A 2-hydroxybutyrate-mediated feedback loop regulates muscular fatigue
Figures
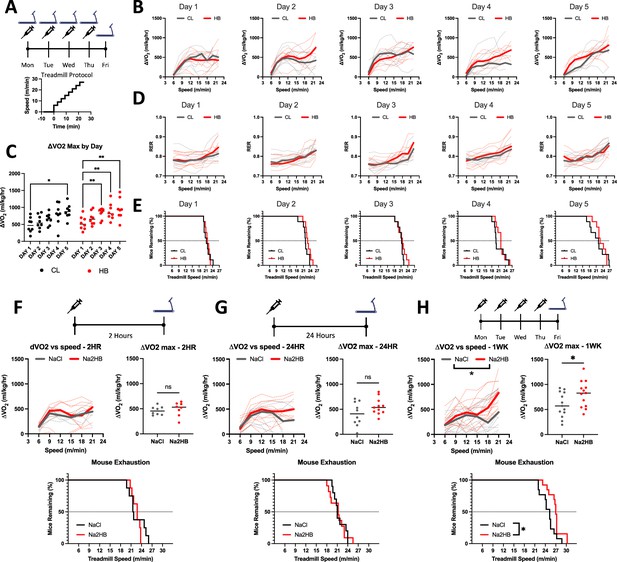
Daily 2-hydroxybutyrate treatment recapitulates the benefits of exercise training on oxidative capacity.
(A) Mice were subjected to five days of daily incremental exercise tests to exhaustion followed immediately by injection with 1 mmol/kg of NaCl or Na2HB, N=8. (B) Change in VO2 relative to baseline (ΔVO2) versus treadmill speed. (C) Maximum ΔVO2 compared with Day 1. Dunnett’s multiple comparisons test, * p<0.05, ** p<0.01. (D) Respiratory exchange ratio (RER) versus treadmill speed. (E) Time-to-exhaustion. Mice were subject to a single incremental exercise test (F) 2 hr (N=8) or (G) 24 hr (N=11) after a single dose, or (H) after four daily doses (N=13) of NaCl or Na2HB. For B,D, and F-H, solid lines indicate median values and dashed lines show each replicate. Median plots clipped at 21 m/min due to mouse dropout, although mice may continue up to 30 m/min. Speed vs ΔVO2 plot shows two-way ANOVA main effect * p<0.05, ΔVO2 max comparison shows student’s t-test * p<0.05. Time-to-exhaustion plots show Log-Rank test, * p<0.05.
-
Figure 1—source data 1
Raw data for Figure 1.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig1-data1-v2.xlsx
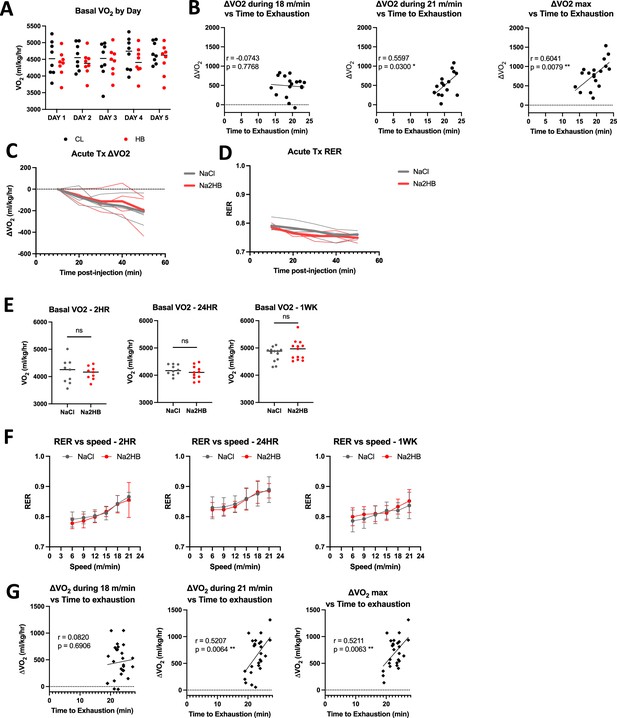
Supplemental data regarding exercise experiments.
