Illuminating T cell-dendritic cell interactions in vivo by FlAsHing antigens
Figures
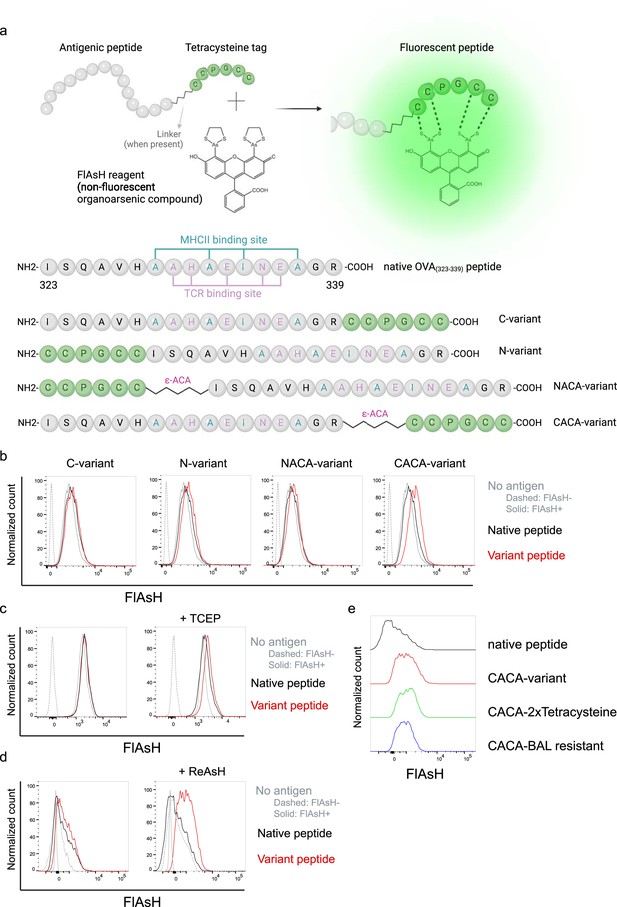
Fluorescein-AsH (FlAsH) labeling elicits fluorescence depending on the location of the tetracysteine tag.
(a) Arsenical Hairpin (AsH)- probe labeling strategy for the tetracysteine-tagged peptides and the four OVA323-339 variants generated for the study. (b) FlAsH signal intensity on splenic dendritic cells (DCs) pulsed with tagged OVA323-339 variants. (c) FlAsH signal intensity on splenic DCs pulsed with CACA-variant following treatment with Tris Carboxy Ethyl Phosphene (TCEP), a strong reducing agent. (d) Signal intensity following pre-treatment of splenic DCs with ReAsH, followed by pulsing with CACA-variant and FlAsH treatment. (e) FlAsH signal intensity of modified CACA-variant peptide with a duplicated CCPGCC sequence (CACA-2xTetracysteine) and CACA-variant peptide flanked with BAL-resistant peptide sequences (CACA-BAL resistant). Data in (b–d) are representative of three independent experiments, each performed with three biological replicates.
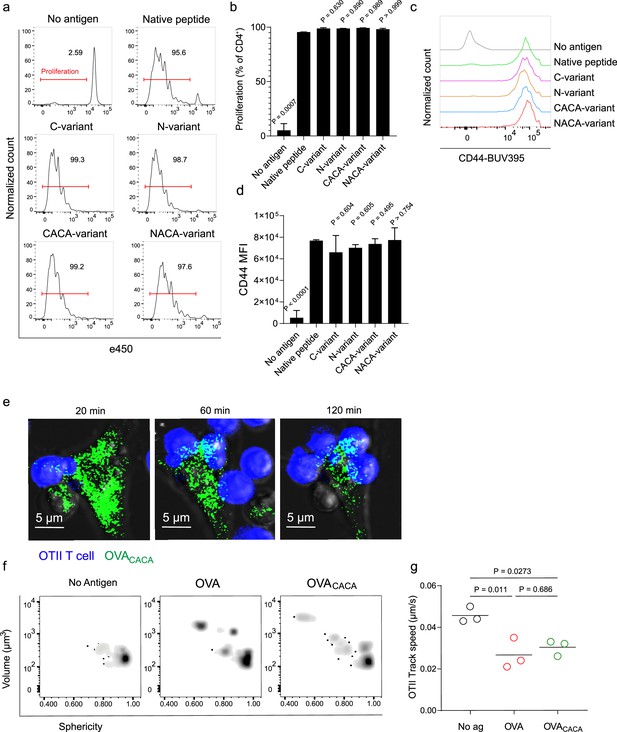
Tetracysteine tag does not alter the T cell priming ability of the native peptide.
