Senescence of endplate osteoclasts induces sensory innervation and spinal pain
Figures
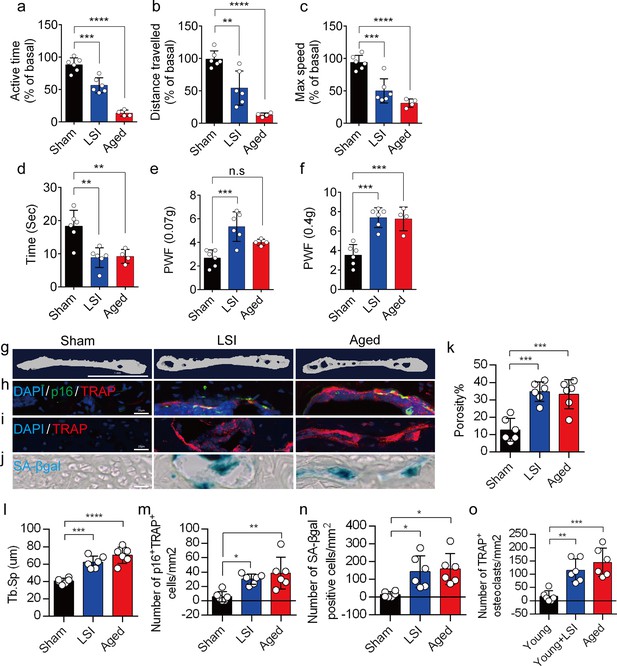
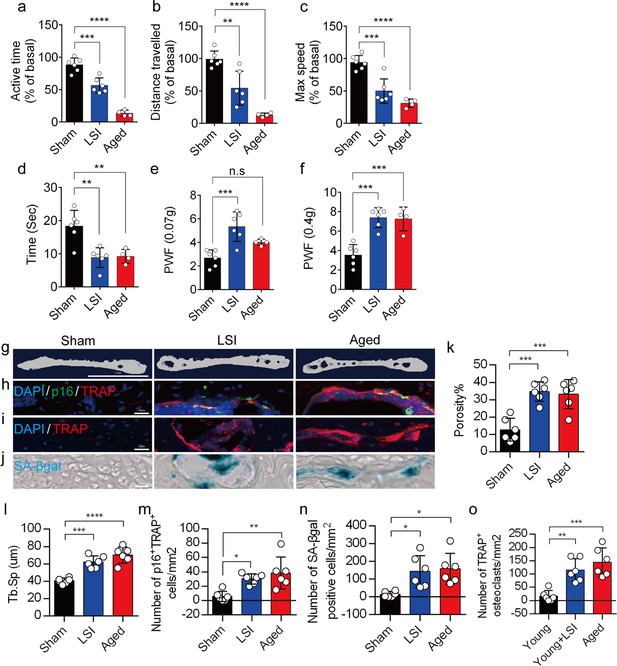
A greater number of senescent osteoclasts (SnOCs) are associated with endplate degeneration and spinal hypersensitivity in the lumbar spine instability (LSI) and aged mouse models.
(a–c) Spontaneous activity, including active time (a), distance traveled (b), and maximum speed (c) on the wheel within 48 hr in the sham, LSI injury, and aged mice. (d) Time in seconds spent on a hot plate in the three groups of mice. (e, f) The frequency of hind paw withdrawal (PWF) in response to mechanical stimulation (von Frey test, 0.07 g (e) and 0.4 g (f)) in the sham, LSI injury, and aged mice. (g) Microcomputed tomography (μCT) images of coronal caudal endplate sections of L4–5 from 3-month-old sham and LSI and 24-month-old aged mice. (h) Immunofluorescent (IF) staining of p16 (green), tartrate-resistant acid phosphatase positive (TRAP) (red), and 4′,6-diamidino-2-phenylindole (DAPI) (blue) of the endplates of sham, LSI, and aged mice. (i and j) IF staining of TRAP (red) and DAPI (blue) (i) and senescence-associated beta-galactosidase (SA-βGal) (blue) staining (j) of endplate serial sections of sham, LSI surgery, and aged mice. (k and l) Microcomputed tomography (μCT) quantitative analysis of the porosity percentage (k) and trabecular separation (Tb.Sp) (l) of the endplates in the indicated groups. (m) Number of SnOCs (p16-positive and TRAP-positive cells) per mm2 in the indicated groups. (n) Number of SA-βGal (blue) positive cells per mm2 in the endplates in the indicated groups. (o) Number of TRAP (red) positive cells per mm2 in the endplates in the indicated groups. n≥4 per group. Scale bar, 1 mm (g) and 20 μm (h, i, j). Statistical significance was determined by one-way ANOVA, and all data are shown as means ± standard deviations.