(A) Basal VO2 for mice in Figure 1A–E subject to daily incremental exercise tests followed by injection with either 1 mmol/kg of NaCl or Na2HB. Basal VO2 is measured on each experiment day during a 5-min rest period before treadmill begins moving. N=8. (B) ΔVO2 during indicated speed phase or maximum ΔVO2 plotted against time-to-exhaustion for mice from Figure 1A–E on day 5. Pearson correlation coefficients and p values displayed in figure. Data support that mice increase their VO2 to match the work rate after the initial boost to VO2 above baseline. (C) Mice (N=3) injected with 1 mmol/kg of NaCl or Na2HB immediately prior to sitting in metabolic chamber at rest show no effect of 2HB on VO2 in first 60 min. (D) RER for mice in C. For C,D, solid line displays median, each replicate displayed in dashed lines. (E) Basal VO2 for mice in Figure 1F–H treated with 1 mmol/kg of NaCl or Na2HB 2 hr, 24 hr, or for 4 days (label 1 WK) prior to exercise test. (F) RER for mice in E, mean ± SEM. For E and F, N=8–13. (G) Pearson correlation as in B for mice from Figure 1H. N=13.
-
Figure 1—figure supplement 1—source data 1
Raw data for Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig1-figsupp1-data1-v2.xlsx
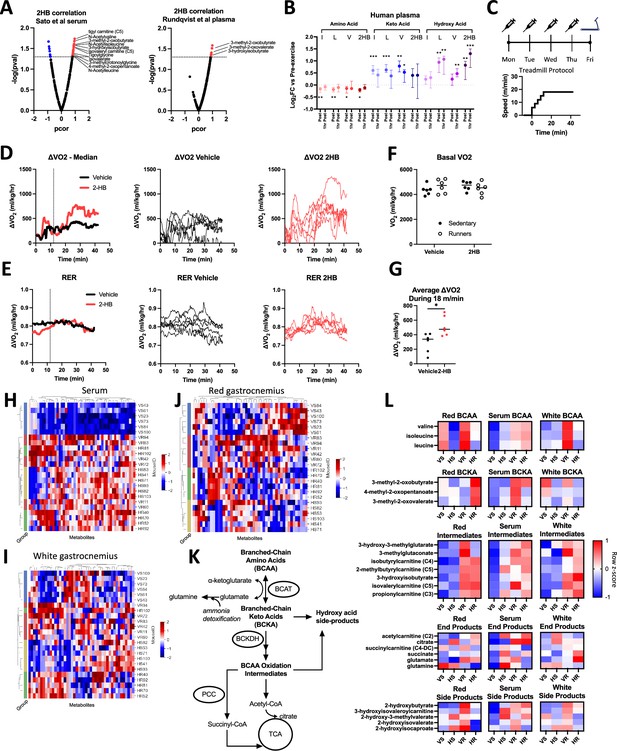
Exercise datasets suggest relation between 2-hydroxybutyrate and BCAA metabolism.
(A) Volcano plots display partial correlation coefficients for metabolites present in the blood that correlate with 2HB levels in mice that have undergone exercise. BCAA degradation metabolites indicated. Data in A are from Sato et al., 2022 (left) and Rundqvist et al., 2020 (right). (B) Levels of amino acid, keto-acid, and hydroxy acid versions of the BCAA and 2HB are displayed for human subjects comparing pre-exercise measures with those immediately post exercise and 1-hr post-exercise. Data from Rundqvist et al., 2020, N=8, median ±interquartile range. Dunnett’s multiple comparisons test made against pre-exercise levels, *** p<0.001, ** p<0.01, * p<0.05. (C) Mice were treated with 1 mmol/kg of NaCl or Na2HB for four days prior to a single endurance exercise protocol consisting of a gradual warm-up and 30 min of running at 18 m/min. Sham control mice placed on motionless treadmill. (D) ΔVO2 and (E) RER plots, Left-to-Right: group medians, vehicle-treated, and 2HB-treated. (F) No effect of 2HB on basal VO2, Dunnett’s multiple comparisons test. (G) 2HB-treated mice show increased average ΔVO2. Student’s t-test, * p<0.05. Untargeted metabolomics was conducted on serum, red gastrocnemius, and white gastrocnemius from mice in figures C-G. Heat maps of top 50 most differentially abundant metabolites in (H) serum, (I) white gastrocnemius, and (J) red gastrocnemius. Unique mouse ID displayed with group abbreviations: ‘VS’ vehicle-sedentary, ‘HS’ 2HB-sedentary, ‘VR’ vehicle-runner, ‘HR’ 2HB-runner. (K) BCAA degradation pathway. (L) Heat map displays metabolites in the BCAA degradation pathway. For D-L, N=6. Heat maps display standardized row medians.
