Fundamental processes in sensorimotor learning: Reasoning, refinement, and retrieval
Figures
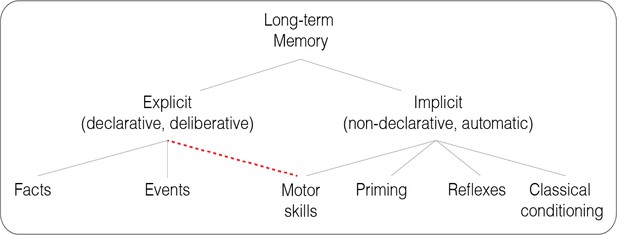
Classic and revised taxonomies of long-term memory.
A revision of the classic taxonomy proposed by Squire and Zola, 1996 (gray lines), with motor skills tapping into both explicit and implicit memory (dashed red line).
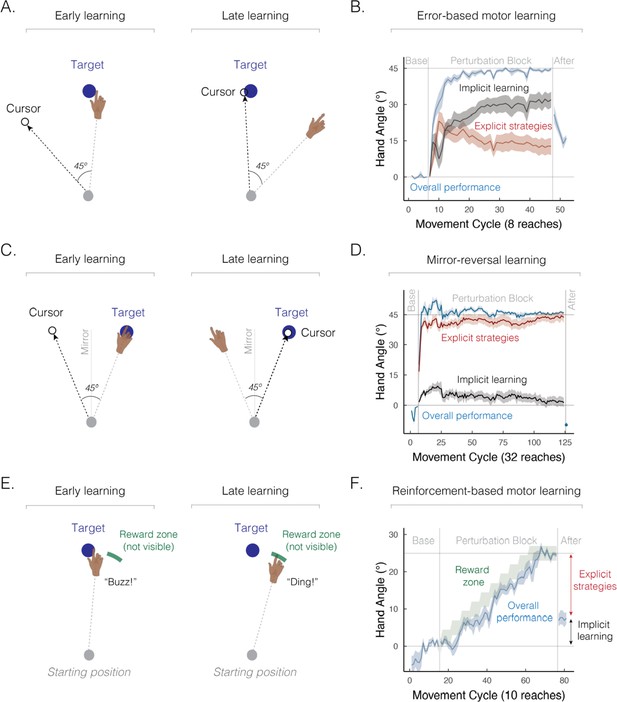
Implicit and explicit learning processes contribute to a wide range of sensorimotor learning tasks.
(A) Schematic of an error-based motor learning task. The 45° rotated cursor feedback (white dot) was provided throughout the movement. (B) Mean time course of hand angle (light blue line) during baseline, perturbed feedback, and aftereffect (no feedback) phases. Red line denotes the time course of strategy use, measured by verbal reports of the aiming location using a number wheel. Black line denotes the time course of implicit learning, estimated by subtracting verbal reports of aiming location from overall performance. Hand angle is presented relative to the target (0°). Figure adapted from Figure 2C in Taylor et al., 2014. (C) Schematic of a mirror-reversal task. The visual cursor feedback (white dot) was reflected over the vertical axis and provided throughout the movement. (D) Mean time course of hand angle in a mirror reversal task. Figure adapted from Figure 10B in Wilterson and Taylor, 2021. (E) Schematic of a reinforcement-based motor learning task. A pleasant auditory ‘ding’ was provided when the movement passed within the reward zone (green arc); otherwise, an unpleasant ‘buzz’ was played. (F) Mean time course of hand angle during the reinforcement learning task. The reward zone (green zone) was gradually shifted, leading to learning (light blue line). Figure adapted from Figure 2A–B in van Mastrigt et al., 2023.
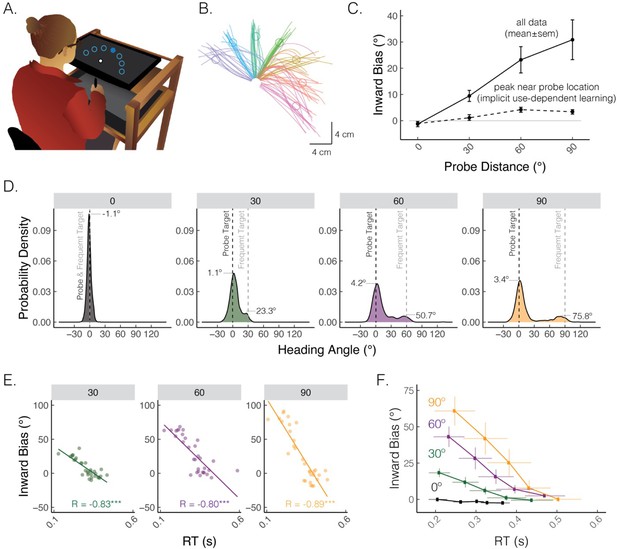
A default motor plan toward a repeated movement direction can be strategically overridden.