(a–d) Dendritic cells (DCs) were loaded with the indicated peptides (5 μM) and co-cultured with e450-labeled naïve OT-II T cells (5 × 104) at a 1:10 DC to T cell ratio. (a) Histograms (a) and the bar graph (b) demonstrate the proliferation status of T cells at 72 hr. Histograms (c) and the bar graph (d) depict the surface expression of CD44, a T cell activation marker. The bars (b,d) indicate the mean of three biological replicates; the error bars show the standard deviation. Data are representative of three independent experiments. p-values refer to comparisons between each group and the native peptide, calculated using one-way ANOVA with Dunnett’s multiple comparisons test. (e–g) Peptide-pulsed DCs (2 × 105) were treated with Fluorescein-AsH (FlAsH), cocultured 1:1 with e450-labeled naïve OT-II T cells and imaged for real-time interactions for 3 hr (blue, OTII; green, FlAsH). (e) Time-lapse images depict the clustering of OTII T cells around OVACACA-pulsed DCs. (f) Histocytometry plots for OTII T cell volume and sphericity. (g) Graph shows the average track speed of OTII T cells. The lines mark the means of n=3 replicates (one per symbol). Data are representative of two independent experiments. p-values were calculated using a one-way ANOVA with Tukey’s test.
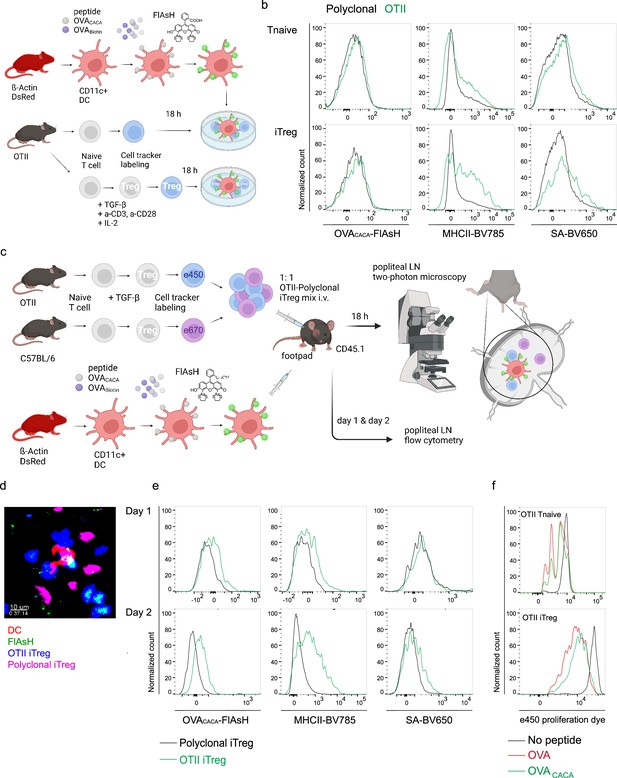
OVA323-339-specific Treg cells acquire OVACACA-Fluorescein-AsH (FlAsH) from immune synapse.
(a–b) Naïve OTII and C57BL/6 (Polyclonal) T cells were differentiated into iTreg cells for 3–4 days. DsRed+ DCs were double-pulsed with 5 μM OVACACA and OVA-biotin and cocultured 1:1 with e450-labeled naïve T cells or iTregs for 18 hr. (a) Experimental scheme. (b) Overlaid histograms for the polyclonal and OTII T cell and iTreg levels of OVACACA (FlAsH), MHCII, and OVA-biotin (detected with streptavidin-BV650). Data are representative of two independent experiments performed with n=3 biological replicates. (c–f) Naïve OTII and C57BL/6 (Polyclonal) T cells were differentiated into induced Treg (iTreg) cells, labeled with e450 and e670, respectively, and injected i.v. into CD45.1 recipients (1–2 × 106 cells/mouse per Treg type). 2–4 × 106 DsRed+ DCs were double-pulsed with 5 μM OVACACA and 5 μM OVA-biotin and adoptively transferred via footpad. Popliteal lymph nodes were either imaged at 18 hr or analyzed by flow cytometry at 18 hr or 48 hr post-transfer. (c) Experimental approach. (d) Summary data from live microscopy depicting DC-Treg cell interaction. (e) Treg levels of OVACACA (FlAsH), MHCII, and OVA-biotin (detected with streptavidin-BV650) at 18 hr (top) and 48 hr (bottom) following adoptive transfer. (f) Proliferation of OTII Tnaive and iTreg cells at 48 hr following adoptive transfer with DCs that had been pulsed with indicated peptides. Imaging data are representative of two independent experiments with n=2 mice. Flow cytometry data are representative of three independent experiments with n=3 mice per group.