-
Figure 2—source data 1
Raw data for Figure 2.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig2-data1-v2.xlsx
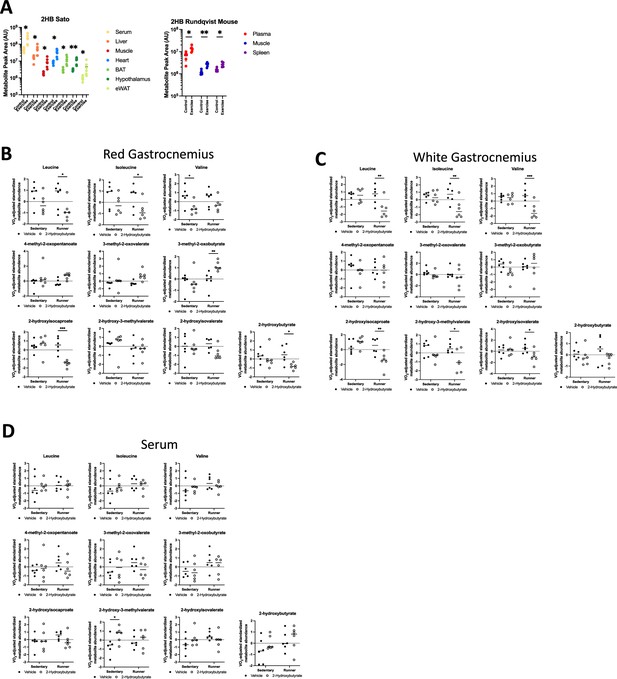
Supplemental data regarding 2-hydroxybutyrate and BCAA metabolomics.
(A) Raw 2HB measures from metabolomics datasets for each organ assessed in Sato et al. and Rundqvist et al. studies. Sidak multiple comparison test results displayed. Plots display VO2-adjusted standardized values for the amino, oxo-, and hydroxy- versions of the branched chain amino acids, and 2HB for mice corresponding to Figure 2C–L, for (B) red gastrocnemius, (C) white gastrocnemius, and (D) serum. All data are N=6. Comparisons made between vehicle and 2HB-treated groups of matching exercise condition. Fisher’s LSD test results displayed. * p<0.05, ** p<0.01, *** p<0.001.
-
Figure 2—figure supplement 1—source data 1
Raw data for Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig2-figsupp1-data1-v2.xlsx
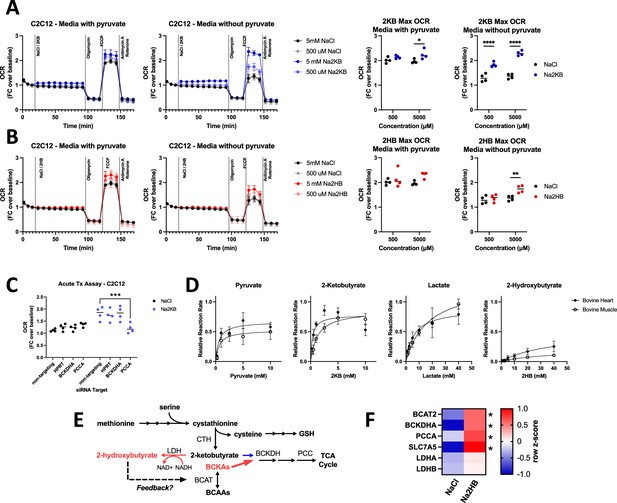
2-Ketobutyrate oxidation depends upon an in tact BCAA degradation pathway.
