Reorganization of the flagellum scaffolding induces a sperm standstill during fertilization
Figures
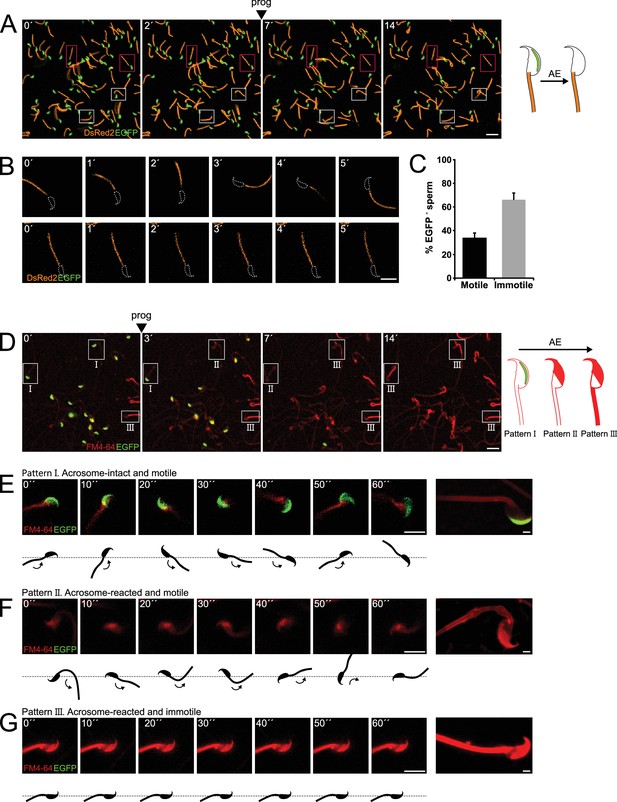
Sperm motility loss and FM4-64 fluorescence dynamics in acrosome-reacted transgenic EGFP-DsRed2 sperm.
(A) Representative time series of transgenic EGFP-DsRed2 sperm attached to concanavalin A-coated coverslips, with acrosomal exocytosis (AE) induced by 100 μM progesterone. White squares indicate cells with spontaneous AE (prior to induction), while pink squares highlight cells with progesterone-induced AE. A schematic representation of AE in this transgenic model is shown on the right side of the panel. Scale bar = 20 μm. (B) Representative time series of transgenic EGFP-DsRed2 sperm that have already experienced AE, attached to laminin-coated coverslips. The upper panel displays a cell with motility after AE, and the lower panel shows an immotile cell. DsRed2 is presented in orange, and EGFP in green. Scale bar = 10 μm. (C) Quantification of motile and immotile acrosome-reacted sperm (EGFP-). A total of 235 cells were counted across at least three independent experiments. (D) Representative time series of transgenic EGFP-DsRed2 sperm stained with 10 μM FM4-64 and attached to concanavalin A-coated coverslips, with AE induced by 100 μM progesterone. White squares indicate cells exhibiting patterns I, II, or III after progesterone induction. Scale bar = 20 μm. A schematic representation of AE in this transgenic model stained with FM4-64 is shown on the right side of the panel. (E–G) Representative images of capacitated transgenic EGFP-DsRed2 sperm stained with 10 μM FM4-64. Panel (E) displays an acrosome-intact, motile sperm (Pattern I), (F) shows an acrosome-reacted sperm with motility and low FM4-64 midpiece fluorescence (Pattern II), and (G) presents an acrosome-reacted sperm with no motility and high FM4-64 midpiece fluorescence (Pattern III). In all three cases, cells were induced with 100 μM progesterone. Scale bar = 10 μm. Enlarged images of each pattern are shown on the right panel. Scale bar = 2 μm. Representative images from at least five independent experiments are displayed.
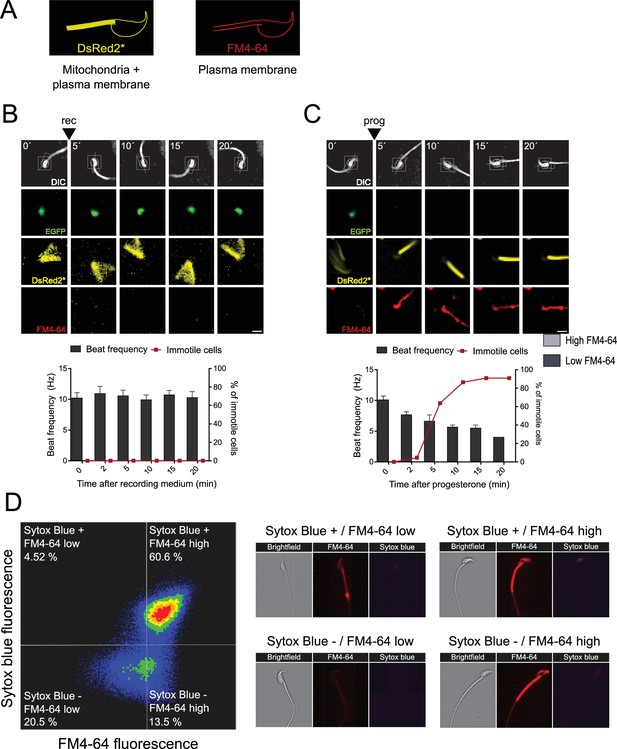
Gradual decrease in flagellar beat frequency following acrosomal exocytosis.
Motility analysis was conducted for capacitated EGFP-DsRed2 sperm. Cells were immobilized on laminin-coated coverslips and incubated in a recording medium (rec) containing 10 μM FM4-64. (A) Due to the spectral characteristics of DsRed2 (in the transgenic model), FM4-64, and the microscope configuration, both DsRed2 and FM4-64 emissions were observed using the U-FGWA excitation 530–550 nm/emission 575–625 nm filter (shown in yellow). In contrast, using the ETCY5 excitation 590–650 nm/emission 665–735 nm filter allowed for the visualization of only FM4-64 emission (in red). As a result, the images display the plasma membrane and mitochondria in yellow (with DsRed2* notation), while the plasma membrane appears in red. (B, C) The figure presents a representative time series of sperm without acrosomal exocytosis (AE) (B) and progesterone-induced AE (C, prog, 100 μM). The upper panel displays DIC images processed by a cell tracking system to measure flagellar beat frequency, along with EGFP, DsRed2*, and FM4-64 images. Scale bar = 10 μm. The lower graphs illustrate the frequency analysis (right y-axis) for control sperm (with the addition of rec, indicated by an arrowhead) and progesterone-induced AE. The percentage of immotile cells is shown on the left y-axis. These images represent at least five independent experiments, with 11 cells analyzed for the control (rec) group and 22 cells for the progesterone-induced AE group. (D) Relationship between cell death and FM4-64 fluorescence capacitated sperm stimulated with ionomycin. Image-based flow cytometry analysis of non-capacitated mouse sperm loaded with FM4-64 and Sytox Blue dyes, with 1 and 2 min of incubation time, respectively. The quadrants in the left panel show: Sytox Blue+/FM4-64 low (4.52%), Sytox Blue+/FM4-64 high (60.6%), Sytox Blue-/FM4-64 low (20.5%), and Sytox Blue-/FM4-64 high (13.5%). Each quadrant indicates the percentage of the total sperm population exhibiting the corresponding staining pattern. Axes are presented on a log10 scale of arbitrary units of fluorescence. The right panel shows representative single-cell images corresponding to the four categorized sperm populations from the flow cytometry analysis in the left panel.
Representative movie of transgenic EGFP-DsRed2 sperm attached to concanavalin A-coated coverslips, with acrosomal exocytosis (AE) induced by 100 μM progesterone as indicated with Prog.
DsRed2 is presented in orange, and EGFP in green. White squares indicate cells with spontaneous AE (prior to induction), while pink squares highlight cells with progesterone-induced AE. Related to Figure 1A.
Representative movie of transgenic EGFP-DsRed2 sperm that have already experienced acrosomal exocytosis (AE), attached to laminin-coated coverslips.
