FAK loss reduces BRAFV600E-induced ERK phosphorylation to promote intestinal stemness and cecal tumor formation
Figures
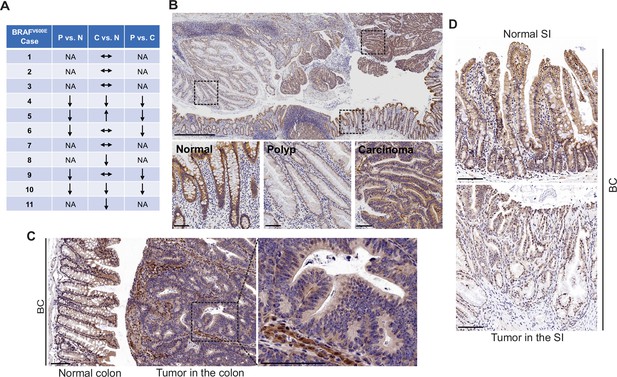
FAK downregulation in serrated tumors.
(A) Summary of FAK IHC staining in 11 human BRAFV600E-mutant CRC samples. N represents normal colon; P represents polyp; C represents carcinoma; NA, not applicable; ↔ represents no change; ↑ represents an increase. ↓ represents a decrease. (B) Representative IHC staining of BRAFV600E-mutant patient SSA/P, serrated colorectal adenoma, and adjacent normal tissues. (C) IHC staining of Fak in small intestine tumors in a 12-month-old BC mouse. (D) Representative IHC staining of Fak in colon tumor in 12-month-old BC mice. Scale bars in (B) 1 mm (upper panel) and 100 µm (lower panel). Scale bars in (C) and (D) 100 µm.
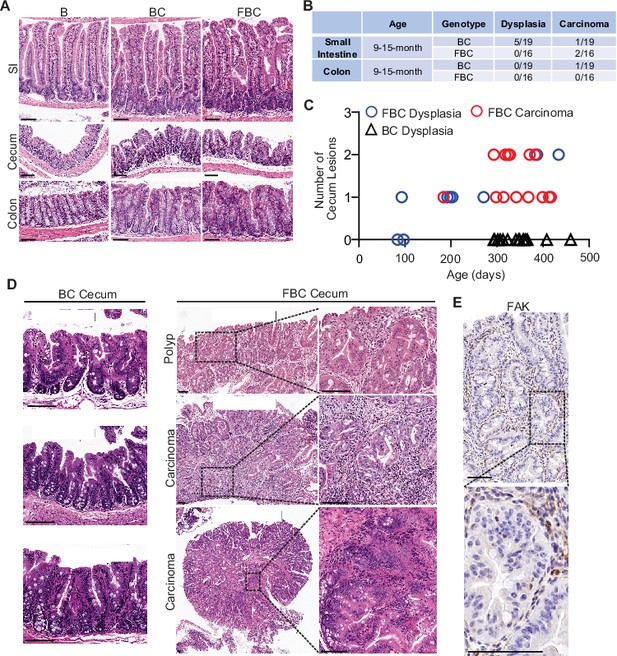
Fak loss enhances BRAFV600E-driven cecal tumorigenesis in mice.
(A) Representative hematoxylin and eosin (H&E) staining of the small intestine, cecum, and colon from indicated 6-week-old mice. (B) Summary of tumor incidence at small intestine and colon in indicted mice at the indicated age. (C) Summary of tumor incidence and tumor stage at cecum in indicated mice at the indicated age. (D) H&E staining of the cecum in BC mice and cecal serrated adenoma/polyp and carcinoma in FBC mice at the indicated age. (E) Representative IHC staining of Fak in cecal tumors in FBC mice. Scale bars: 100 µm.