(A,B) Oxygen consumption rate (OCR) during mitochondrial stress test with acute administration of (A) Na2KB or (B) Na2HB as indicated. N=4 independent assays, mean ± SD. Basal and maximum OCR values displayed with Sidak’s multiple comparisons test displayed, * p<0.05, **** p<0.0001. (C) Maximum OCR values from mitochondrial stress tests in C2C12 transfected with siRNA against indicated targets, N=4 independent assays mean ± SD. Sidak’s multiple comparisons test, *** p<0.001. (D) Relative reaction rate of LDH assays using indicated substrate. Data are normalized to the maximum reaction rate for a given experiment day for each reaction direction. Data are a summary of five independent experiment days, mean ± SD. (E) Summary figure; we propose that accumulation of 2HB, as occurs post-exertion, is an indicator of saturation in the BCAA degradation pathway, leading to reduction of 2 KB instead of mitochondrial oxidation and the potential for metabolic feedback via 2HB. (F) Heatmap summarizing RT-qPCR for C2C12 myoblasts treated with 500 μM of Na2HB or NaCl for 8 hr. N=4 independent assays, median row z-score displayed with Sidak’s multiple comparisons test, * p<0.05.
-
Figure 3—source data 1
Raw data for Figure 3.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig3-data1-v2.xlsx
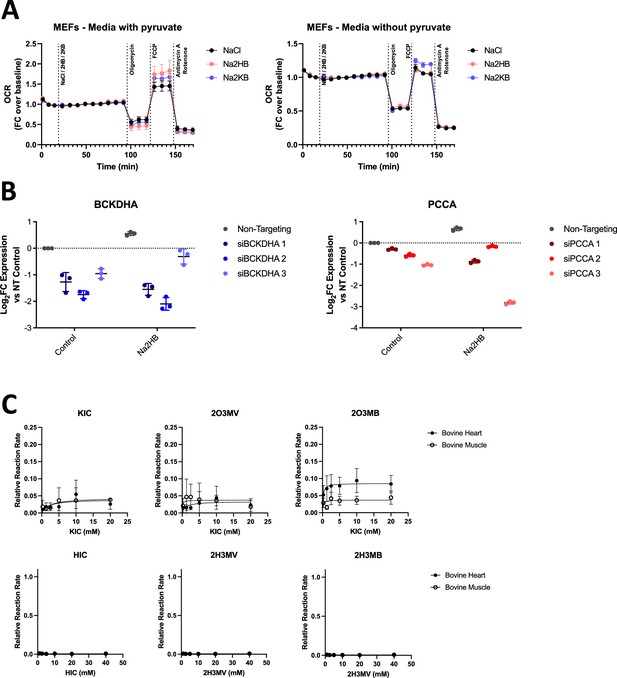
Supplemental data regarding the BCAA degradation pathway.
(A) OCR during mitochondrial stress test with acute administration of 500 μM Na2HB or Na2KB as indicated. N=4 independent assays, mean ± SD. (B) qPCR validation of reduced target gene expression for siRNA transfected cells. Final siRNA selection was based on knockdown of target gene expression in control and 2HB-treated cells; siBCKDHA #2 and siPCCA #3. (C) LDH activity assays using keto- and hydroxy-acid versions of BCAA as substrates. From left to right, metabolites are the leucine, isoleucine, and valine derived metabolites; KIC = ketoisocaproate, 2O3MV = 2-oxo-3-methylvalerate, 2O3MB = 2-oxo-3-methylbutyrate, HIC = hydroxyisocaproate, 2H3MV = 2-hydroxy-3-methylvalerate, and 2H3MB = 2-hydroxy-3-methylbutyrate. Data are normalized to the maximum reaction rate in the given reaction direction for the matched experiment day. Data represent mean ± SD of three independent experiment days.