The left panel displays a cell with motility after AE, and the right panel shows an immotile cell. DsRed2 is presented in orange, and EGFP in green. Related to Figure 1B.
Representative movie of transgenic EGFP-DsRed2 sperm stained with 10 μM FM4-64 and attached to concanavalin A-coated coverslips, with acrosomal exocytosis (AE) induced by 100 μM progesterone as indicated with Prog.
FM4-64 is presented in red, and EGFP in green. White squares indicate cells exhibiting patterns I, II, or III after progesterone induction. Related to Figure 1D.
Representative movies of capacitated transgenic EGFP-DsRed2 sperm stained with 10 μM FM4-64.
Left panel displays an acrosome-intact, motile sperm (Pattern I), middle panel shows an acrosome-reacted sperm with motility and low FM4-64 midpiece fluorescence (Pattern II), and right panel presents an acrosome-reacted sperm with no motility and high FM4-64 midpiece fluorescence (Pattern III). In all three cases, cells were induced with 100 μM progesterone. FM4-64 is presented in red, and EGFP in green. Related to Figure 1E–G.
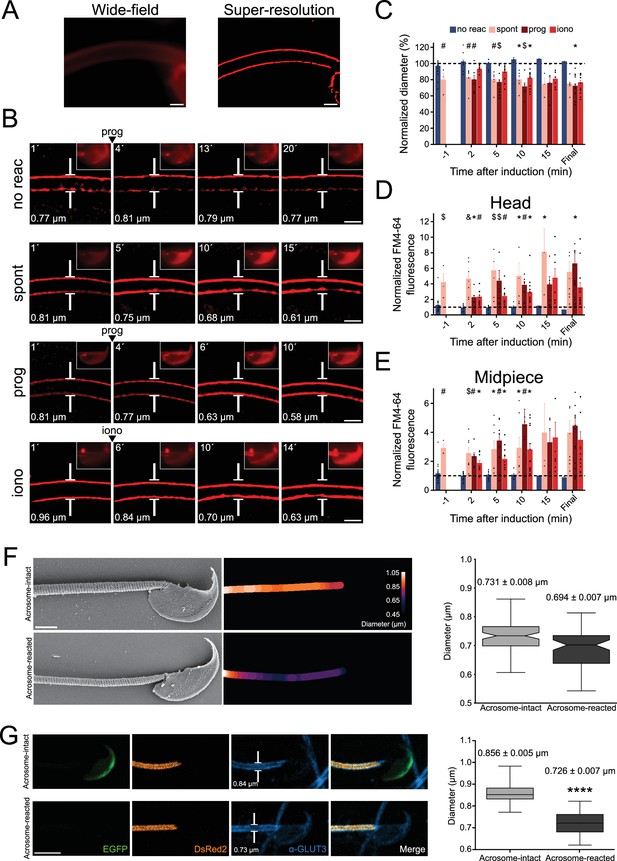
Midpiece contraction coincides with the onset of acrosomal exocytosis (AE).
(A) Left panel displays a wide-field fluorescence image of capacitated CD1 sperm membrane stained with 0.5 μM FM4-64, while the right panel shows its super-resolution radial fluctuations (SRRF) reconstruction. Scale bar = 1 μm. (B) Representative time series of sperm midpiece with no AE (no reac), spontaneous exocytosis (spont), progesterone (prog, 100 μM), and ionomycin-induced (iono, 10 μM) exocytosis, respectively. Following acquisition, images were analyzed using SRRF. Insets in the sperm head show wide-field images of AE. The midpiece diameter value is displayed in the bottom-left corner for each time point. Scale bar = 1 μm. (C) Quantification of midpiece diameter changes for each experimental group across time. Data are presented as a percentage of the initial diameter value before induction for each cell. (D, E) Quantification of FM4-64 fluorescence in the sperm head and midpiece, respectively, for each experimental group across time. Data are presented as times of increases compared to initial fluorescence before AE induction. *p<0.05; #p<0.01; $p<0.001, and &p<0.0001 compared to the non-reacted group. A nonparametric Kruskal–Wallis test was performed in combination with Dunn’s multiple-comparisons test. Representative images of at least five independent experiments are shown, with 36 cells analyzed. (F) Comparison of the midpiece architecture in acrosome-intact (AI, upper panel) and acrosome-reacted (AR, lower panel) sperm using scanning electron microscopy. Representatives images are shown, middle panels show quantification of these images whereas the left panel shows the quantification of all replicates. Data is presented as mean ± SEM, Kruskal–Wallis test was employed, p=0.013 (AI n = 85, AR n = 72). Scale bar = 2 μm. (G) Capacitated transgenic EGFP-DsRed2 sperm were induced by 100 µM progesterone. Cells were fixed and immunostained against ɑ-GLUT3 in order to see the plasma membrane in the midpiece. Representative images of at least two independent experiments are shown. Left panel shows quantification of midpiece diameter in acrosome-intact and acrosome-reacted EGFP-DsRed2 sperm. Data is presented as mean ± SEM. A nonparametric Mann–Whitney test was performed, ****p<0.001 (AI n = 84, AR n = 47). Scale bar = 5 μm.
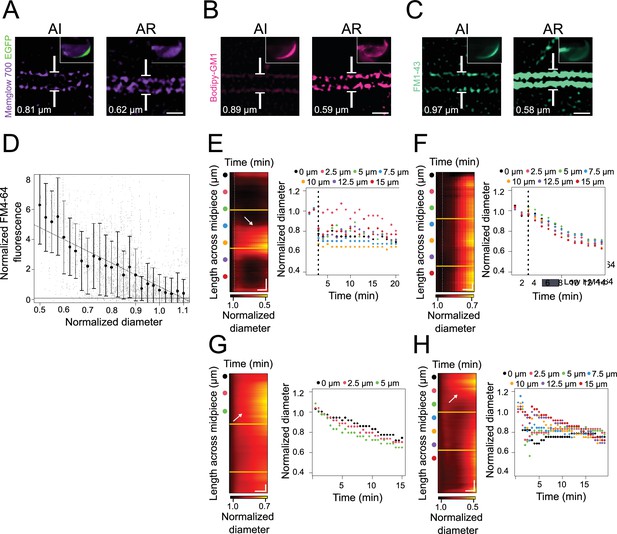
Correlation between FM4-64 increase and midpiece contraction.
(A–C) Representative mean shift super-resolution (MSSR)-processed images of acrosome-intact (AI) and acrosome-reacted (AR) EGFP-DsRed2 transgenic sperm stained with membrane dyes Memglow 700 (A, violet), Bodipy-GM1 (B), and FM1-43 (C). Insets in the sperm head images reveal acrosomal exocytosis (AE) in wide-field views: in (A), AE is visible with EGFP in green and the membrane in violet, while in (B) and (C), the acrosome has the same color as the membrane. Scale bar = 1 μm. (D) Correlation between FM4-64 midpiece fluorescence (y-axis) and normalized diameter (x-axis), with data normalized to the mean of the frames prior to AE induction. (E–H) Representative contraction kymographs and diameter measurements for ionomycin-induced AE (10 μM, E–F) and spontaneous AE (G–H), respectively, with one or multiple contraction foci. In the contraction kymographs, yellow lines indicate the separation between midpiece sections, and colored spots mark the locations where super-resolution kymographs were generated. A dotted vertical line in both kymograph and diameter measurement graphs signifies the point of induction. The data presented are derived from at least five independent experiments, with 36 cells analyzed. For (E), horizontal scale bar = 5 min and vertical scale bar = 2 μm; for (F), horizontal scale bar = 4 min and vertical scale bar = 2 μm; for (G), horizontal scale bar = 5 min and vertical scale bar = 1 μm; and for (H), horizontal scale bar = 5 min and vertical scale bar = 2 μm.
Representative movie of capacitated CD1 sperm stained with 0.5 μM FM4-64 64 and attached to concanavalin A-coated coverslips with spontaneous acrosomal exocytosis (AE).