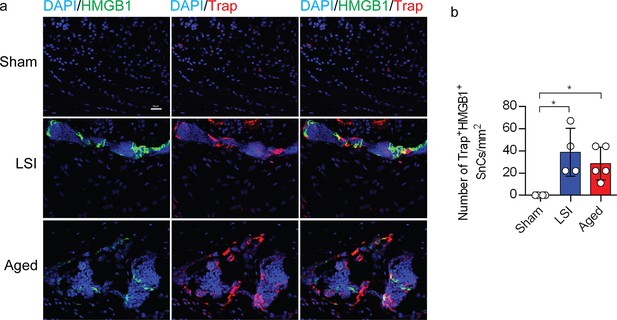
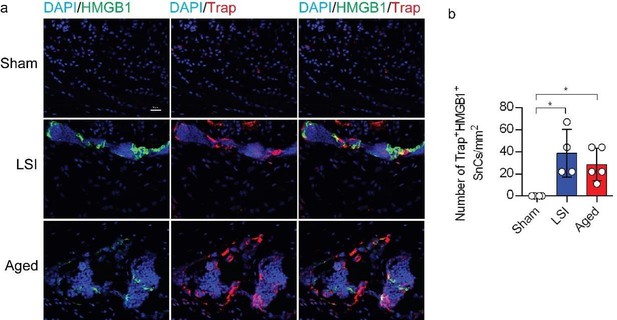
Increased occurrence of SnOCs in the endplates of two LBP models compared to the control young sham mice.
(a) Representative images of immunofluorescent analysis of HMGB1, a senescent marker (green), tartrate-resistant acid phosphatase positive (TRAP), an osteoclast marker (red) and nuclei (4′,6-diamidino-2-phenylindole [DAPI]; blue) of adult sham, lumbar spine instability (LSI), and aged mice. (b) Quantitative analysis of the number of TRAP+HMGB1+ senescent osteoclasts (SnOCs) per mm2. n≥4 per group. Statistical significance was determined by one-way ANOVA, and all data are shown as means ± standard deviations.
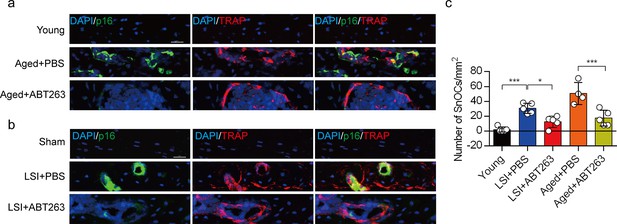
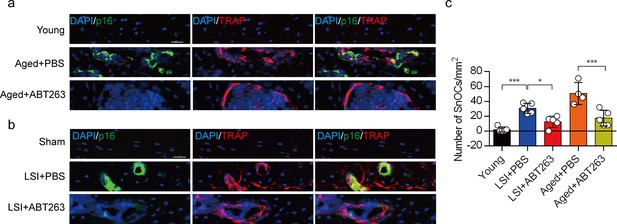
ABT263 effectively depletes endplate senescent osteoclasts (SnOCs) in the lumbar spine instability (LSI) and aging mouse models.
(a–c) Immunofluorescent staining of p16 (green), tartrate-resistant acid phosphatase (TRAP) (red), and nuclei (4′,6-diamidino-2-phenylindole [DAPI]; blue) of the endplates in aged (a) and LSI mice (b) injected with PBS (control) or ABT263 and the quantitative analysis of SnOCs based on dual staining for p16 and TRAP (c). n≥4 per group. Scale bar, 20 μm. Statistical significance was determined by one-way ANOVA, and all data are shown as means ± standard deviations.