(A) Reaching set-up showing locations of frequent and rare probe targets. Only one of seven targets (filled blue circle) was visible on each trial. (B) Movement trajectories from a representative participant who exhibited straight and curved movements. The frequent target is denoted by the green circle, whereas rare targets are denoted by the purple, blue, turquoise, yellow, red, and magenta circles (clockwise). (C) Average inward bias measured early in movement (at peak velocity) increased as a function of probe distance (solid line). By contrast, the peak of the Gaussian estimated from the distribution near the probe location saturates for larger probe distances (dashed line). (D) Distribution of heading angles for each of the probe distances. Gray dashed line denotes the location of the frequently presented target, and black dashed line (0 on the x-axis) denotes the location of the probe target. The means obtained from the mixture of Gaussians models are provided. (E) Bias as a function of reaction time (RT) for a representative participant. Dots indicate individual reaches, with the thin line showing the best-fitting regression line. R denotes Pearson correlation; ***p<0.001. (F) Group-level analysis of bias as a function of RT. For each individual, RTs were binned into quintiles and mean bias was calculated for each quintile. These data were then averaged across the group. Error bars denote SEM. Figure adapted from Figure 1 in Tsay, Kim et al. (2022).
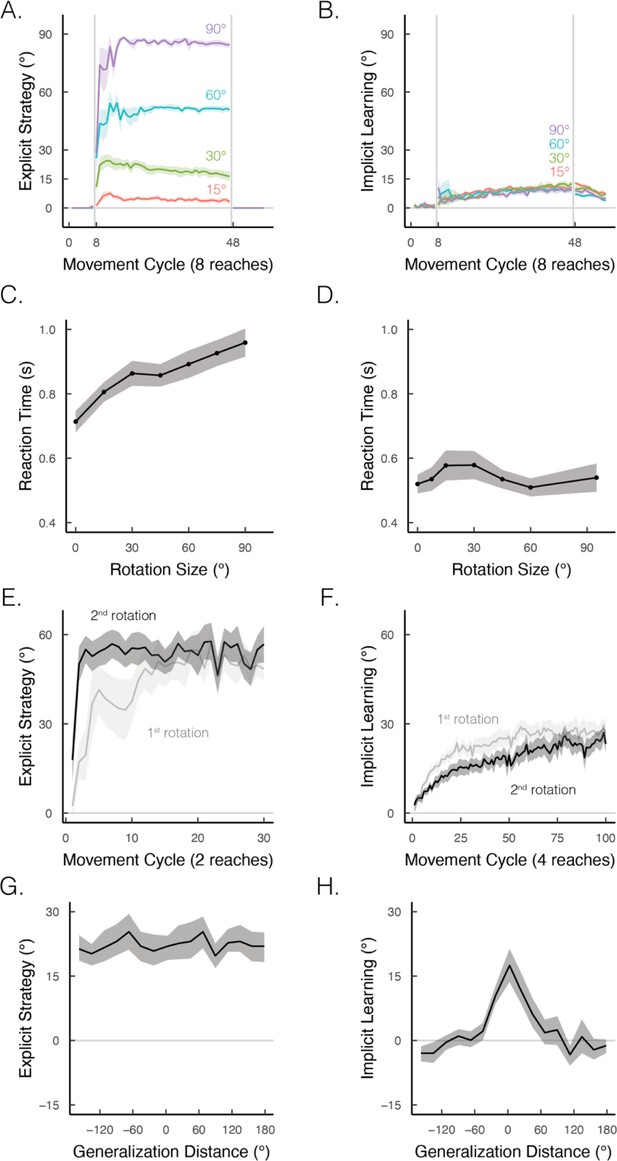
Implicit and explicit learning exhibit contrasting properties in visuomotor rotation tasks.
(A, B) Learning functions requiring strategic re-aiming scale with the size of the rotation, whereas the size of the aftereffect, indicative of the implicit component, does not scale. Figure adapted from Figure 7 in Bond and Taylor, 2015. (C, D) When learning is under explicit control, reaction times scale with the size of the rotation; this effect is not observed when learning is implicit. Figure adapted from Figure 2B in McDougle and Taylor, 2019 and Figure 4 in Morehead et al., 2017. (E, F) Re-exposure to the same rotation enhances explicit learning but attenuates implicit learning. Figure adapted from Figure 1A in Tsay, Schuck et al. (2022) and Figure 2D in Avraham et al., 2021. (G, H) Explicit learning generalizes globally over the entire workspace, whereas generalization from implicit learning is local, centered around the aiming location (i.e., 0° on the x-axis). Figure adapted from Figure 2B in McDougle et al., 2017.