-
Figure 3—figure supplement 1—source data 1
Raw data for Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig3-figsupp1-data1-v2.xlsx
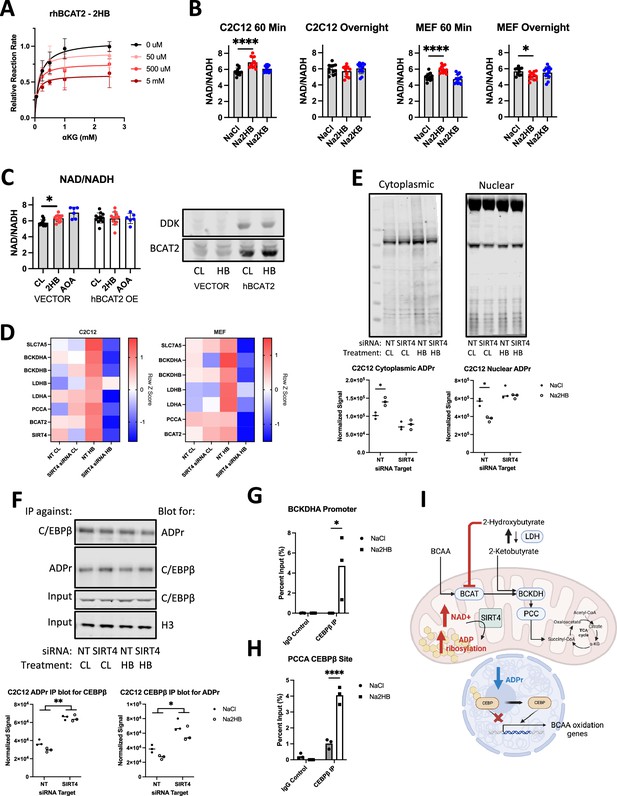
2-Hydroxybutyrate feedback mecanism.
(A) Relative reaction rate for recombinant human BCAT2 activity assays. Leu was kept at a constant concentration of 5 mM, 2HB concentrations are indicated in the legend. Data are normalized to the maximum reaction rate for a given experiment day and data represent three independent experiment days, mean ± SD. (B) Indicated metabolites were added to culture media for indicated time prior to cell harvest for NAD +and NADH assays, NAD+/NADH ratio displayed. N=8 independent assays, Dunnett’s multiple comparisons test, **** p<0.0001, * p<0.05. (C) C2C12 myoblasts were transfected with vector control or DDK-tagged hBCAT2 before repeating the 60-min time point NAD +and NADH assays. 1 mM Aminooxyacetic acid (AOA) included as positive control. N=12 independent assays, Dunnett’s multiple comparisons test, * p<0.05. (D) RT-qPCR gene expression for factors related to 2HB and BCAA metabolism in MEFs and C2C12 transfected with siRNA against SIRT4 or non-targeting control, and treated with 500 μM NaCl or Na2HB for 8 hr. N=4 independent assays, median row z-score displayed. (E) Total protein ADP ribosylation western blots from cytoplasmic and nuclear extracts of C2C12 cells transfected with siRNA against SIRT4 or non-targeting control and treated with 500 μM NaCl or Na2HB for 16 hr. Representative blots shown and quantification from three independent experiments. N=3, Sidak multiple comparisons test, * p<0.05. (F) Immunoprecipitation was conducted on C2C12 nuclear extracts using either antibody against C/EBPβ or poly/mono-ADP ribose, with resultant membranes probed using the opposite antibody. Total C/EBPβ and histone 3 were detected in input samples. N=3, two-way ANOVA main effect, ** p<0.01, * p<0.05. (G) ChIP-qPCR conducted on C2C12 myoblast lysates treated with 500 μM of NaCl or Na2HB for 8 hr. (I) Summary figure displaying proposed mechanism.