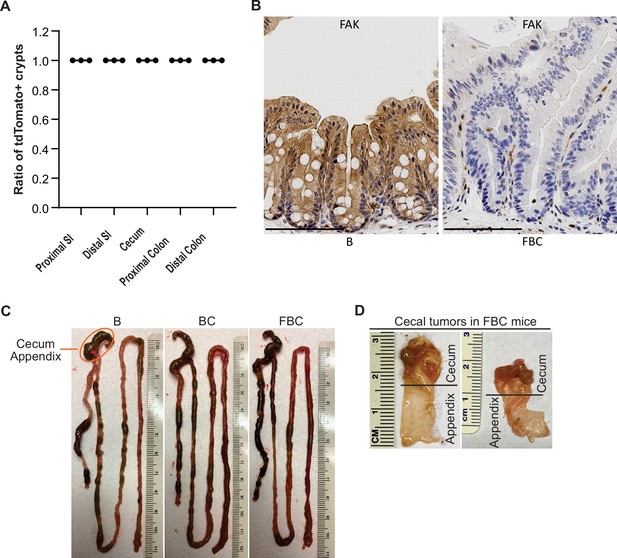
Fak deletion promotes BRAFV600E-induced cecal tumorigenesis.
(A) Cre-mediated recombination efficiency in Villin-Cre; Rosa26LSL-tdTomato/+ mice were scored for 30 crypts at each indicated bowel subsites (n=3). (B) Representative IHC staining of Fak in the cecum from B and FBC mice. (C) Representative examples of intestinal tracts in indicated 6-week-old mice. (D) Representative cecal tumors in 11–13-month-old FBC mice.
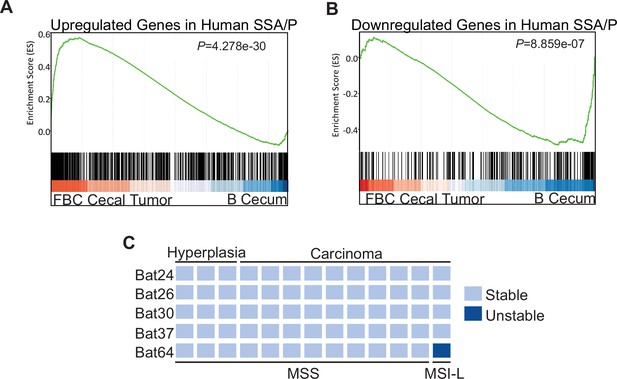
Molecular characterization of cecal tumors in FBC mice.
(A) GSEA plot showing enrichment of human SSA/Ps signature genes (upregulated genes in SSA/Ps) in FBC cecal tumors vs normal cecal mucosa of B mice. (B) GSEA plot showing that downregulated genes in human SSA/Ps were also reduced in FBC cecal tumors. (C) Microsatellite instability status of FBC mice cecal mucosa and cecal carcinomas. Each column represents one sample.
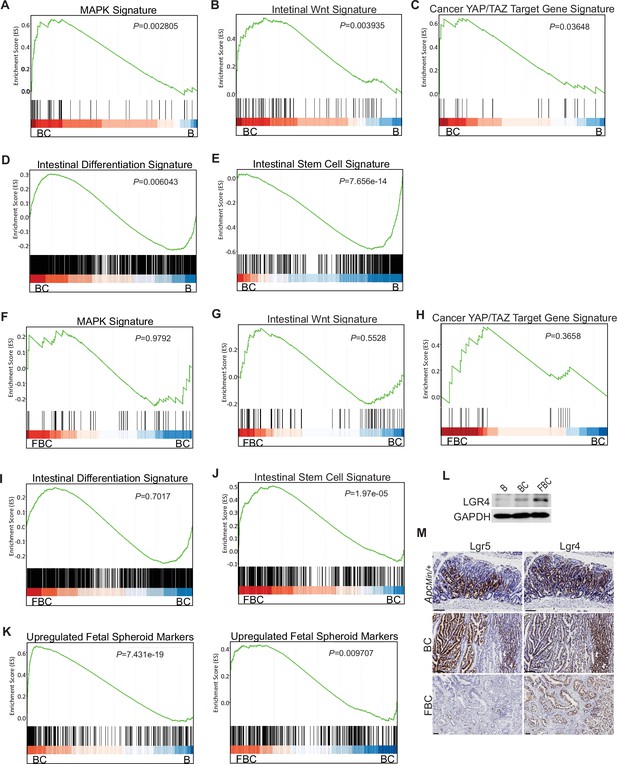
BRAFV600E mutation and Ptk2 loss-mediated changes in signaling pathways.
