gr11: n=6. Gr12: n=6. Gr10 +Gr6: n=7). Gray and red signals represent lower and higher response levels, respectively. (B) The representative two-electrode voltage-clamp traces of oocytes expressing Gr4, Gr5, Gr7, Gr8, Gr11, and Gr5 +Gr6 to eleven sugars at 100 mM and the control (H2O). (A–B) Ara: arabinose; Fru: fructose; Fuc: fucose; Gal: galactose; Glu: glucose; Lac: lactose; Mal: maltose; Man: mannose; Suc: sucrose; Tre: trehalose; Xyl: xylose; Rin: ringer solution. (C) The responses of the oocytes expressing Gr5 +Gr6 to sugars at 100 mM (n=10). Data (mean ± SEM) were analyzed by one-way ANOVA with Tukey’s HSD test, and different letters labeled indicate significant differences (p<0.05).
-
Figure 4—figure supplement 1—source data 1
The inward current responses of Xenopus oocytes expressing sugar GRs of Helicoverpa armigera to sugars.
(Figure 4—figure supplement 1A) The inward current responses of the oocytes expressing sugar GRs to 100 mM sugars. (Figure 4—figure supplement 1C) The inward current responses of the oocytes expressing Gr5 +Gr6 to 100 mM sugars.
- https://cdn.elifesciences.org/articles/91711/elife-91711-fig4-figsupp1-data1-v2.xlsx
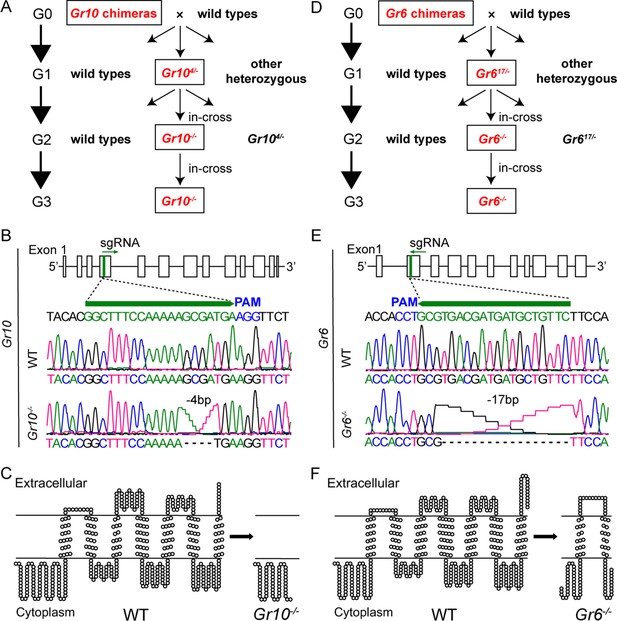
Establishment of Gr10 and Gr6 homozygous mutants (Gr10-/- and Gr6-/-) of Helicoverpa armigera via CRISPR/Cas9.
(A) The cross process of obtaining Gr10-/-. (B) The genomic structure of Gr10, the single-guide RNA (sgRNA) targeting sequence (in green), and representative chromatograms of direct sequencing of the PCR products obtained from wild types (WT) and Gr10-/-, in which 4 bp of the Gr10 sequence were deleted. (C) The predicted secondary structures of the Gr10 protein in WT and the truncated Gr10 protein in Gr10-/-. (D) The cross process of obtaining Gr6-/-. (E) The genomic structure of Gr6, the single-guide RNA (sgRNA) targeting sequence (in green), and representative chromatograms of direct sequencing of the PCR products obtained from WT and Gr6-/-, in which 17 bp of the Gr6 sequence were deleted. (F) The predicted secondary structures of the Gr6 protein in WT and the truncated Gr6 protein in Gr6-/-. (B and E) Boxes represent exons, black lines represent introns, the green arrowhead indicates the direction of sgRNA; the protospacer adjacent motif (PAM) is in blue. (C and F) The secondary structure of Gr10 and Gr6 in WT, Gr10-/- and Gr6-/- was predicted by https://dtu.biolib.com/DeepTMHMM, and the image was constructed by TOPO2 software (http://www.sacs.ucsf.edu/TOPO2).