-
Figure 4—source data 1
Raw data for Figure 4 and western blots.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig4-data1-v2.xlsx
-
Figure 4—source data 2
Labeled western blots.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig4-data2-v2.zip
-
Figure 4—source data 3
Unlabeled western blots.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig4-data3-v2.zip

Supplemental data regarding the 2-hydroxybutyrate feedback mechanism.
(A) Relative reaction rate for recombinant human Got1 activity. Amino-oxyacetate (AOA) was used as a positive control for Got1 inhibition. Data represent mean ± SD of three independent experiment days. (B) Total protein stains for western blots in Figure 4C. (C) RT-qPCR validation of siRNA against SIRT4. siRNA#3 was selected for experiments in main figures. (D) Total protein stains for western blots in Figure 4E.
-
Figure 4—figure supplement 1—source data 1
Raw data for Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig4-figsupp1-data1-v2.xlsx
-
Figure 4—figure supplement 1—source data 2
Labeled western blots.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig4-figsupp1-data2-v2.zip
-
Figure 4—figure supplement 1—source data 3
Unlabeled western blots.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig4-figsupp1-data3-v2.zip
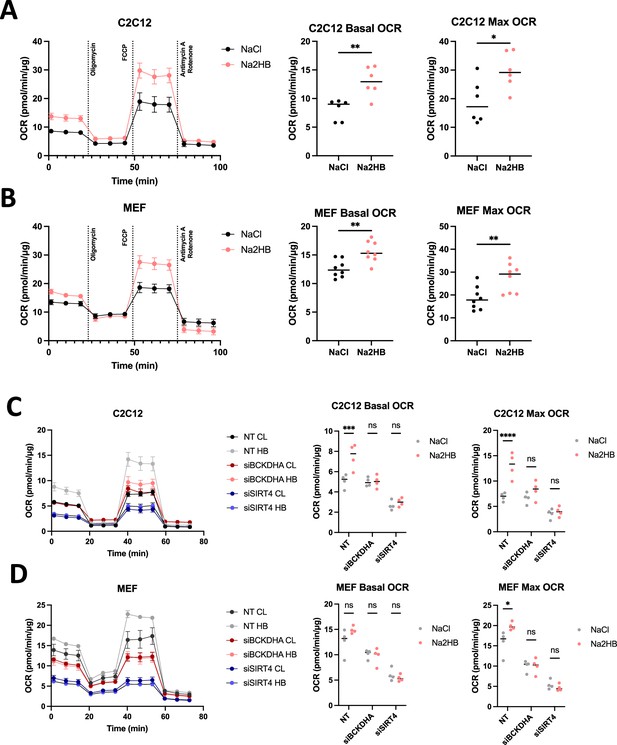
2-Hydroxybutyrate promotes oxidative metabolism in vitro.
(A) C2C12 and (B) MEFs cultured overnight in media containing 500 μM of Na2HB, or NaCl as indicated, prior to mitochondrial stress test, mean ± SD. Basal and maximum OCR values displayed with Student’s t-test results; N=6 independent assays ** p<0.01, * p<0.05. OCR during mitochondrial stress tests, basal OCR, and maximum OCR for (C) C2C12s and (D) MEFs transfected with siRNA against indicated gene targets. N=4 independent assays, mean ± SEM Sidak’s multiple comparisons test, **** p<0.0001, *** p<0.001, * p<0.05.
-
Figure 5—source data 1
Raw data for Figure 5.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig5-data1-v2.xlsx

Supplemental data regarding the effects of 2-hydroxybutyrate treatment in vitro.