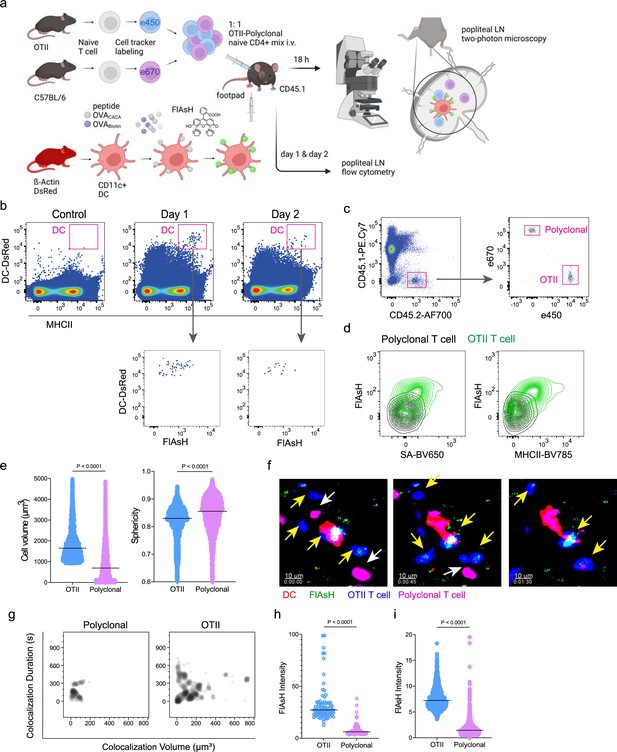
T cells acquire tetracysteine-tagged peptide from immune synapse.
DsRed+ splenic dendritic cells (DCs) were double-pulsed with 5 μM OVACACA and 5 μM OVA-biotin and adoptively transferred into CD45.1 recipients (2 × 106 cells) via footpad. Naïve e450-labeled OTII (1 × 106 cells) and e670-labeled polyclonal T cells (1 × 106 cells) were injected i.v. Popliteal lymph nodes were either imaged at 18 hr or analyzed by flow cytometry at 18 hr or 48 hr post-transfer. (a), Experimental approach. (b) Flow cytometry plots gating on DsRed+ DCs over 48 hr (top panel) and demonstrating the OVACACA levels (Fluorescein-AsH: FlAsH, bottom panel). (c) Gating strategy for T cells of the recipient (CD45.1) and donor (CD45.2). (d) T cell levels of OVACACA (FlAsH), MHCII, and OVA-biotin (detected with streptavidin) at 48 hr following adoptive transfer. (a–d) Data are representative of three independent experiments with n=3 mice per time point. (e–i) Summary data from live microscopy showing OTII cell blasting (e), DC-T cell interaction with 45 min intervals (f), plots for the DC-T cell contact duration and volume, graphs for the FlAsH intensity limited to the DC-T cell contact regions (g–h) and average Flash intensity acquired by T cells (i). Data are representative of four independent experiments with n=2 mice per experiment. p-values were calculated using two-sided Welch’s t-test.