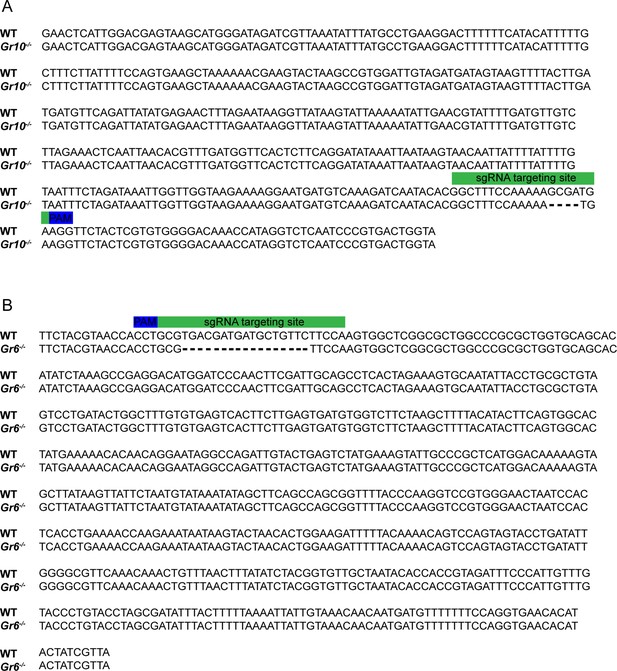
Confirmation of the deletion of Gr10 and Gr6 in Gr10-/- and Gr6-/- mutants at mRNA level.
(A) The alignment of the nucleic acid sequences based on Gr10 transcripts in WT and Gr10-/-. (B) The alignment of the nucleic acid sequences based on Gr6 transcripts in WT and Gr6-/-. The green rectangle corresponds to the position of the sgRNA targeting site, the blue rectangle corresponds to the position of the PAM site.
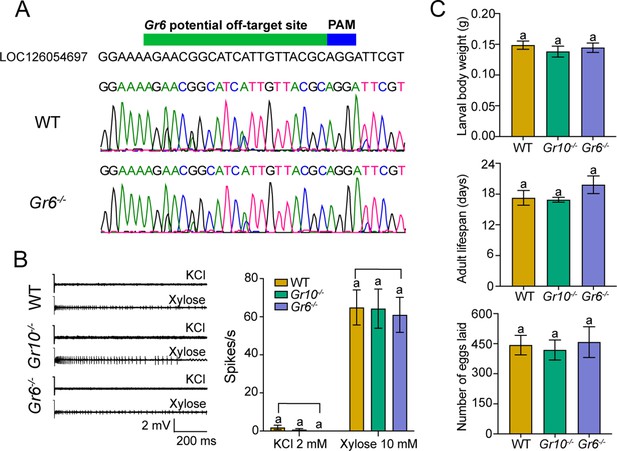
The potential off-target effects detection.
(A) Representative chromatograms of potential off-target PCR products obtained from the wild type (WT) and Gr6-/-. (B) The representative spike traces (the left) and quantifications of the firing rate (the right) of medial sensilla styloconica on larval maxillary galea to 2 mM KCl and 10 mM xylose (n=8). (C) The larval body weight at the beginning of the fifth instar (the upper, n=16), the adult lifespan (the middle, n=16), and the number of eggs laid (the lower, n=5). (B–C) Data are mean ± SEM. Data were analyzed by one-way ANOVA with Turkey’s HSD test, and different letters labeled indicate significant differences (p<0.05).
-
Figure 5—figure supplement 2—source data 1
The source data of potential off-target effects detection.
(Figure 5—figure supplement 2B) The electrophysiological responses of the medial sensilla styloconica of larvae in the wild type (WT) and two mutants of H. armigera to KCl and xylose. (Figure 5—figure supplement 2C) The larval body weight, adult lifespan, and the number of eggs laid by females of the WT and two mutants of H. armigera.