(A) Extracellular acidification rate for C2C12 and MEFs cultured overnight in media containing 500 μM of either NaCl or Na2HB. N=4 independent assays, mean ± SD. Dunnett’s multiple comparisons test, * p<0.05. (B) OCR during mitochondrial stress test and maximum OCR for MEFs and C2C12s treated daily with 500 μM of Na2HB or NaCl supplemented media for 3 days with assays run on day 4. N=4 independent assays, Sidak’s multiple comparisons test, ** p<0.01, * p<0.05. (C) Representative western blot and quantification for histones isolated from MEFs treated for 3 days with 500 μM of Na2HB or NaCl, with or without the DOT1L methyltransferase inhibitor EPZ004777. Amounts of protein loaded are indicated above each blot. (D) Representative western blots and quantification using histones isolated from C2C12 cells treated for 3 days with 2HB or NaCl control at indicated concentrations. EPZ004777 was used as a positive control DOT1L inhibitor. (E) Representative western blot using OXPHOS antibody cocktail from C2C12 (left) and MEF (right) cell lysates following 3 days of treatment with NaCl (Cl), Na2HB (HB), DMSO (D), or ethyl 2HB (Et). ‘+’ indicates positive control sample. Amount of protein loaded in each well is indicated above. Total protein staining and histone methylation markers displayed. For all western blots, total protein for each well was detected using REVERT total protein stain. (F) Quantification from two independent assays of OXPHOS antibody cocktail western blots using lysates from MEFs treated with 2HB for three days. No observed change to any respiratory chain complex from 2HB treatment is suggested.
-
Figure 5—figure supplement 1—source data 1
Raw data for Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig5-figsupp1-data1-v2.xlsx
-
Figure 5—figure supplement 1—source data 2
Labeled western blots.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig5-figsupp1-data2-v2.zip
-
Figure 5—figure supplement 1—source data 3
Unlabeled western blots.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig5-figsupp1-data3-v2.zip
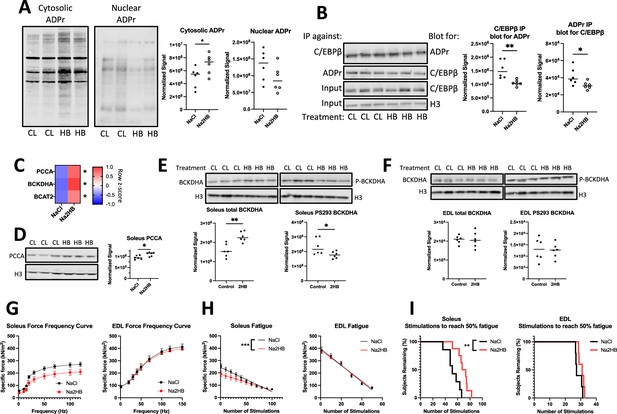
Mice treated with 2-hydroxybutyrate show functional responses intrinsic to skeletal muscle.
Data come from mice treated for four days with 1 mmol/kg NaCl or Na2HB with harvest of soleus and EDL skeletal muscle on the fifth day. (A) Representative western blots and quantification for total protein ADP ribosylation from cytoplasmic and nuclear extracts of soleus muscle. (B) Immunoprecipitation was conducted on soleus nuclear extracts using either antibody against C/EBPβ or poly-ADP ribose, with resultant membranes probed using the opposite antibody. Total C/EBPβ and histone 3 were detected in input samples. For A,B, N=6, Student’s t-test, * p0.05, ** p<0.01. (C) RT-qPCR of soleus muscle. N=4, Sidak multiple comparisons test * p<0.05. Representative western blots displayed using (D,E) soleus and (F) EDL lysates collected after incremental exercise tests showing total PCCA, BCKDHA, and BCKDHA phosphorylated at S293. N=10, student’s t-test, * p<0.05. Ex vivo contractility analysis was conducted on freshly dissected muscle. (G) Force frequency curve, Sidak’s multiple comparisons test found no differences. (H) Force production during fatigue protocol with linear regression line displayed. Slopes of linear regression lines are significantly different; F test *** p<0.001. (I) Time-to-event analysis displaying the number of stimulations in the fatigue protocol for the force output of each muscle to reach 50% of the initial force, log-rank test, ** p<0.01. N=7–8 for soleus, N=5 for EDL. For (G, H) mean ± SEM.