Following acquisition, images were analyzed using SRRF. FM4-64 is presented in red. Related to Figure 2B and C.
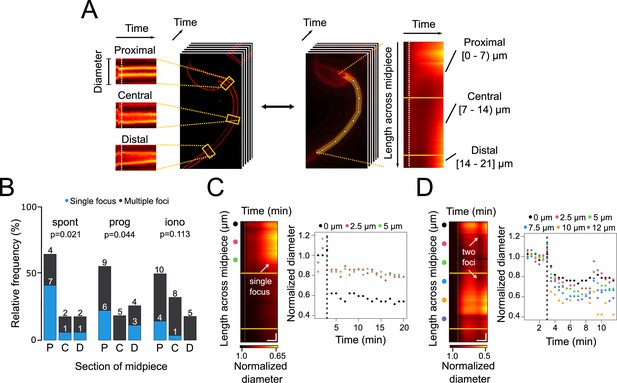
Contraction initiation preferentially occurs near the head-midpiece junction.
(A) Schematic diagram illustrating the generation of super-resolution kymographs from super-resolution radial fluctuations (SRRF)-processed images. Crosslines are drawn every 2.5 μm through the sperm midpiece, and the ImageJ Kymograph builder plug-in is used to create kymographs. The x-axis represents time, and the y-axis shows diameter changes. For wide-field images, a line along the midpiece is drawn to create fluorescence kymographs, with the y-axis representing midpiece length. Three sections of the midpiece are defined: proximal [0–7 μm), central [7–14 μm), and distal [14–21 μm]. (B) Relative frequency graph displaying the distribution of the initiation sites for midpiece contractions in sperm with spontaneous exocytosis (spont), progesterone-induced (prog, 100 μM) exocytosis and ionomycin-induced (iono, 10 μM) exocytosis, respectively. The x-axis indicates the midpiece section where the contraction begins: proximal (P), central (C), or distal (D). A chi2 test was performed using the R language environment. (C, D) Representative contraction kymographs and diameter measurements for progesterone-induced (100 μM) AE with one or two contraction initiation sites, respectively. In contraction kymographs, yellow lines demarcate midpiece sections, and colored spots indicate where super-resolution kymographs were created. Both kymograph and diameter measurement graphs display a dotted vertical line marking the induction point. For (C), horizontal scale bar = 5 min and vertical scale bar = 1 μm and for (D), horizontal scale bar = 3 min and vertical scale bar = 1 μm. Data from at least five independent experiments are shown, with 36 cells analyzed.
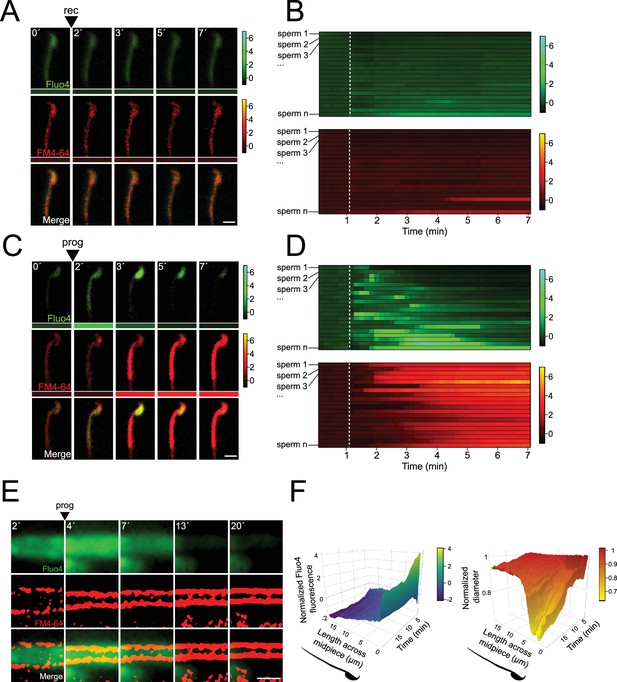
Midpiece contraction is driven by [Ca2+]i changes.
(A) The representative time series demonstrates [Ca2+]i and acrosomal exocytosis (AE) dynamics. Capacitated F1 sperm, loaded with Fluo4 AM, were immobilized on concanavalin A-coated coverslips and incubated in a recording medium (rec) containing 10 μM FM4-64. Rec was added as indicated by arrowheads. Scale bar = 10 μm. Beneath each frame in the Fluo4 (green) and FM4-64 (red) images, a color code displays the normalized intensity of the fluorescence signal (scale bar on the right of the panel). (B) Kymograph-like analysis of the midpiece of 20 sperm following the addition of recording medium. Each row depicting the [Ca2+]i (upper) and membrane (lower) dynamics of a single cell over time. A white dotted line indicates the moment of addition. The images presented are representative of at least five independent experiments. (C) The representative time series demonstrates [Ca2+]i and AE dynamics. Progesterone (prog, 100 μM) was added as indicated by arrowheads. Scale bar = 10 μm. Beneath each frame in the Fluo4 (green) and FM4-64 (red) images, a color code displays the normalized intensity of the fluorescence signal (scale bar on the right of the panel). (D) Kymograph-like analysis of the midpiece of 20 sperm following the addition of prog. Each row depicting the [Ca2+]i (upper) and membrane (lower) dynamics of a single cell over time. A white dotted line indicates the moment of addition. The images presented are representative of at least five independent experiments. Consistently, an [Ca2+]i transient increase precedes contraction, which is proportional to the increase in FM4-64 fluorescence, as shown in Figure 2—figure supplement 1D. (E) Representative time series of [Ca2+]i and midpiece contraction dynamics. Capacitated CD1 sperm were loaded with Fluo4 AM, immobilized on concanavalin A-coated coverslips, and incubated in a recording medium containing 0.5 μM FM4-64. AE was induced with 100 μM progesterone (prog, arrowhead). Fluo4 images are widefield images, while FM4-64 images are super-resolution radial fluctuations (SRRF)-processed (super-resolution). Scale bar = 1 μm. (F) 3D kymographs of [Ca2+]i (left) and contraction (right) dynamics. Data are normalized to the mean of the frames before the induction of AE. Representative images from at least five independent experiments are shown, with 36 cells analyzed.
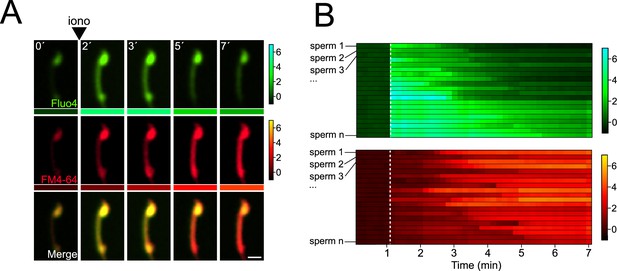
[Ca2+]i concentration drives midpiece contraction in Ionomycin-stimulated sperm.
(A) The representative time series demonstrates [Ca2+]i and acrosomal exocytosis (AE) dynamics. Capacitated F1 sperm, loaded with Fluo4 AM, were immobilized on concanavalin A-coated coverslips and incubated in a recording medium containing 10 μM FM4-64. Ionomycin (iono, 10 μM) was added as indicated by arrowheads. Beneath each frame in the Fluo4 (green) and FM4-64 (red) images, a color code displays the normalized intensity of the fluorescence signal (scale bar on the right of the panel). Scale bar = 10 μm. (B) The kymograph-like analysis of 20 ionomycin-stimulated cells shows each row depicting the [Ca2+]i (upper) and membrane (lower) dynamics of a single cell over time. A white dotted line indicates the moment of ionophore addition. The images presented are representative of at least five independent experiments.
Representative movies showing [Ca2+]i and acrosomal exocytosis (AE) dynamics for a control case (addition of recording medium, rec), progesterone (Prog, 100 μM), and ionomycin-induced AE (Iono, 10 μM).
Capacitated F1 sperm, loaded with Fluo4 AM, were immobilized on concanavalin A-coated coverslips and incubated in a recording medium containing 10 μM FM4-64. Additions were added when indicated with Rec, Prog, or Iono. Fluo4 is presented in green and FM4-64 in red. Related to Figure 4, Figure 4—figure supplement 1A.