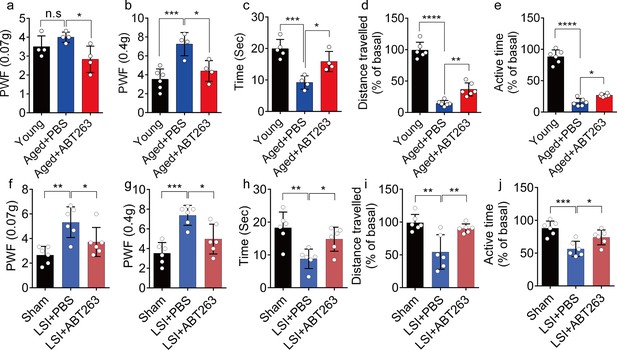
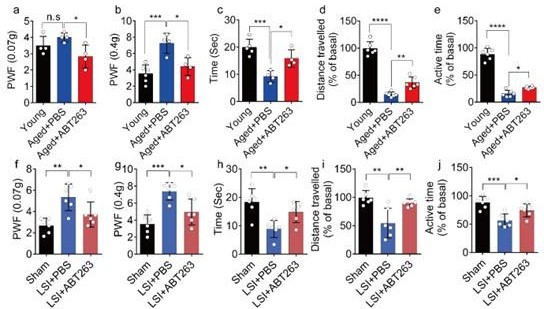
ABT263 treatment improves the symptomatic spinal pain behavior in the aged and lumbar spine instability (LSI) mouse models.
(a, b) The paw withdrawal frequency (PWF) in response to mechanical stimulation (von Frey test, 0. 07 g (a) and 0.4 g (b)) in aged mice treated with PBS or ABT263 compared to young adult mice. (c–e) Time (in seconds) spent on a hot plate (c), as well as spontaneous activity, including distance traveled (d) and active time (e), on the wheel within 48 hr in aged mice treated with PBS or ABT263 compared to young adult mice. (f, g) The PWF in response to mechanical stimulation (von Frey test, 0. 07 g (f) and 0.4 g (g)) in the LSI mouse model treated with PBS or ABT263 compared to sham-operated mice. (h–j) Time (in seconds) spent on a hot plate (h), as well as spontaneous activity analysis, including distance traveled (i) and active time (j) on the wheel within 48 hr in the sham and LSI mice treated with PBS or ABT263. n≥4 per group. Statistical significance was determined by one-way ANOVA, and all data are shown as means ± standard deviations.
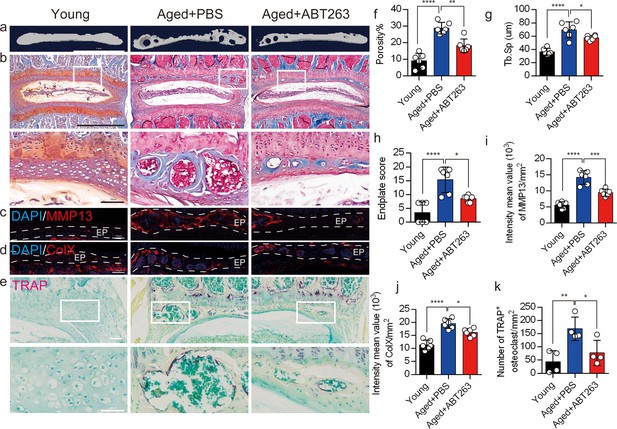
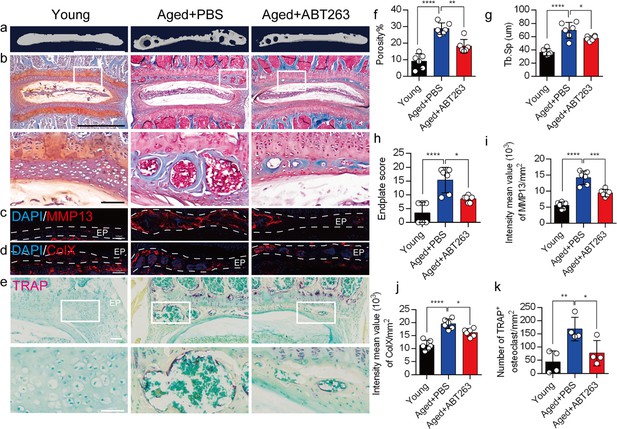
Depletion of senescent osteoclasts (SnOCs) reduces spinal degeneration and sustains endplate microarchitecture in aged mice.