© 2015, American Physiology Society. Figure 4A-B is taken from Figure 7 in Bond and Taylor, 2015, Journal of Neurophysiology. It is not covered by the CC-BY 4.0 license and further reproduction of this panel would need permission from the copyright holder.
© 2017, Nature Communications. Figure 4D is taken from Figure 4 in Morehead et al., 2017, Journal of Cognitive Neuroscience. It is not covered by the CC-BY 4.0 license and further reproduction of this panel would need permission from the copyright holder.
© 2017, American Physiology Society. Figure 4G-H is taken from Figure 2B in McDougle et al., 2017. Journal of Neurophysiology. It is not covered by the CC-BY 4.0 license and further reproduction of this panel would need permission from the copyright holder.
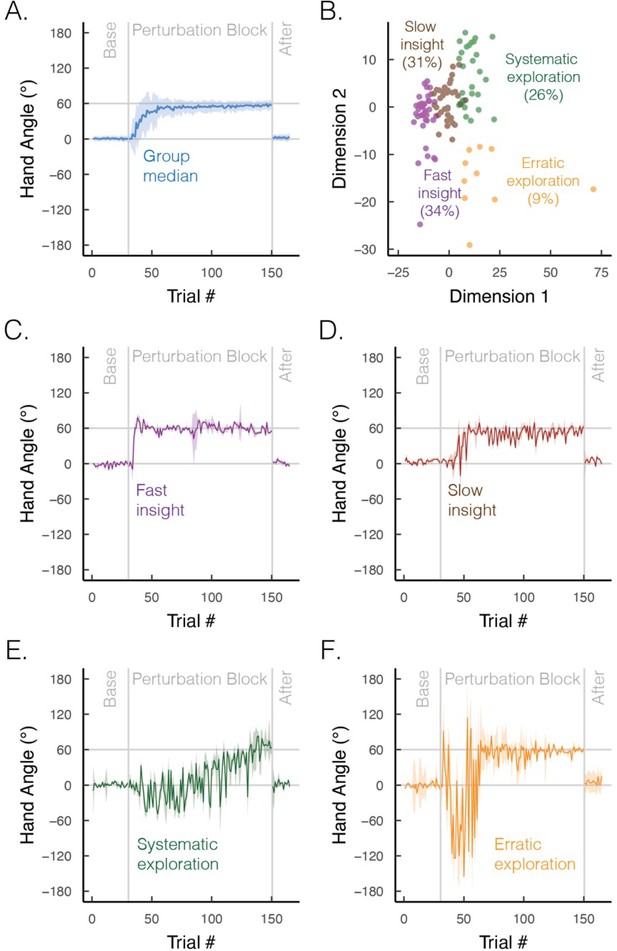
Group and subgroup performance in an explicit motor learning task.
(A) Median time course of heading angle during baseline, perturbed feedback, and aftereffect (no feedback) phases. During the perturbation phase, the feedback cursor was rotated 60° from the actual position of the hand when the movement distance equaled the target amplitude. To isolate strategy use, we abolished implicit adaptation by delaying the onset of the feedback by 800 ms. The effectiveness of this manipulation is given by the fact that there is no evidence of an aftereffect. (B) Results from an unsupervised clustering algorithm. We used a dynamic time warping approach to quantify the degree of dissimilarity between participants’ time series (Jeong et al., 2011; Sidarta et al., 2022). We then applied a k-means clustering algorithm, which identified four optimal clusters via the elbow method of within-cluster sum of squared errors (colors denote different clusters; points denote different individuals). (C–F) Median time course of heading angle for the four subgroups that exhibit (C) fast insight, (D) slow insight, (E) systematic exploration (sign flips of ±60°), and (F) erratic exploration. Shaded error bars denote first/third interquartile range. Figures generated by data from Cisneros et al., 2024.
Tables
Reasoning, refinement, and retrieval in different motor skill learning contexts.
| Learning to bike | Learning to play tennis | Learning to play piano | Learning to walk | |
|---|---|---|---|---|
| Reasoning | Understanding the mapping between arm movements and direction of the bike | Understanding how different arm and wrist movements affect the trajectory of the ball | Understanding the relationship between musical notes and the required finger movements | Developing an intuition for how to distribute weight to achieve balance |
| Refinement | Fine-tuning the amplitude of movement for smooth and efficient cycling. | Fine-tuning the angle of the stroke and racquet grip to accurately hit the ball | Fine-tuning the force and timing to enhance emotional expression | Fine-tuning the muscular coordination to maintain balance while walking |
| Retrieval | Performing a flawless ‘Wheelie Drop’ when encountering stairs. | Executing a complex spin serve when finding the opponent in a favorable receiving position | Improvising new musical pieces by combining learned melodies | Rapid recovery from stumbles |