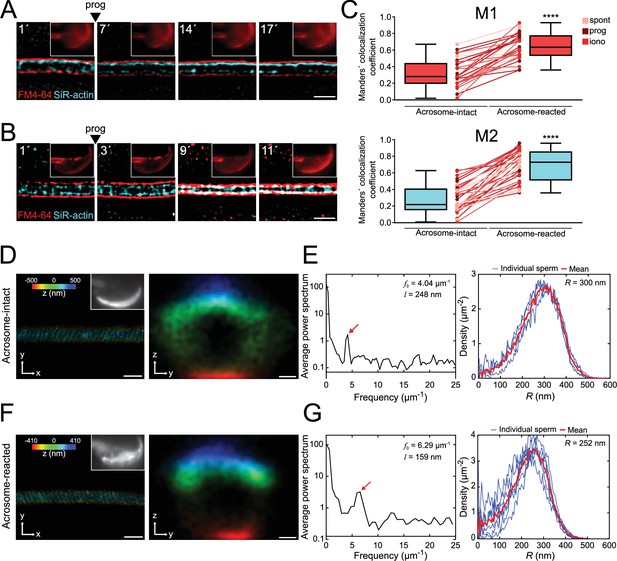
The flagellar membrane approaches the actin cytoskeleton in the midpiece of the sperm flagellum during midpiece contraction and acrosomal exocytosis (AE).
(A, B) Representative time series of plasma membrane and actin cytoskeleton colocalization in the midpiece in the absence of AE (A) and during progesterone-induced AE (B, prog, 100 μM; FM4-64 shown in red, SiR-actin shown in cyan). Capacitated CD1 sperm were loaded with 100 nM SiR-actin, immobilized on concanavalin A-coated coverslips, and incubated in a recording medium containing 0.5 μM FM4-64. Scale bar = 1 μm. Representative images from at least five independent experiments are shown, with 36 cells analyzed. (C) Manders' colocalization coefficients for acrosome-intact and acrosome-reacted cells in the midpiece. M1 was assigned to FM4-64, and M2 to SiR-actin. Data are presented as mean ± SEM. ****p<0.0001 represents statistical significance. Paired t-test was performed. (D–G) Representative images of sperm midpiece stained with the acrosome marker PNA (left panel, upper right insets, epifluorescence) and phalloidin (actin filaments, STORM) for acrosome-intact (D, E) and acrosome-reacted (F, G) cells. The left panel displays a longitudinal section of the midpiece (scale bar = 10 μm), while the right panel illustrates the radial distribution (scale bar = 0.2 μm). (E, G) Schematics of the analyzed actin double helix parameters in the midpiece: helical pitch (l, distance between turns of the helix, left panel), helical pitch frequency (f0, number of turns the helix makes per 1 μm), and radial distribution (R, radius of the double helix, right panel). Representative images from at least three independent experiments are shown. Four acrosome-intact cells and seven acrosome-reacted cells were analyzed.
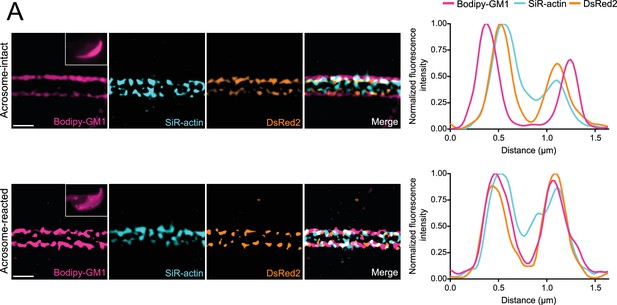
Unaltered distance between F-actin and mitochondrial network after acrosomal exocytosis (AE) indicates consistent mitochondrial network organization in EGFP-DsRed2 transgenic sperm.
(A) Representative super-resolution images of the plasma membrane (Bodipy-GM1), actin cytoskeleton (SiR-actin), and mitochondria (DsRed2) for acrosome-intact (upper panel) and acrosome-reacted (lower panel) EGFP-DsRed2 transgenic sperm. Insets in the Bodipy-GM1 images display the sperm head with AE in wide-field images. Scale bar = 1 μm. The left panel illustrates the normalized fluorescence intensity for all three dyes.
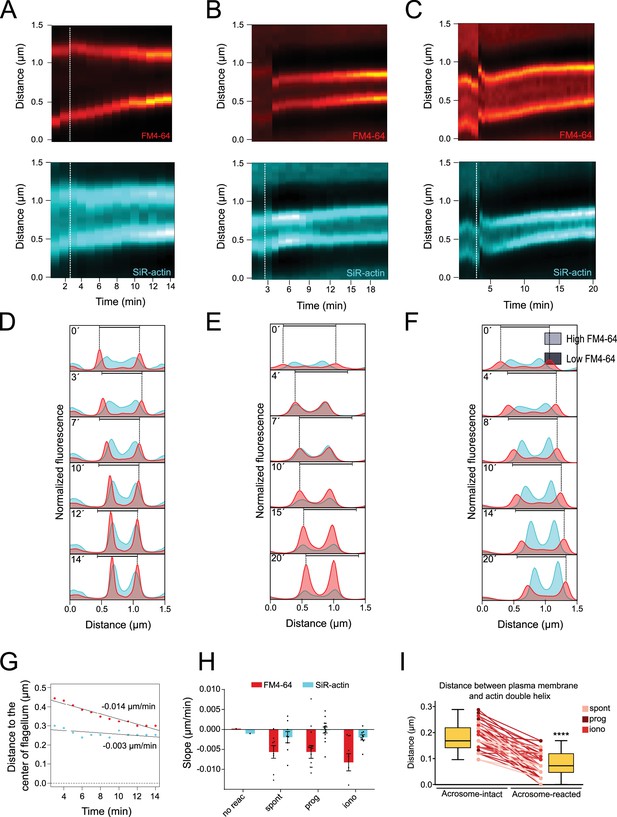
Plasma membrane approaches actin cytoskeleton during midpiece contraction.
(A–C) Super-resolution kymographs display FM4-64 (upper) and SiR-actin (lower) dynamics, computed at the proximal region of the midpiece. A dotted white line indicates the point of induction for ionomycin-induced acrosomal exocytosis (AE) (10 μM, A), progesterone-induced AE (100 μM, B), and spontaneous AE (C). (D–F) Normalized fluorescence of FM4-64 (red) and SiR-actin (blue) across the midpiece of the flagellum is shown over time for ionomycin-induced AE (10 μM, D), progesterone-induced AE (100 μM, E), and spontaneous AE (F). A horizontal black bar represents the initial distance between FM4-64 peaks. (G) Using the data from panels (A) and (D), this panel presents an analysis of the changes in the positions of fluorescence peaks, measured as the distance to the center of the flagella, over time. FM4-64 peaks are shown in red, and SiR-actin peaks are shown in blue. The numbers near the lines indicate the slopes, represented in μm/min⁻¹. (H) Slope values for no reaction, spontaneous, progesterone, and ionomycin-induced AE for FM4-64 and SiR-actin. Data are presented as mean ± SEM, and the images shown are representative of at least five independent experiments, with 36 cells analyzed. (I) The distance between the plasma membrane and actin double helix in the midpiece for acrosome-intact and reacted sperm is analyzed and represented as box plots. A box plot is a graphical representation of data that displays the median, quartiles, and a summary of the data distribution. Wilcoxon test was performed, ****p<0.001.