(a) Microcomputed tomography (μCT) images of the aged mouse caudal endplates of L4–L5 injected with PBS or ABT263. Scale bar, 1 mm. (b) Representative images of Safranin O and fast green staining of coronal sections of the caudal endplates of L4–5 in aged mice caudal endplates of L4–L5 injected with PBS or ABT263, respectively. Lower panners are zoomed in images from upper white boxes. Scale bar, 1 mm (upper panels) and 100 μm (lower panels). (c) Representative images of spine degeneration marker MMP13 (red) and nuclei (4′,6-diamidino-2-phenylindole [DAPI]; blue) staining in aged mouse caudal endplates of L4–L5 injected with PBS or ABT263. Scale bar, 100 μm. (d) Representative images of spine degeneration marker ColX (red) and nuclei (DAPI; blue) staining in aged mouse caudal endplates of L4–L5 injected with PBS or ABT263. Scale bar, 100 μm. (e) Representative images of tartrate-resistant acid phosphatase (TRAP) (magenta) staining of coronal sections of the caudal endplates of L4–5 in aged mice caudal endplates of L4–L5 injected with PBS or ABT263, respectively. Lower panners are zoomed-in images from upper white boxes. Scale bar, 100 μm. (f) The quantitative analysis of the porosity percentage. (g) The quantitative analysis of the trabecular separation. (h) The endplate score based on the Safranin O and fast green staining. (i) Quantitative analysis of the intensity mean value of MMP13 in endplates per mm2. (j) Quantitative analysis of the intensity mean value of ColX in endplates per mm2. (k) The quantitative analysis of the number of TRAP-positive cells in the endplate per mm2. n≥3 per group. Statistical significance was determined by one-way ANOVA, and all data are shown as means ± standard deviations.
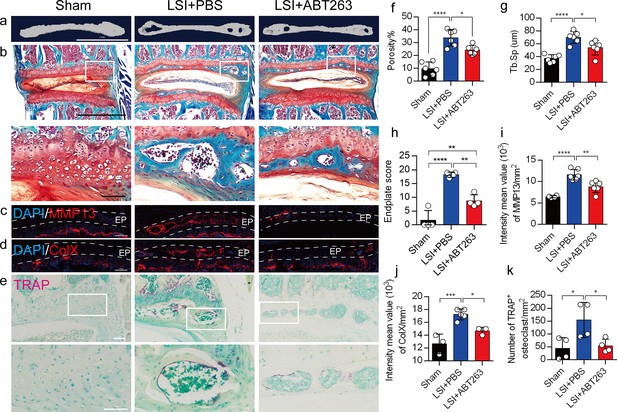
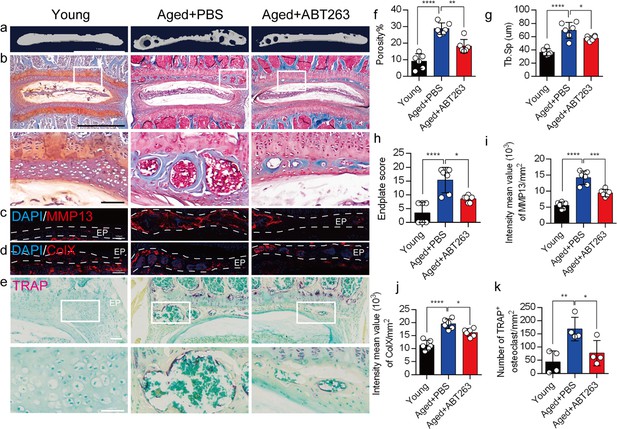
Depletion of senescent osteoclasts (SnOCs) reduces spinal degeneration and sustains endplate microarchitecture in lumbar spine instability (LSI) mice.