GSEA analysis showing upregulation of MAPK signature (A), intestinal WNT signaling (B), YAP/TAZ target gene signature (C) and intestinal differentiation signature (D), and downregulation of intestinal stem cell signature (E) in the cecum of BC mice vs B mice (n=4 per group). GSEA plots revealed no significant change in MAPK signature (F), intestinal WNT signaling (G), YAP/TAZ target gene signature (H), and intestinal differentiation signature (I) in the cecum of FBC mice vs BC mice, but enrichment of stem cell signature in FBC mice (J) (n=4 per group). (K) GSEA analysis showing upregulation of upregulated fetal spheroid markers in the cecum of BC mice vs B mice, and further enrichment in the cecum of FBC mice vs BC mice (n=4 per group). (L) Immunoblotting analysis of LGR4 in the cecum from indicated 6-week-old mice. (M) Representative in situ hybridization (ISH) staining of tumor sections from ApcMin/+, BC, and FBC mice using Lgr4 and Lgr5 probes. Scale bars: 100 µm.
-
Figure 4—source data 1
Uncropped and labelled gels for (Figure 4).
- https://cdn.elifesciences.org/articles/94605/elife-94605-fig4-data1-v1.pdf
-
Figure 4—source data 2
Raw unedited gels for (Figure 4).
- https://cdn.elifesciences.org/articles/94605/elife-94605-fig4-data2-v1.zip
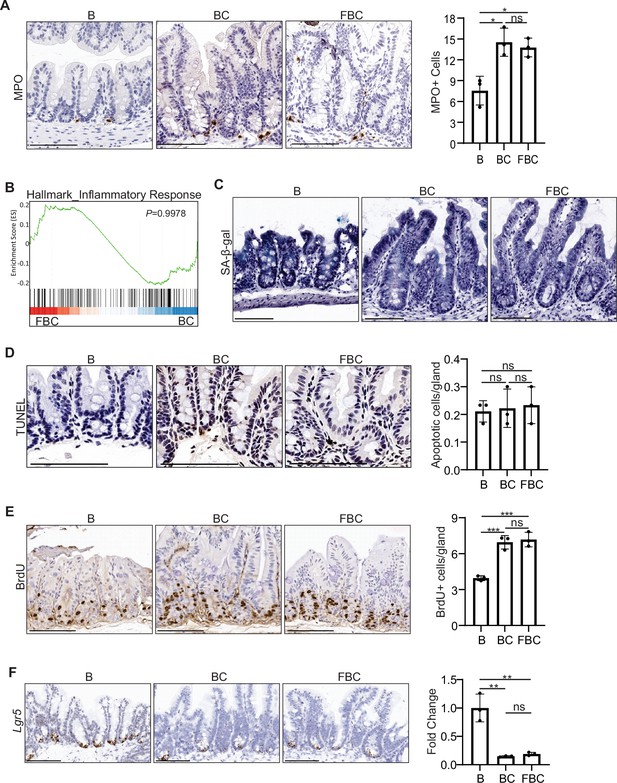
Fak loss has no impact on inflammation, senescence, apoptosis, proliferation, and Lgr5 expression in the cecum in FBC mice.
(A) Left panel: representative IHC staining of MPO in indicated mice. Right panel: quantification of MPO+ cells per 500 µm-length of cecum on the tissue sections from indicted mice (n=3 per group). (B) GSEA plots reveal no change of inflammatory response gene signature in the cecum of FBC mice vs BC mice. (C) SA-β-galactosidase staining in indicated mice. (D) Left panel: representative images of TUNEL staining in indicated mice. Right panel: quantification of apoptotic cells per gland in cecum from indicated mice (n=3 per group). (E) Left panel: representative IHC staining of BrdU in indicated mice. Right panel: quantification of BrdU+ cells per gland in cecum from indicated mice (n=3 per group). (F) Left panel: representative RNA in situ hybridization (ISH) staining of Lgr5 in indicated mice. Right panel: qRT-PCR of Lgr5 in cecum from indicated mice (n=3 per group). All samples were collected from 6-week-old mice. Data presented as mean ± SD (ns, not significant; *p<0.05; **p<0.01; ***p<0.001, Student’s t-test, two-tailed). Scale bars: 100 µm.