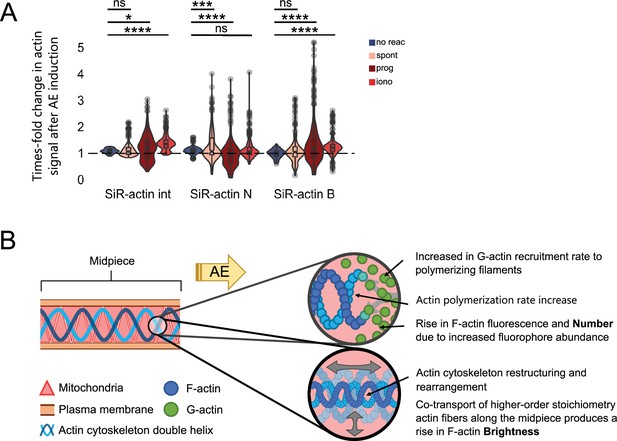
Actin cytoskeleton reorganization in the midpiece during acrosomal exocytosis (AE).
(A) Changes in SiR-actin fluorescence signal intensity (int), number (N), and brightness (B) following AE induction. After 2–3 min of time-lapse recording, progesterone (prog, 100 μm) or ionomycin (iono, 10 μm) was added to the media. Using the generated regions of interest (ROIs) for each cell (Figure 5—figure supplement 3B), SiR-actin signal was measured at each of these regions before and after induction (at its maximum increase point). The reported values indicate the maximum times-fold increase in SiR-actin int/N/B. The sample size n of this analysis represents the total number of ROIs analyzed across all cells for each condition: negative control (non-reacted sperm): n = 72, cells = 5, mice = 2; progesterone: n = 315, cells = 28, mice = 11; ionomycin: n = 324, cells = 22, mice = 10; spontaneous: n = 184, cells = 14, mice = 5. Statistical significance assessed by non-parametric Wilcoxon test. (B) Proposed model of the structural rearrangements of the actin cytoskeleton in the midpiece during AE: Based on the results obtained from the moment-based analysis shown in (A), this work suggests that the actin filament network in the midpiece experiences dynamic polymerization, evidenced by the increase in SiR-actin fluorescence and number. Additionally, reorganization is indicated by the overall increase in the magnitude and dispersion of the brightness measurement, as a consequence of the AE.
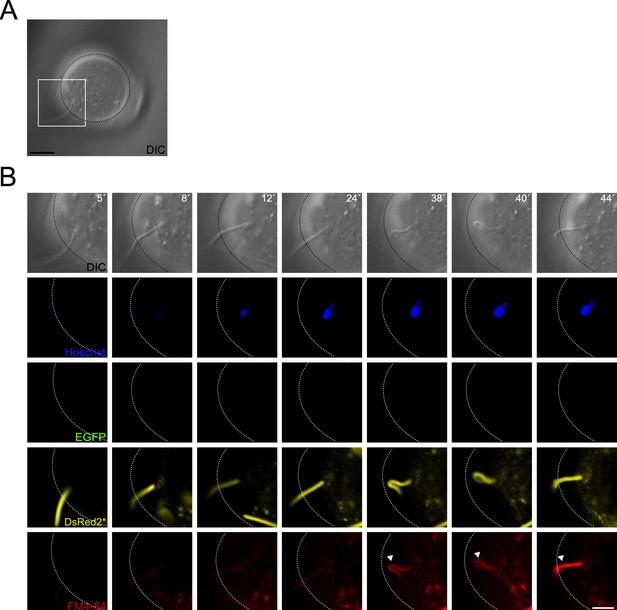
Midpiece contraction occurs following sperm-egg fusion.
(A) DIC image of a sperm-oocyte interaction, with the area depicted in higher magnification in (B). Scale bar = 20 μm. (B) Representative time series of sperm-oocyte fusion assay experiments using EGFP-DsRed2 transgenic sperm. Oocytes were stained with 1 μg/ml Hoechst and 10 μM FM4-64. DIC, Hoechst, EGFP, DsRed2*, and FM4-64 images are shown over time. Scale bar = 10 μm. The images shown are representative of at least four independent experiments.
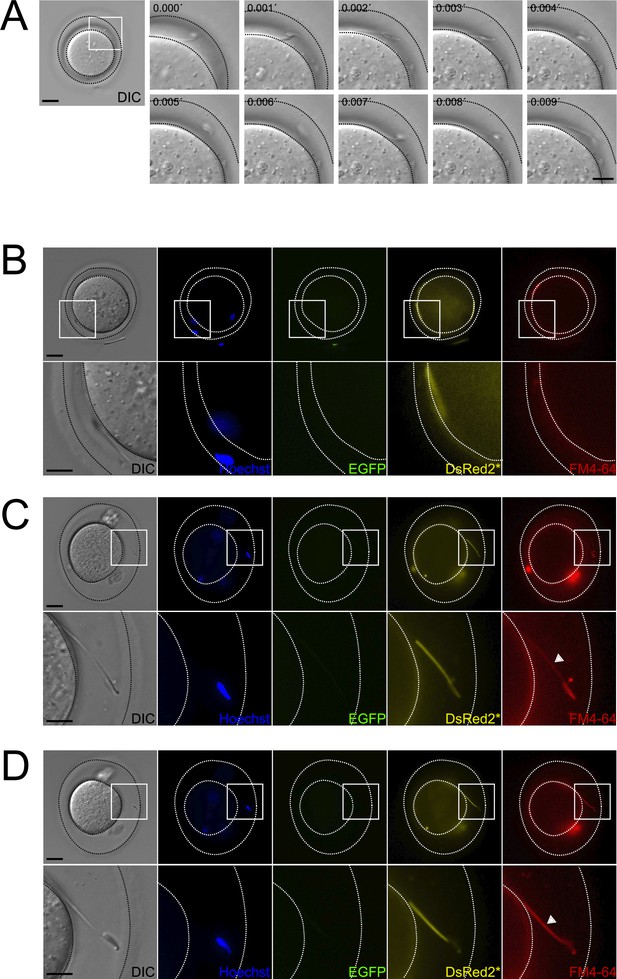
Occurrence of midpiece contraction in sperm located within the perivitelline space.
Representative images of in vitro fertilization (IVF) experiments using EGFP-DsRed2 sperm. Oocyte-sperm complexes were stained with 10 μg/ml Hoechst and 10 μM FM4-64. (A) Representative time series of DIC images showing a sperm moving within the perivitelline space. Scale bar in right panel = 20 μm, scale bar in left panel = 10 μm. (B) DIC, Hoechst, EGFP, DsRed2*, and FM4-64 images are shown for the case depicted in (A), note that, as the sperm is moving, it is located in a different position in the perivitelline space. The area depicted in the upper panel is shown in higher magnification in the lower panel. Scale bar in upper panel = 20 μm, scale bar in lower panel = 10 μm. (C, D) DIC, Hoechst, EGFP, DsRed2*, and FM4-64 images are shown for a (C) sperm that have passed through the ZP, displaying acrosomal exocytosis (AE) with an initially non-contracted midpiece. After 20 min, as shown in (D), the midpiece becomes contracted. The area depicted in the upper panel is shown in higher magnification in the lower panel. Scale bar in upper panel = 20 μm, scale bar in lower panel = 10 μm. Representative images from at least six independent experiments are shown. A total of 23 oocytes and 69 sperm were analyzed.
Representative movie of in vitro fertilization (IVF) assay.
DIC images are shown. Related to Figure 6A.
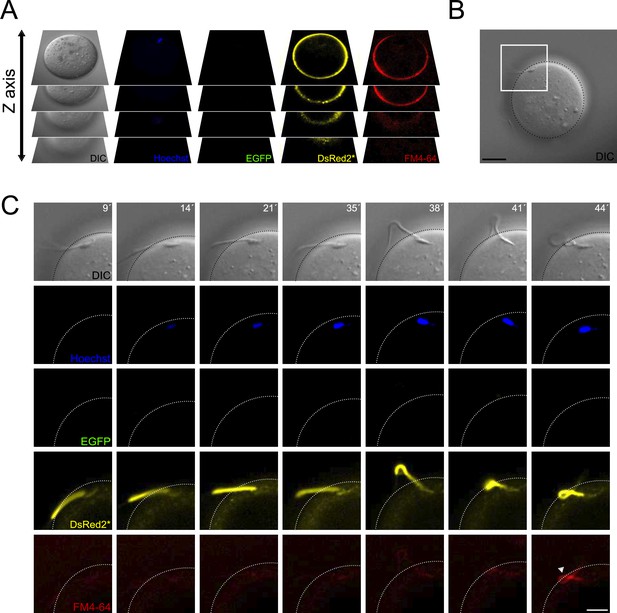
Contraction of the midpiece occurs after sperm-egg fusion.