(a) Microcomputed tomography (μCT) images of adult sham mice and 3-month-old LSI model mice caudal endplates of L4–L5 injected with PBS or ABT263. Scale bar, 1 mm. (b) Representative images of Safranin O and fast green staining in different groups. Lower panners are zoomed-in images from upper white boxes. Scale bar, 1 mm (upper panels) and 100 μm (lower panels). (c) Representative images of immunofluorescent staining of spine degeneration marker MMP13 (red) and nuclei (4′,6-diamidino-2-phenylindole [DAPI]; blue). Scale bar, 100 μm. (d) Representative images of immunofluorescent staining of spine degeneration marker ColX (red) and nuclei (DAPI; blue). Scale bar, 100 μm. (e) Representative images of tartrate-resistant acid phosphatase (TRAP) (magenta) staining in different groups. Lower panners are zoomed-in images from upper white boxes. Scale bar, 100 μm. (f) The quantitative analysis of the porosity percentage of the mouse caudal endplates of L4–5 measured by the μCT. (g) The quantitative analysis of the trabecular separation (Tb.Sp) of the mouse caudal endplates of L4–5 measured by the μCT. (h) The endplate score based on the Safranin O and fast green staining. (i) Quantitative analysis of the intensity mean value of MMP13 in endplates per mm2. (j) Quantitative analysis of the intensity mean value of ColX in endplates per mm2. (k) The quantitative analysis of the number of TRAP-positive cells in the endplate per mm2. n≥3 per group. Statistical significance was determined by one-way ANOVA, and all data are shown as means ± standard deviations.
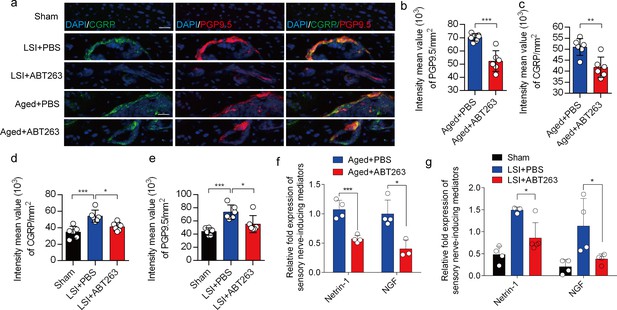
Depletion of senescent osteoclasts (SnOCs) abrogates sensory innervation and pain in aged and lumbar spine instability (LSI) mouse models.
(a) Representative images of immunofluorescent analysis of calcitonin gene-related peptide (CGRP) (green), PGP9.5 (red), and nuclei (4′,6-diamidino-2-phenylindole [DAPI]; blue) of adult sham, LSI, and aged mice injected with PBS or ABT263. Scale bar, 20 μm. (b) Quantitative analysis of the intensity mean value of PGP9.5 per mm2 in aged mice. (c) Quantitative analysis of the intensity mean value of CGRP per mm2 in aged mice. (d) Quantitative analysis of the intensity mean value of PGP9.5 per mm2 in the LSI mouse model. (e) Quantitative analysis of the intensity mean value of CGRP per mm2 in the LSI mouse model. (f, g) Relative fold expression of Ntn and Ngf in aged mice (f) or LSI mice (g) with or without ABT263 treatment. n≥4 per group. Statistical significance in panels b, c, and f are analyzed using t-tests, while panels d, e, and g are subjected to one-way ANOVA. All data are shown as means ± standard deviations.