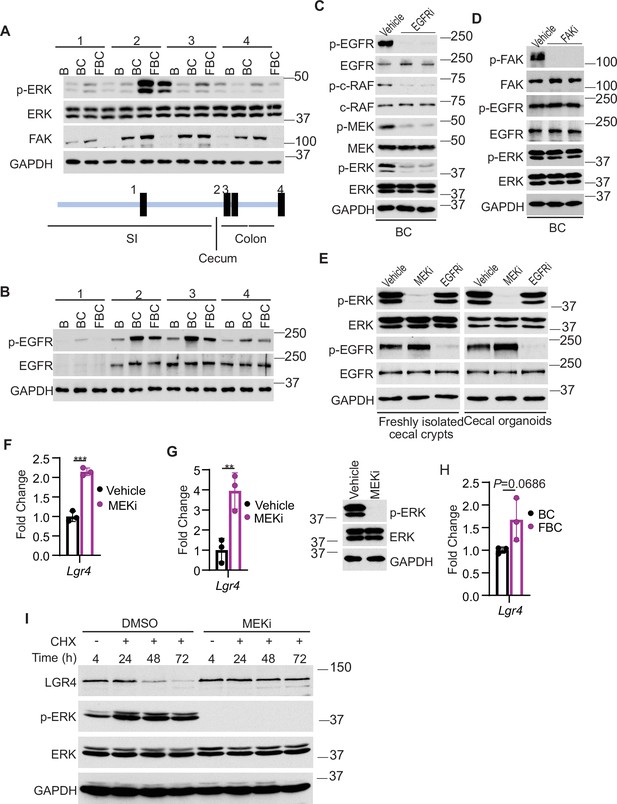
Fak loss inhibits ERK phosphorylation and upregulates Lgr4.
(A andB) Immunoblotting analysis of intestinal mucosa lysates from indicated bowel subsites in indicated 6-week-old mice. (C) Immunoblotting analysis of cecum lysates from 6-week-old BC mice treated with vehicle or EGFR inhibitor erlotinib for 4 hr. Each lane represented a single mouse. (D) Immunoblotting analysis of cecum lysates from 6-week-old BC mice treated with vehicle or FAK inhibitor PF-562271 for 4 hr. Each lane represented a single mouse. (E) Immunoblotting analysis of lysates from freshly isolated cecal crypts and cecal organoids treated with DMSO, MEK inhibitor PD0325901, or erlotinib, respectively as described in Methods. (F) qRT-PCR of Lgr4 using lysates from HT-29 cells treated with the vehicle and MEKi for 4 hr. Data presented as mean ± SD (***p<0.001; Student’s t-test, two-tailed). (G) qRT-PCR of Lgr4 using cecum lysates from BC mice treated with vehicle or MEKi for 6 hr. Data presented as mean ± SD (**p<0.01; Student’s t-test, two-tailed). Abrogation of ERK phosphorylation at T202/Y204 in the cecum was confirmed by western blot. (H) qRT-PCR of Lgr4 in cecum from BC and FBC mice (n=3 per group). Data presented as mean ± SD (p value calculated using two-tailed Student’s t-test). (I) Immunoblotting analysis of the lysates from HT-29 cells treated with cycloheximide (100 μg/ml) and/or MEK inhibitor PD0325901 (10 μM) as indicated.
-
Figure 5—source data 1
Uncropped and labelled gels for (Figure 5).
- https://cdn.elifesciences.org/articles/94605/elife-94605-fig5-data1-v1.zip
-
Figure 5—source data 2
Raw unedited gels for (Figure 5).
- https://cdn.elifesciences.org/articles/94605/elife-94605-fig5-data2-v1.zip
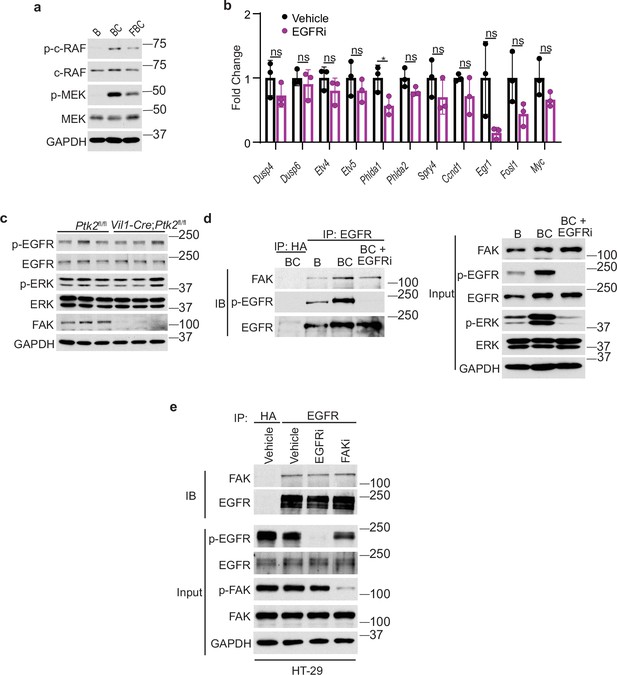
Fak loss downregulates BRAFV600E-induced ERK phosphorylation.