(A) Schematic representation of the acquisition settings for the sperm-oocyte fusion assay. Images were taken every 7 μm along the z-axis. (B) DIC image of a sperm-oocyte complex, with the area depicted in higher magnification in panel (C). Scale bar = 20 μm. (C) Representative time series of sperm-oocyte fusion assay experiments using EGFP-DsRed2 sperm. Oocytes were stained with 1 μg/ml Hoechst and 10 μM FM4-64. DIC, Hoechst, EGFP, DsRed2*, and FM4-64 images are shown over time. Scale bar = 10 μm. Note that midpiece contraction occurs after sperm-egg fusion and is proportional to the increase in FM4-64 fluorescence, as shown in Figure 2—figure supplement 1D, highlighting its potential importance in the fertilization process. Representative images from at least four independent experiments are displayed.
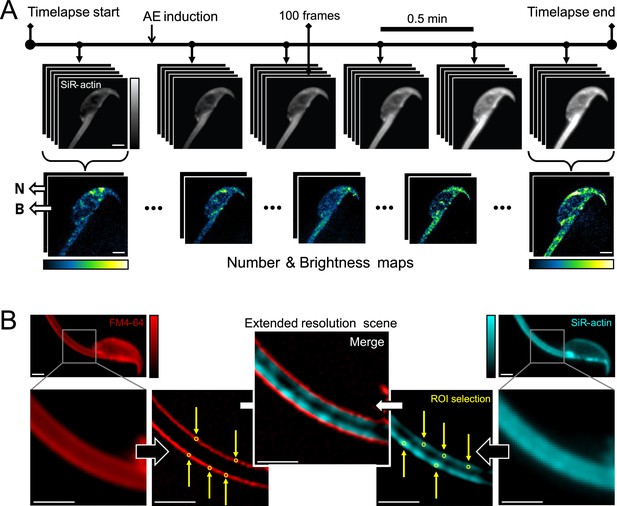
Moment-based analysis overview.
(A) The diagram illustrates the acrosomal exocytosis (AE) induction time-lapse recording process. Imaging was performed using an ONI Nanoimager-S microscope, featuring a 100 ×1.49 NA oil-immersion objective. Excitation of FM4-64 and SiR-actin was achieved using 561 nm and 640 nm lasers, respectively. Scale bar = 2 μm. (B) Time-lapse recordings from both FM4-64 (red) and SiR-actin (cyan) channels were employed to generate an extended-resolution image reconstruction. Scale bar = 2 μm. This allowed for the detection of nanoscale localized enrichments of polymerized actin. Number and brightness analysis was executed using a custom RStudio code. The following mean shift super-resolution (MSSR) parameters were applied: AMP = 3; FWHM of PSF = 2.44; Order = 0; Interpolation = bicubic; Temporal analysis = mean (100 frames).
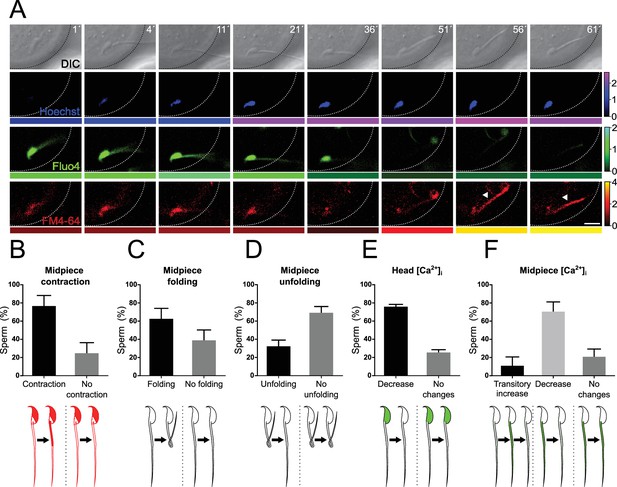
Midpiece contraction occurs in sperm-egg fusion after a decrease in [Ca2+]i.
(A) Representative time series of sperm-oocyte fusion assay experiments using wild-type sperm loaded with 1 μM Fluo-4. Oocytes were stained with 1 μg/ml Hoechst and 10 μM FM4-64. DIC, Hoechst, Fluo-4, and FM4-64 images are shown over time. Scale bar = 10 μm. The color code below each frame in the Hoechst (shown in blue), Fluo-4 (shown in green), and FM4-64 (shown in red) images indicates the normalized intensity of the fluorescence signal (scale bar on the right of the panel). (B–F) Quantification of sperm showing midpiece contraction (B, indicated by increased FM4-64 fluorescence), midpiece folding (C), midpiece unfolding (D), Fluo-4 fluorescence dynamics in the head (E), and different patterns in the midpiece (F) during fusion. Data are presented as the mean ± SEM of the percentage of sperm counted for each experiment. Representative images and data from at least three independent experiments are shown. A total of 74 oocytes and 136 sperm were analyzed. Note that midpiece contraction occurs in sperm-egg fusion after a decrease in Fluo-4 fluorescence.
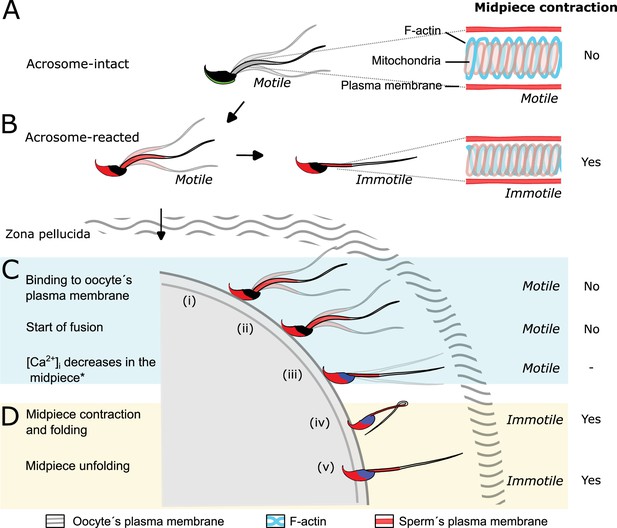
Proposed model of the structural reorganization of the sperm actin cytoskeleton during key events of fertilization.
The double helix actin network surrounding the mitochondrial sheath of the midpiece undergoes structural changes prior to the motility cessation. This structural modification is accompanied by a decrease in diameter of the midpiece and is driven by intracellular calcium changes that occur concomitant with a reorganization of the actin helicoidal cortex. Although midpiece contraction may occur in a subset of cells that undergo acrosomal exocytosis (AE) (A, B), the midpiece contraction occurs prior to motility cessation observed after sperm-egg fusion (C, D).

Fi Image based flow cytometry analysis (ImageStream Merk II), of non-capacitated mouse sperm, showing the distribution of EGFP signal (acrosome integrity) against Sytox Blue staining (cell viability).
(A) The quadrants show: Sytox Blue + / EGFP low (17.6%), Sytox Blue + / EGFP high (40.1%), Sytox Blue - / EGFP high (20.2%), and Sytox Blue - / EGFP low (21.7%). Each quadrant indicates the percentage of the total sperm population exhibiting the corresponding staining pattern. Axes are presented in a log10 scale of arbitrary units of fluorescence. (B) Representative single-cell images corresponding to the four categorized sperm populations from the flow cytometry analysis in panel (A). The top row displays sperm with compromised plasma membrane integrity (Sytox Blue +), showing low (left) and high (right) EGFP signals. The bottom row shows sperm with intact plasma membrane (Sytox Blue -), displaying high (left) and low (right) EGFP signal. It is worth noting that when analyzing the percentages in (A), we observed that the data also encompass a population of headless flagella, which was present in all observed categories. Therefore, the percentages should be interpreted with caution.