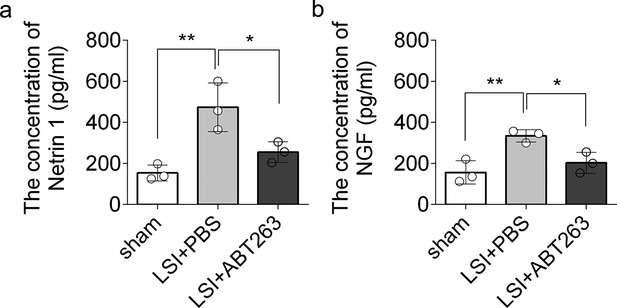
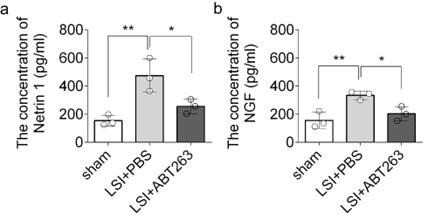
ELISA analysis of Netrin-1 and NGF in L3–5 endplates of sham, LSI+PBS, and LSI+ABT263 mice.
(a) Enzyme-linked immunosorbent assay (ELISA) analysis showing the concentration of Netrin-1 in L3–5 endplates of adult sham, lumbar spine instability (LSI) + PBS, and LSI + ABT263 mice. (b) ELISA analysis showing the concentration of NGF in L3–5 endplates of adult sham, LSI + PBS, and LSI + ABT263 mice. n=3 per group. Statistical significance was determined by one-way ANOVA, and all data are shown as means ± standard deviations.
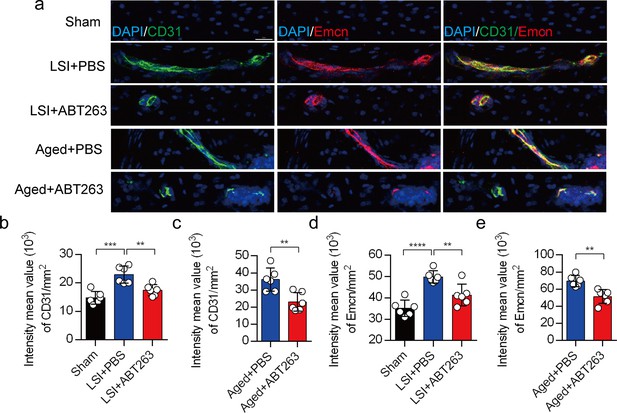
Depletion of senescent osteoclasts (SnOCs) abrogates blood vessels innervation in aged and lumbar spine instability (LSI) mouse models.
(a) Representative images of immunofluorescent analysis of CD31, an angiogenesis marker (green), Emcn, an endothelial cell marker (red) and nuclei (4′,6-diamidino-2-phenylindole [DAPI]; blue) of adult sham, LSI, and aged mice injected with PBS or ABT263. Scale bar, 20 μm. (b) Quantitative analysis of the intensity mean value of CD31 per mm2 in sham, LSI mice treated with PBS or ABT263. (c) Quantitative analysis of the intensity mean value of CD31 per mm2 in aged mice treated with PBS or ABT263. (d) Quantitative analysis of the intensity mean value of Emcn per mm2 in sham, LSI mice treated with PBS or ABT263. (e) Quantitative analysis of the intensity mean value of Emcn per mm2 in aged mice treated with PBS or ABT263. n≥4 per group. Statistical significance was determined by one-way ANOVA, and all data are shown as means ± standard deviations.
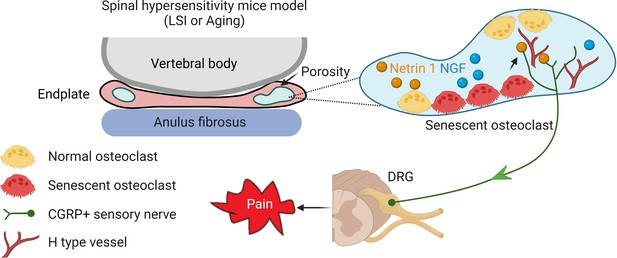
Schematic diagram of senescent osteoclasts (SnOCs) in porous endplate-induced spinal pain.
In lumbar spine instability (LSI) or aging mouse models there is an induction of spinal hypersensitivity due to increased numbers of SnOCs in the endplate, leading to excessive secretion of sensory nerve mediators, such as Netrin-1, to attract calcitonin gene-related peptide (CGRP+) sensory nerve innervation. Additionally, aberrant microarchitecture and spinal degeneration are associated with increased wiring of sensory nerve fibers and H-type vessels in the endplate, all further contributing to lower back pain.