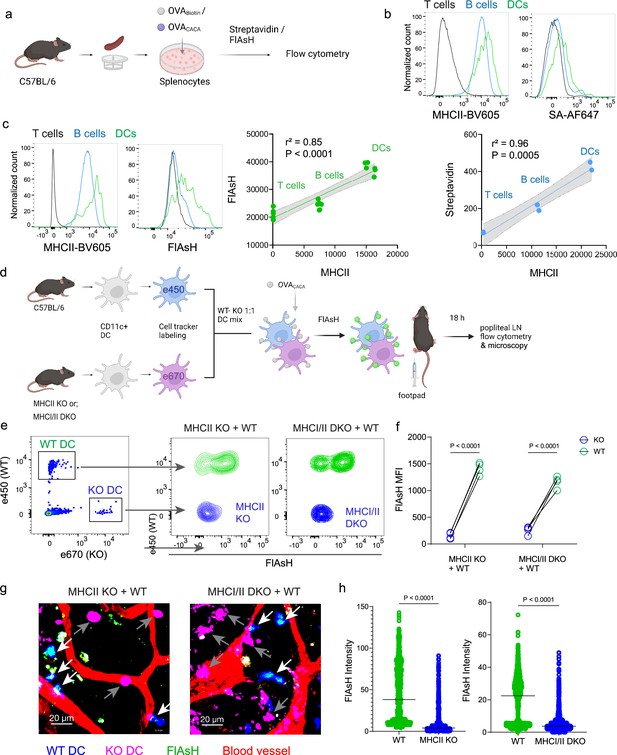
Arsenical Hairpin (AsH) probe reports the peptide-loaded on the MHCII.
(a-c), Relationship between surface MHCII expression and peptide load. (a) Experimental scheme. (b) Plots (top) show the surface levels of MHCII and OVA-biotin, graph (bottom) shows their correlation. Data are representative of two independent experiments performed with n=2 biological replicates (one per symbol). (c) Surface levels of MHCII and OVACACA (Fluorescein-AsH, FlAsH) and their correlation. Data are representative of two independent experiments performed with n=6 biological replicates (one per symbol). Simple linear regression and Pearson correlation were used to demonstrate the relationship between surface MHCII and peptide load, gray area and dotted lines mark the standard error of the fitted line (b, c). (d) Schematic depicting in vivo adoptive transfer approach. Wild-type (WT) and KO DCs were labeled, mixed, pulsed with 5 μM OVACACA, labeled with FlAsH and adoptively transferred to WT recipients via footpad (1–2 × 106 cells). (e–f) Flow cytometry plots (e), and graphs (f) showing FlAsH intensity of WT, MHCII-KO, or MHCI/II double-KO DCs that had migrated to the lymph node within 18 h following adoptive transfer. Data are representative of two independent experiments with n=4 mice per group. p-values were calculated using two-sided Student’s t-test. (g–h) Microscopy images of popliteal lymph node following anti-CD31 (red) antibody injection i.v., yellow arrows point to WT, white arrows point to KO DCs (g), FlAsH intensity of adoptively transferred dendritic cells (DCs) (h). Data are representative of four independent experiments with n=2 mice per experiment. p-values were calculated using two-sided Welch’s t-test.
Sequential gating strategy for splenocytes to analyze CD4+ T cells, B cells, and dendritic cells (DCs).
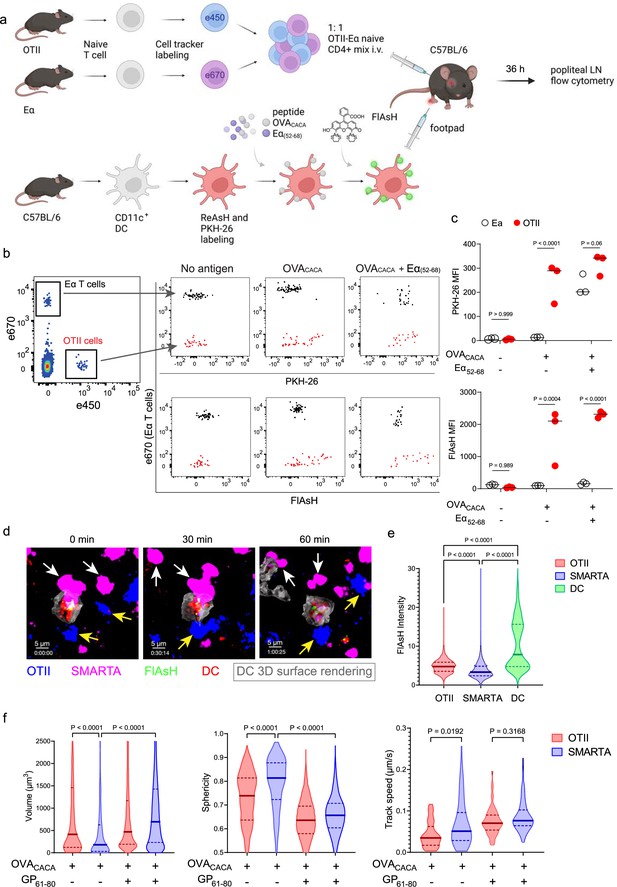
FlAsH-pMHCII is incorporated by cognate T cells in an antigen-specific manner.