- https://cdn.elifesciences.org/articles/91711/elife-91711-fig5-figsupp2-data1-v2.xlsx
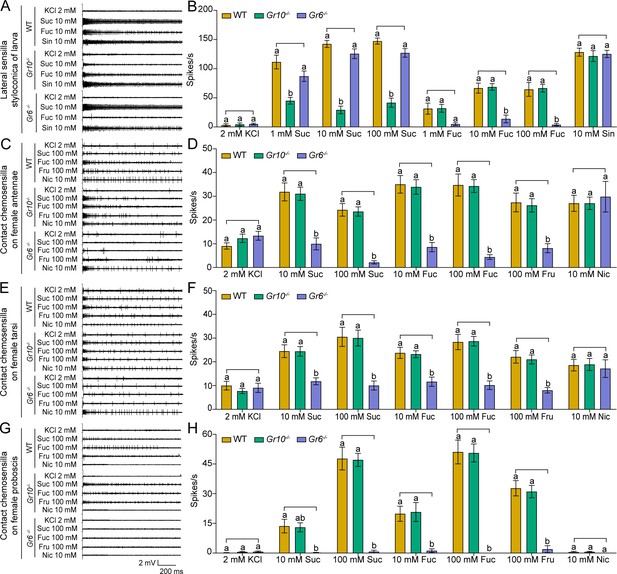
Electrophysiological responses of larval and adult contact chemosensilla in WT, Gr10-/- and Gr6-/- of Helicoverpa armigera to sucrose and other compounds.
(A) The representative spike traces of lateral sensilla styloconica on larval maxillary galea. (B) Quantifications of the firing rates of the lateral sensilla styloconica on larval maxillary galea (mean ± SEM; WT, n=12; Gr6-/-, n=13; Gr10-/-, n=13). (C) The representative spike traces of contact chemosensilla on female antennae. (D) Quantifications of the firing rates of the contact chemosensilla on female antennae (mean ± SEM; WT, n=20. Gr6-/-: nicotine, n=17; other compounds, n=18. Gr10-/-, n=20). (E) The representative spike traces of contact chemosensilla on female tarsi. (F) Quantifications of the firing rates of the contact chemosensilla on female tarsi (mean ± SEM; WT, n=18; Gr6-/-: nicotine, n=14. other compounds, n=18. Gr10-/-, n=18). (G) The representative spike traces of contact chemosensilla on female proboscis. (H) Quantifications of the firing rates of the contact chemosensilla on female proboscis (mean ± SEM; WT: sucrose 100 mM, n=17; other compounds, n=18. Gr6-/-: sucrose 100 mM, n=17; other compounds, n=18. Gr10-/-, n=18). (B, D, F, and H) Two-way ANOVA with post hoc Tukey’s multiple comparison was used separately for sucrose and fucose, and one-way ANOVA with Tukey’s HSD test was used for KCl, sinigrin, fructose, and nicotine. Different letters labeled indicate significant differences (P<0.05). Suc: sucrose; Fuc: fucose; Sin: sinigrin; Fru: fructose; Nic: nicotine.
-
Figure 6—source data 1
Electrophysiological responses of larval and adult contact chemosensilla in WT, Gr10-/- and Gr6-/- of Helicoverpa armigera to sucrose and other compounds.
(Figure 6B) The electrophysiological responses of the lateral sensilla styloconica on the maxillary galea of larvae in the WT and two mutants of H. armigera to test compounds. (Figure 6D) The electrophysiological responses of the contact chemosensilla on the antennae of female adults in the WT and two mutants of H. armigera to test compounds. (Figure 6F) The electrophysiological responses of the contact chemosensilla on the fore leg tarsi of female adults in the WT and two mutants of H. armigera to test compounds. (Figure 6H) The electrophysiological responses of the contact chemosensilla on the proboscis of female adults in the WT and two mutants of H. armigera to test compounds.