-
Figure 6—source data 1
Raw data for Figure 6.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig6-data1-v2.xlsx
-
Figure 6—source data 2
Labeled western blots.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig6-data2-v2.zip
-
Figure 6—source data 3
Unlabeled western blots.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig6-data3-v2.zip
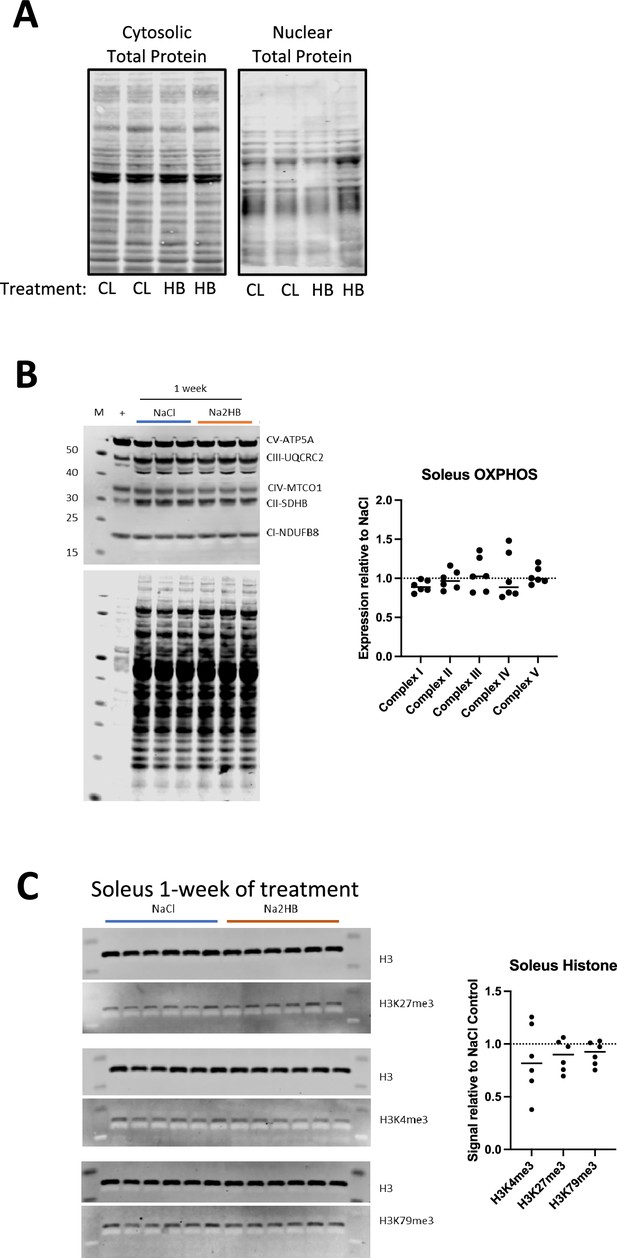
Supplemental data regarding skeletal muscle response to 2-hydroxybutyrate treatment.
(A) Total protein stain for western blot membrane corresponding to Figure 6A. (B) Quantification and representative western blot using OXPHOS antibody cocktail detection of electron transport chain members in soleus lysates from mice treated with 1 mmol/kg of NaCl or Na2HB for 4 days. (C) Quantification and representative western blot of histone methylation markers in soleus muscle treated as in B. For B and C, N=6. Data are expressed as fold change relative to average signal for NaCl group on the same membrane.
-
Figure 6—figure supplement 1—source data 1
Raw data for Figure 6—figure supplement 1.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig6-figsupp1-data1-v2.xlsx
-
Figure 6—figure supplement 1—source data 2
Labeled western blots.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig6-figsupp1-data2-v2.zip
-
Figure 6—figure supplement 1—source data 3
Unlabeled western blots.
- https://cdn.elifesciences.org/articles/92707/elife-92707-fig6-figsupp1-data3-v2.zip
Additional files
-
Supplementary file 1
2HB and 2KB IC50 for isolated PHD and KDM enzymes.
- https://cdn.elifesciences.org/articles/92707/elife-92707-supp1-v2.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/92707/elife-92707-mdarchecklist1-v2.pdf