(a–c) Dendritic cells (DCs) were labeled with ReAsH and PKH-26, single/ double-pulsed with 5 μM OVACACA, Eα(52-68), or left unpulsed. They were labeled with Fluorescein-AsH (FlAsH) and adoptively transferred into wild-type (WT) mice (0.5–1 × 106 cells) via footpad. Naïve e450-labeled OTII and e670-labeled Eα-specific T cells were mixed 1:1 (0.25–0.5 × 106 / T cell type) and injected i.v. Popliteal lymph node was analyzed by flow cytometry at 36–42 hr post-transfer. (a) Experimental scheme. (b) Summary plots. (c) PKH-26 and FlAsH MFI in OTII and Eα-specific T cells. Data are representative of two independent experiments with n=3 mice per group. p-values were calculated using two-way ANOVA with Sidak’s multiple comparisons test. (d-f) DCs were labeled with ReAsH and single/double-pulsed with 5 μM OVACACA, LCMV GP61-80, labeled with FlAsH and adoptively transferred into WT mice (1–2 × 106 cells) via footpad. Naïve e450-labeled OTII and e670-labeled SMARTA T cells were mixed 1:1 (0.5–1 × 106 / T cell type) and injected i.v. Live popliteal lymph node sections were imaged at 18 hr. (d) Time series representative of DC-T cell interactions. (e) Average Flash intensity of the adoptively transferred cells. (f) T cell blasting (left and middle panels) and motility (right) following adoptive transfer with DCs pulsed with the indicated peptides. Data are representative of three independent experiments with n=2 mice per group. p-values were calculated using one-way ANOVA with Tukey’s multiple comparisons test.
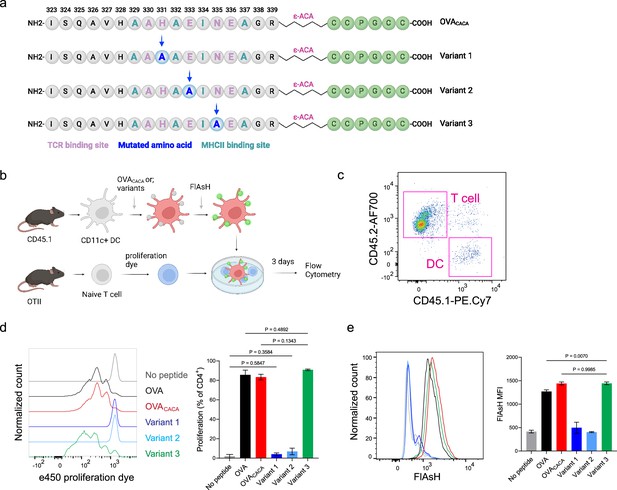
Nuanced TCR-pMHCII interactions can be discerned by Fluorescein-AsH (FlAsH) labeling of peptides.
(a–b) Graphical depiction of the alanine-mutants for OVACACA TCR binding site and experimental approach. 0,5 × 105 CD45.1+ DCs were pulsed with the indicated peptides, labeled with FlAsH, and cocultured with 2,5 × 105 e450-labeled OTII cells for 3 days. (c) Gating strategy for T cells and dendritic cell (DCs). (d) Representative flow cytometry plots for OTII T cell proliferation with a partial agonist (top) and two antagonist peptides (bottom). (e) FlAsH signal intensity of DCs and OTII cells with partial agonist (top) and antagonist peptides (bottom) adoptive transfer with the indicated peptides followed by FlAsH treatment. Data are representative of two independent experiments performed with n=3 biological replicates.
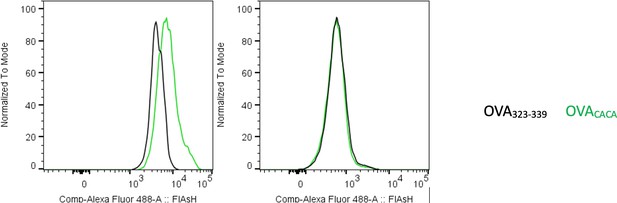
Preconjugation yields poor FlAsH signal.
Splenic DCs were pulsed with peptide then treated with FlAsH or incubated with peptide-FlAsH preconjugates. Overlaid histograms show the FlAsH intensities on DCs following the two-step labeling (left) and preconjugation (right). Data are representative of two independent experiments, each performed with three biological replicates.