- https://cdn.elifesciences.org/articles/91711/elife-91711-fig6-data1-v2.xlsx
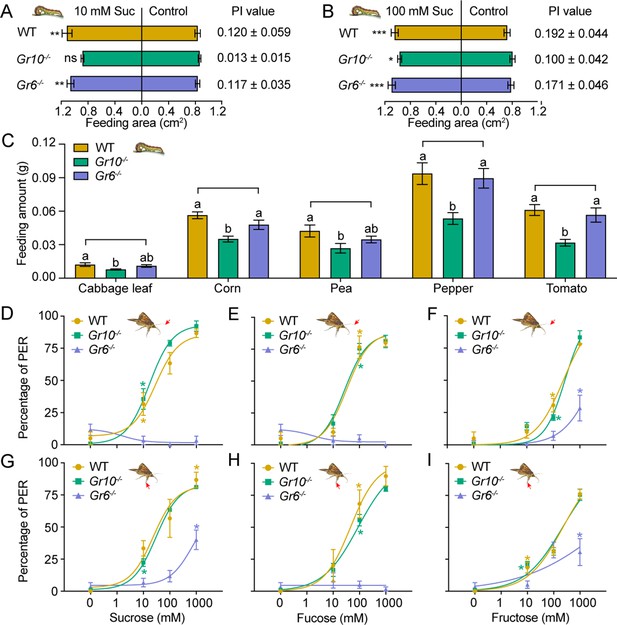
Behavioral responses of WT, Gr10-/- and Gr6-/- larvae and adults of Helicoverpa armigera to sugars and plant tissues.
(A) Feeding area of 5th instar larvae in two-choice tests and the PI value to 10 mM sucrose (n=20). ** p<0.01; ns indicates no significance, p ≥ 0.05 (paired t test). Suc, sucrose. (B) Feeding area of 5th instar larvae in two-choice tests and the PI value to 100 mM sucrose (n=20). * p<0.05; ** p<0.01; *** p<0.001 (paired t test). Suc, sucrose. (C) Feeding amount of 5th instar larvae on cabbage leaves, corn kernels, pea seeds, pepper fruits, and tomato fruits in no-choice tests (n=20). Data were analyzed by one-way ANOVA with Tukey’s HSD test, and different letters labeled on the data of WT, Gr10-/- and Gr6-/- for each plant tissue indicate significant differences (p<0.05). Proboscis extension reflex (PER) in adult females upon (D) antennal stimulation by sucrose concentrations (n=3), (E) antennal stimulation by fucose concentrations (n=3), (F) antennal stimulation by fructose concentrations (n=3), (G) tarsal stimulation by sucrose concentrations (n=3), (H) tarsal stimulation by fucose concentrations (n=3), and (I) tarsal stimulation by fructose concentrations (n=3). (A to I) Data are mean ± SEM. (D to I) Data were analyzed by two-way ANOVA with post hoc Tukey’s multiple comparison. * p<0.05. The red arrow indicates the stimulating site.
-
Figure 7—source data 1
Behavioral responses of WT, Gr10-/- and Gr6-/- larvae and adults of Helicoverpa armigera to sugars and plant tissues.
(Figure 7A) Feeding area of the WT, Gr10-/- and Gr6-/- 5th instar larvae of H. armigera in two-choice tests of 10 mM sucrose. (Figure 7B) Feeding area of the WT, Gr10-/- and Gr6-/- 5th instar larvae of H. armigera in two-choice tests of 100 mM sucrose. (Figure 7C) The feeding amount of the 5th instar larvae in no-choice tests of cabbage leaf, corn seed, pea seed, pepper fruit, and tomato fruit. (Figure 7D) Proboscis extension reflex (PER) in females of the WT and Gr10-/- and Gr6-/- mutants of H. armigera upon antennal stimulation by sucrose concentrations. (Figure 7E) PER in females of the WT and Gr10-/- and Gr6-/- mutants of H. armigera upon antennal stimulation by fucose concentrations. (Figure 7F) PER in females of the WT and Gr10-/- and Gr6-/- mutants of H. armigera upon antennal stimulation by fructose concentrations. (Figure 7G) PER in females of the WT and Gr10-/- and Gr6-/- mutants of H. armigera upon tarsal stimulation by sucrose concentrations. (Figure 7H) PER in females of the WT and Gr10-/- and Gr6-/- mutants of H. armigera upon tarsal stimulation by fucose concentrations. (Figure 7I) PER in females of the WT and Gr10-/- and Gr6-/- mutants of H. armigera upon tarsal stimulation by fructose concentrations.