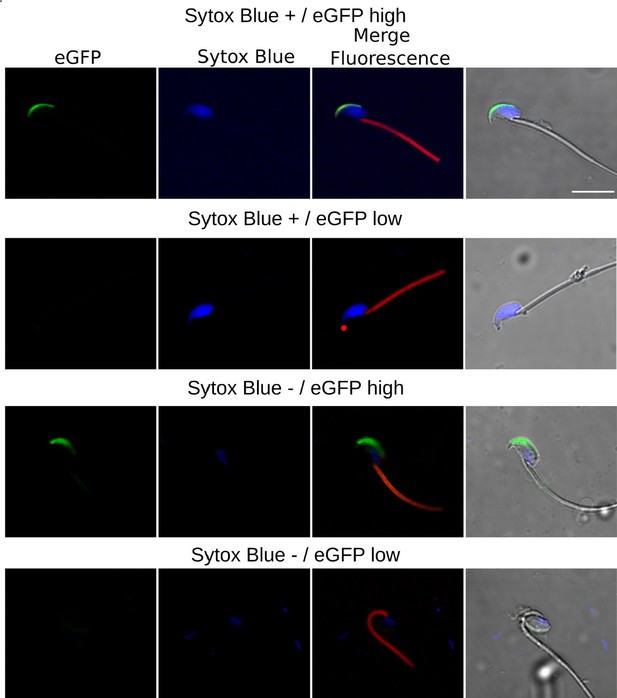
Confocal Microscopy Examples of AE and cell viability.
The top row features sperm with compromised plasma membrane integrity (Sytox Blue +) and high EGFP expression; the second row displays sperm with compromised membrane and low EGFP expression; the third row illustrates sperm with intact membrane (Sytox Blue -) and high EGFP expression; the bottom row shows sperm with intact membrane and low EGFP expression.
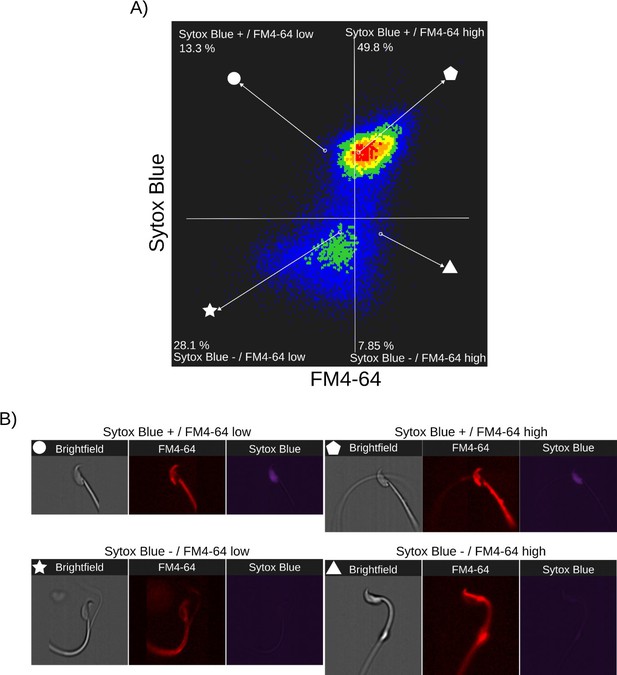
Relationship between cell death and FM4-64 fluorescence in capacitated sperm without inductor of RA.
Image-based flow cytometry analysis of non-capacitated mouse sperm loaded with FM464 and Sytox Blue dyes, with one and two minutes of incubation time, respectively.
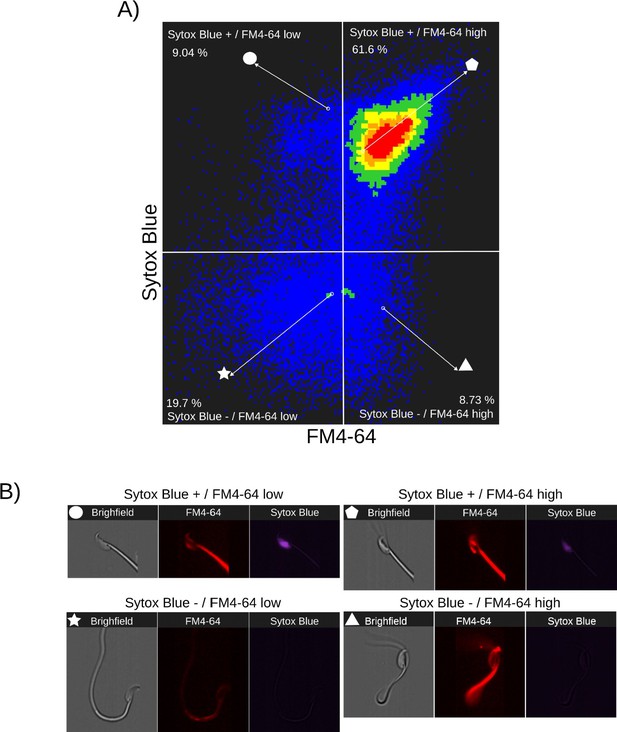
Relationship between cell death and FM4-64 fluorescence capacitated sperm stimulated with progesterone.
Image-based flow cytometry analysis of non-capacitated mouse sperm loaded with FM4-64 and Sytox Blue dyes, with one and two minutes of incubation time, respectively. (A) The quadrants show: Sytox Blue+ / FM4-64 low (9.04%), Sytox Blue+ / FM4-64 high (61.6%), Sytox Blue- / FM4-64 low (19.7%), and Sytox Blue- / FM4-64 high (8.73%). Each quadrant indicates the percentage of the total sperm population exhibiting the corresponding staining pattern. Axes are presented on a log10 scale of arbitrary units of fluorescence. (B) Representative single-cell images corresponding to the four categorized sperm populations from the flow cytometry analysis in panel (A)
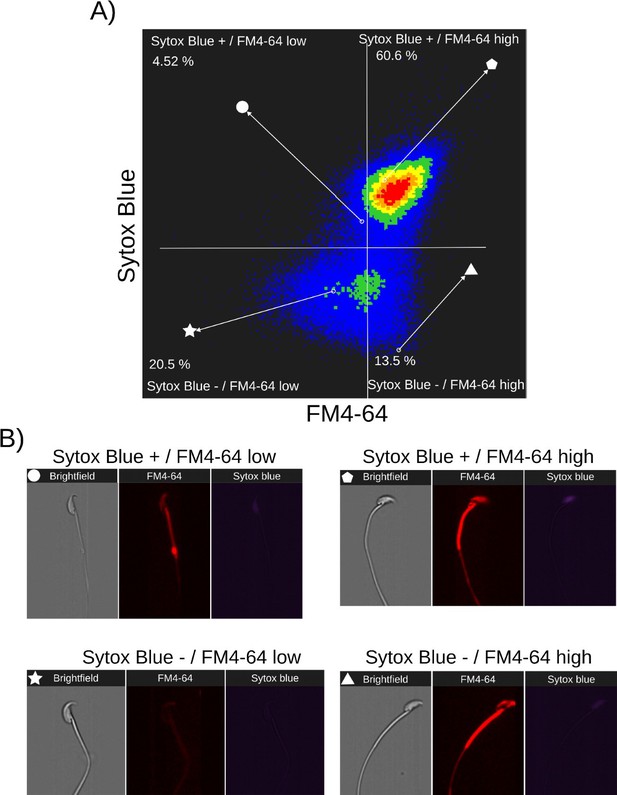
Relationship between cell death and FM4-64 fluorescence capacitated sperm stimulated with ionomycin.