ReAsH and DsRed are not picked up by T cells during immune synapse.
DsRed+ DCs were labeled with ReAsH, pulsed with 5 μM OVACACA, labeled with FlAsH and adoptively transferred into CD45.1 congenic mice mice (1-2 × 106 cells) via footpad. Naïve e450-labeled OTII and e670-labeled polyclonal CD4+ T cells were mixed 1:1 (0.25-0.5 × 106/ T cell type) and injected i.v. Popliteal lymph nodes were removed at 42 h post-transfer and analyzed by flow cytometry. Overlaid histograms show the ReAsh/DsRed, MHCII and FlAsH intensities of the T cells. Data are representative of two independent experiments with n=2 mice per group.

FlAsH signal acquisition by antigen specific T cells becomes more prominent at 36-48 h post-transfer.
DsRed+ splenic DCs were double-pulsed with 5 μM OVACACA and 5 μM OVA-biotin and adoptively transferred into CD45.1 recipients (2 × 106 cells) via footpad. Naïve e450-labeled OTII (1 × 106 cells) and e670-labeled polyclonal T cells (1 × 106 cells) were injected i.v. Popliteal lymph nodes were analyzed by flow cytometry at 18 h or 48 h post-transfer. Overlaid histograms show the T cell levels of OVACACA (FlAsH). Data are representative of three independent experiments with n=3 mice per time point
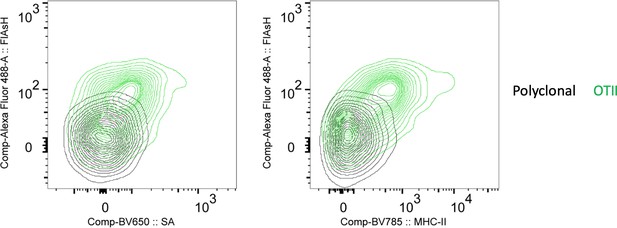
FlAsH signal acquisition by antigen specific T cells is correlates with the OVA-biotin (SA) and MHCII uptake.
DsRed+ splenic DCs were double-pulsed with 5 μM OVACACA and 5 μM OVA-biotin and adoptively transferred into CD45.1 recipients (2 × 106 cells) via footpad. Naïve e450-labeled OTII (1 × 106 cells) and e670-labeled polyclonal T cells (1 × 106 cells) were injected i.v. Popliteal lymph nodes were analyzed by flow cytometry. Overlaid histograms show the T cell levels of OVACACA (FlAsH) at 48 h post-transfer. Data are representative of three independent experiments with n=3 mice.
Videos
OVACACA-pulsed DsRed+ dendritic cells (DCs) were adoptively transferred via footpad.
Naïve e450-labeled OTII and e670-labeled polyclonal T cells (1 × 106 cells) were injected i.v. via retroorbital sinus. Popliteal lymph nodes were imaged by intravital two-photon microscopy at 18 hr after adoptive transfer. Video shows movement and interaction of naive OT-II (blue) cells with DCs (red). OVACACA-Fluorescein-AsH, FlAsH is visualized as cyan-green speckles in DCs and OTII cells. Polyclonal T cells (magenta) don’t engage in sustained interactions with DCs and don’t acquire FlAsH. Data are representative of four independent experiments.
ReAsH-labeled dendritic cells (DCs) were double-pulsed with OVACACA and LCMV GP61-80, treated with Fluorescein-AsH (FlAsH) (green) and adoptively transferred via footpad.
Naïve e450-labeled OTII and e670-labeled SMARTA T cells were mixed 1:1 and injected i.v. via retroorbital sinus. Popliteal lymph nodes of the recipient mice were surgically removed 18 hr after transfer, sectioned, and recovered at 37 °C until the resumption of cell migration in the tissue. Sections were then imaged at 37 °C by confocal microscopy for 4 hr. Video shows that both OTII (blue) and SMARTA T cells (magenta) have sustained contacts with the DC (Red). Gray: 3D surface rendering for the DCs. Data are representative of three independent experiments.
Additional files
-
Supplementary file 1
The sequences and molecular weights of the peptides used in the study.
- https://cdn.elifesciences.org/articles/91809/elife-91809-supp1-v1.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/91809/elife-91809-mdarchecklist1-v1.pdf