- https://cdn.elifesciences.org/articles/91711/elife-91711-fig7-data1-v2.xlsx
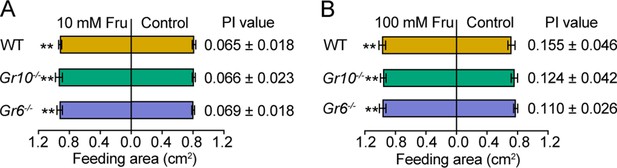
Feeding responses and the PI value of 5th instar larvae of WT, Gr10-/- and Gr6-/- of Helicoverpa armigera to fructose painted on the cabbage leaf discs in two-choice tests.
(A) Feeding area and the PI value to 10 mM fructose. (B) Feeding area and the PI value to 100 mM fructose. Data are mean ± SEM, n=20, paired t test, ** p<0.01. Fru, fructose. Supplementary file 1. GenBank accession numbers for sugar gustatory receptors used in this study.
-
Figure 7—figure supplement 1—source data 1
Behavioral responses of WT, Gr10-/- and Gr6-/- larvae and adults of Helicoverpa armigera to fructose.
(Figure 7—figure supplement 1A) Feeding area of the WT, Gr10-/- and Gr6-/- 5th instar larvae of H. armigera in two-choice tests of 10 mM fructose. (Figure 7—figure supplement 1B) Feeding area of the WT, Gr10-/- and Gr6-/- 5th instar larvae of H. armigera in two-choice tests of 100 mM fructose.
- https://cdn.elifesciences.org/articles/91711/elife-91711-fig7-figsupp1-data1-v2.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Helicoverpa armigera) | Gr4 | GenBank | OP251144 | More details about this gene see Supplementary file 1. |
| Gene (H. armigera) | Gr5 | GenBank | OP251145 | More details about this gene see Supplementary file 1 |
| Gene (H. armigera) | Gr6 | GenBank | OP251146 | More details about this gene see Supplementary file 1 |
| Gene (H. armigera) | Gr7 | GenBank | OP251147 | More details about this gene see Supplementary file 1 |
| Gene (H. armigera) | Gr8 | GenBank | OP251148 | More details about this gene see Supplementary file 1 |
| Gene (H. armigera) | Gr9 | GenBank | XM_049843199 | More details about this gene see Supplementary file 1 |
| Gene (H. armigera) | Gr10 | GenBank | OP251149 | More details about this gene see Supplementary file 1 |
| Gene (H. armigera) | Gr11 | GenBank | OP251150 | More details about this gene see Supplementary file 1 |
| Gene (H. armigera) | Gr12 | GenBank | OP251151 | More details about this gene see Supplementary file 1 |
| Commercial assay or kit | RNeasy Plus Universal Mini Kit | Qiagen | Cat# 73404 | |
| Commercial assay or kit | Q5 High-Fidelity DNA Polymerase | NEB | Cat# M0491 | |
| Commercial assay or kit | TransStart FastPfu DNA Polymerase | TransGen Biotech | Cat# AP221-01 | |
| Commercial assay or kit | M-MLV reverse transcriptase | Promega | Cat# M1701 | |
| Commercial assay or kit | SYBR Premix Ex Taq | Takara | Cat# RR820 | |
| Commercial assay or kit | mMESSAGE mMACHINE SP6 | Ambion | Cat# AM1340 | |
| Commercial assay or kit | GeneArt gRNA Clean Up Kit | Invitrogen | Cat#A29377 | |
| Commercial assay or kit | GeneArt gRNA Prep Kit | Invitrogen | Cat#A29377 | |
| Commercial assay or kit | TrueCut Cas9 protein 2 | Invitrogen | Cat#A36498 | |
| Commercial assay or kit | Animal Tissue PCR Kit | TransGen Biotech | Cat#AD201-01 | |
| Chemical compound, drug | L - (+) - Arabinose | Sigma-Aldrich | CAS: 5328-37-0 | |
| Chemical compound, drug | D - (-) - Fructose | Sigma-Aldrich | CAS: 57-48-7 | |
| Chemical compound, drug | L - (-) - Fucose | Sigma-Aldrich | CAS: 2438-80-4 | |
| Chemical compound, drug | D - (+) - Galactose | Sigma-Aldrich | CAS: 59-23-4 | |
| Chemical compound, drug | D - (+) - Glucose | Sigma-Aldrich | CAS: 50-99-7 | |
| Chemical compound, drug | D - (+) - Mannose | Sigma-Aldrich | CAS: 3458-28-4 | |