Image-based flow cytometry analysis of non-capacitated mouse sperm loaded with FM464 and Sytox Blue dyes, with one and two minutes of incubation time, respectively. (A) The quadrants show: Sytox Blue+ / FM4-64 low (4.52%), Sytox Blue+ / FM4-64 high (60.6%), Sytox Blue- / FM4-64 low (20.5%), and Sytox Blue- / FM4-64 high (13.5%). Each quadrant indicates the percentage of the total sperm population exhibiting the corresponding staining pattern. Axes are presented on a log10 scale of arbitrary units of fluorescence. (B) Representative single-cell images corresponding to the four categorized sperm populations from the flow cytometry analysis in panel (A).
SEM image showing the proximity between plasma membrane and mitochondria.
Scale bar 100 nm.
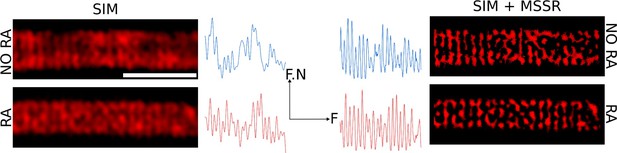
Organization of mitochondria observed via FF-SRM and SIM.
Scale bar 2 µm. F.N:Fluorescence normalized. F:Frequency
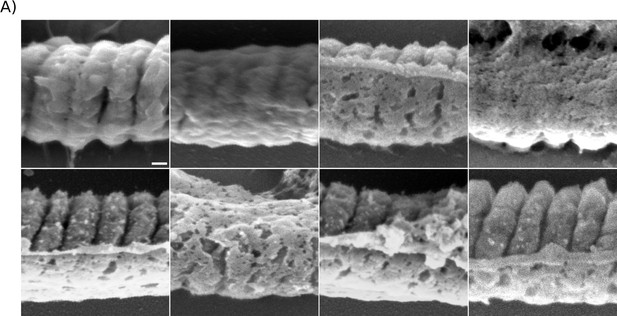
Comparison of the midpiece architecture in acrosome-intact and acrosome-reacted sperm using scanning electron microscopy (SEM).
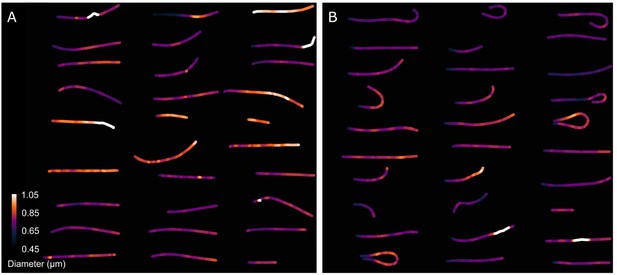
Comparison of the midpiece architecture in acrosome-intact (A) and acrosome-reacted (B) sperm using scanning electron microscopy (SEM).
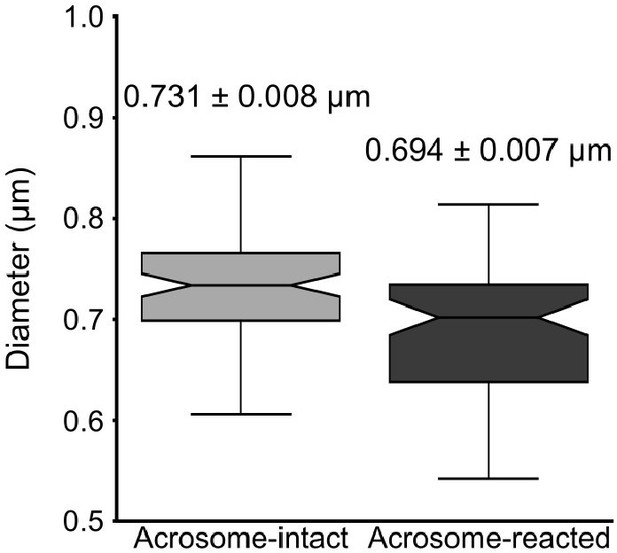
Quantification of the midpiece diameter of the sperm flagellum in acrosome-intact and acrosome-reacted sperm analyzed by scanning electron microscopy (SEM).
Data is presented as mean ± SEM. Kruskal-Wallis test was employed, p = 0.013 (AI n = 85 , AR n = 72).
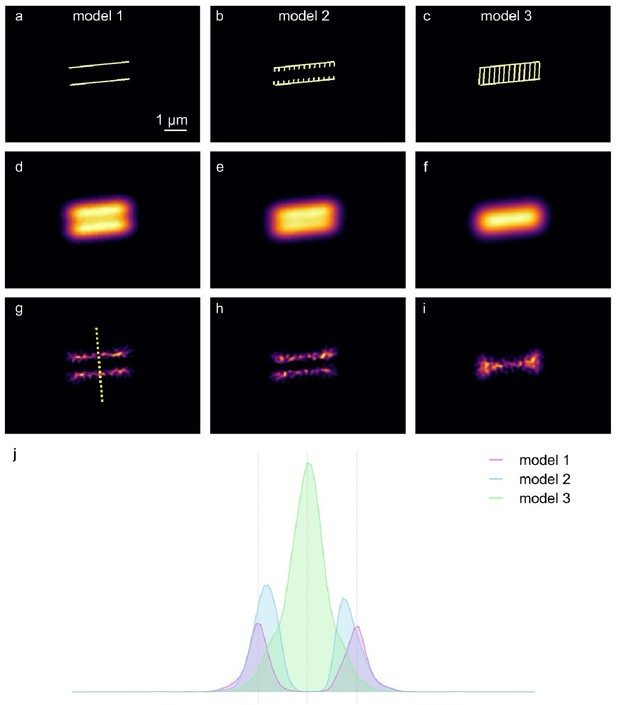
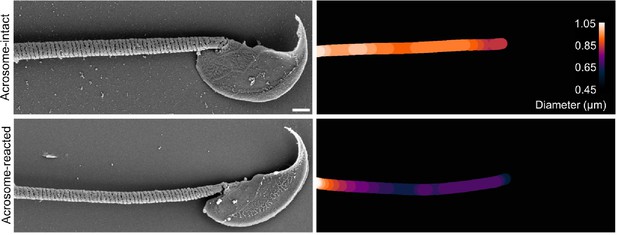
Modeling three scenarios of midpiece staining with membrane fluorescent dyes.
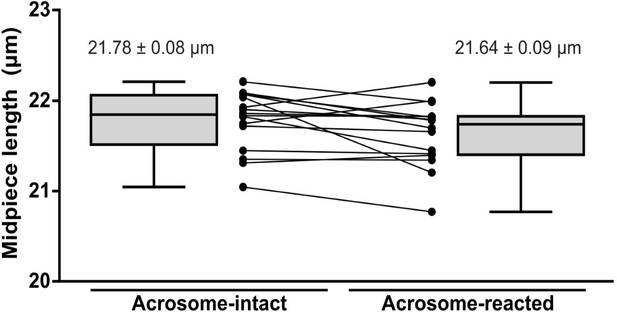
Midpiece length measured by the length of mitochondrial DsRed2 fluorescence in EGFP-DsRed2 sperm.
Measurements were done before (acrosome-intact) and after (acrosome-reacted) acrosome exocytosis and midpiece contraction. Data is presented as the mean ± sem of 14 cells induced by 10 µM ionomycin. Paired t-test was performed, resulting in no statistical significance.
Videos
Representative movie of sperm-oocyte fusion assay experiments using EGFP-DsRed2 sperm.
Oocytes were stained with 1 μg/ml Hoechst and 10 μM FM4-64. DIC images are presented in gray scales, Hoechst in blue, EGFP is green, DsRed2* in yellow, and FM4-64 in red. Sperm of interest is indicated with a white arrow.
Representative movie of sperm-oocyte fusion assay experiments using wild-type sperm loaded with 1 μM Fluo-4.
Oocytes were stained with 1 μg/ml Hoechst and 10 μM FM4-64. DIC images are presented in gray scales, Hoechst in blue, Fluo4 in green, and FM4-64 in red.
Additional files
-
Supplementary file 1
Sample details, optical equipment, imaging conditions, and probes used.
- https://cdn.elifesciences.org/articles/93792/elife-93792-supp1-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/93792/elife-93792-mdarchecklist1-v1.docx