| Chemical compound, drug | D - (+) - Xylose | Sigma-Aldrich | CAS: 58-86-6 | |
| Chemical compound, drug | D - Lactose monohydrate | Sigma-Aldrich | CAS: 64044-51-1 | |
| Chemical compound, drug | D - (+)-Maltose monohydrate | Sigma-Aldrich | CAS: 6363-53-7 | |
| Chemical compound, drug | Sucrose | Sigma-Aldrich | CAS: 57-50-1 | |
| Chemical compound, drug | D - (+) - Trehalose dihydrate | Sigma-Aldrich | CAS: 6138-23-4 | |
| Chemical compound, drug | Sodium chloride | Sigma-Aldrich | CAS: 7647-14-5 | |
| Chemical compound, drug | Potassium chloride | Sigma-Aldrich | CAS: 7447-40-7 | |
| Chemical compound, drug | Magnesium chloride hexahydrate | Sigma-Aldrich | CAS: 7791-18-6 | |
| Chemical compound, drug | HEPES | Sigma-Aldrich | CAS: 7365-45-9 | |
| Software, algorithm | SAPID Tools software version 3.5 | Smith et al., 1990; | ||
| Software, algorithm | Autospike 3.7 | Syntech | ||
| Software, algorithm | MAFFT version 7.455 | Rozewicki et al., 2019 | ||
| Software, algorithm | trimAI version 1.4 | Capella-Gutiérrez et al., 2009; | ||
| Software, algorithm | IQ-tree version 6.8 | Nguyen et al., 2015 | http://iqtree.org/ | |
| Software, algorithm | pCLAMP software version 10.4.2.0 | Axon Instruments Inc | RRID:SCR_011323 | |
| Software, algorithm | SnapGene software version 4.3.8 | Insightful Science | https://www.snapgene.com/ | |
| Software, algorithm | SeqMan software version 7.1 | DNASTAR | https://www.dnastar.com/ | |
| Software, algorithm | SPSS 20 | IBM | https://www.ibm.com | |
| Software, algorithm | GraphPad Prism 8.2.1 | Dotmatics | https://www.graphpad.com/ | |
| Other | Primers for full-length cloning of GRs | This paper | see Supplementary file 2 | |
| Other | Primers for qRT-PCR | This paper | see Supplementary file 3 | |
| Other | Primers for Xenopus oocytes expression system | This paper | see Supplementary file 4 | |
| Other | Primers for experiments of mutant strains establishment | This paper | see Supplementary file 5 |
Additional files
-
Supplementary file 1
GenBank accession numbers for sugar gustatory receptors used in this study.
- https://cdn.elifesciences.org/articles/91711/elife-91711-supp1-v2.xlsx
-
Supplementary file 2
The primer sequences used in PCR for full-length cloning of GRs.
F: forward strand; R: reverse strand.
- https://cdn.elifesciences.org/articles/91711/elife-91711-supp2-v2.xlsx
-
Supplementary file 3
The primer sequences used in qRT-PCR for GR gene expression quantification.
F: forward strand; R: reverse strand.
- https://cdn.elifesciences.org/articles/91711/elife-91711-supp3-v2.xlsx
-
Supplementary file 4
The primer sequences used in cDNA synthesis for Xenopus oocytes expression system.
The italic sequences are protective bases, underline sequences are restriction enzymes, bold sequences are Kozak sequences. F: forward strand; R: reverse strand.
- https://cdn.elifesciences.org/articles/91711/elife-91711-supp4-v2.xlsx
-
Supplementary file 5
The primer sequences used in the experiments of Gr10 and Gr6 mutants establishment.
F: forward strand; R: reverse strand.
- https://cdn.elifesciences.org/articles/91711/elife-91711-supp5-v2.xlsx
-
Supplementary file 6
Summary of the CRISPR/Cas9 directed mutation rates from G0 to G2 in the establishment of Gr10 and Gr6 mutants.
- https://cdn.elifesciences.org/articles/91711/elife-91711-supp6-v2.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/91711/elife-91711-mdarchecklist1-v2.docx