(A) Immunoblotting analysis of cecal lysates from indicated 6-week-old mice. (B) qRT-PCR of selected ERK transcriptional output markers in cecum from vehicle- and erlotinib-treated BC mice (n=3 per group). Data presented as mean ± SD (ns, not significant; *p<0.05; Student’s t-test, two-tailed). (C) The cecal mucosa lysates from 6-week-old Ptk2fl/fl and Vil1-Cre;Ptk2fl/fl mice were used for immunoblotting. Each lane represented a single mouse. (D) The cecal mucosa lysates from 6-week-old vehicle-treated B and BC mice and EGFR inhibitor erlotinib-treated BC mice were used for immunoprecipitation and immunoblotting with the indicated antibodies. (E) The lysates from HT-29 cells treated with vehicle, EGFR inhibitor erlotinib, or FAK initiator PF-562271 were used for immunoprecipitation and immunoblotting with the indicated antibodies.
-
Figure 5—figure supplement 1—source data 1
Uncropped and labelled gels for (Figure 5—figure supplement 1).
- https://cdn.elifesciences.org/articles/94605/elife-94605-fig5-figsupp1-data1-v1.zip
-
Figure 5—figure supplement 1—source data 2
Raw unedited gels for (Figure 5—figure supplement 1).
- https://cdn.elifesciences.org/articles/94605/elife-94605-fig5-figsupp1-data2-v1.zip
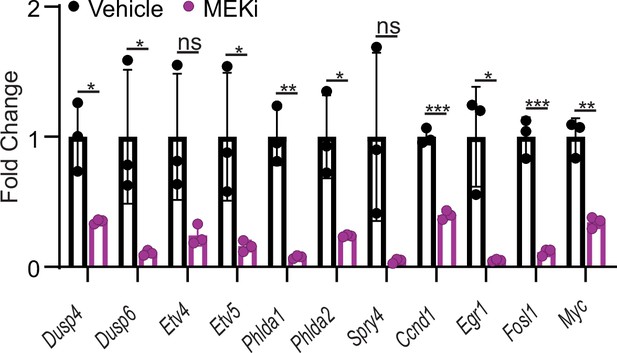
qRT-PCR of selected ERK transcriptional output markers in cecum from vehicle- and MEKi-treated BC mice (n=3 per group).
Data presented as mean ± SD (ns, not significant; *p<0.05; **p<0.01; ***p<0.001, Student’s t-test, two-tailed).
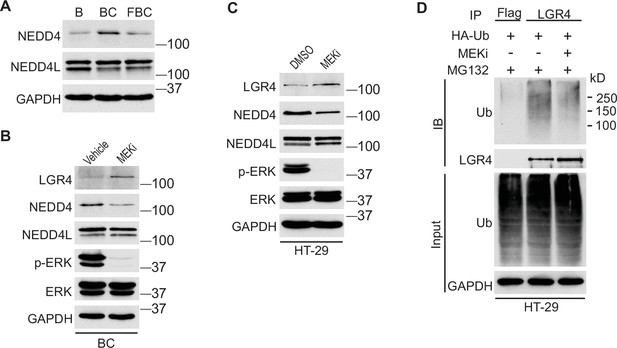
Inhibition of ERK phosphorylation stabilizes LGR4 through downregulating NEDD4.
(A) Immunoblotting analysis of cecum lysates from indicated 6-week-old mice. (B) Immunoblotting analysis of cecum lysates from 6-week-old BC mice treated with vehicle or MEK inhibitor PD0325901. MEK inhibitor was given to the mice at a dose of 25 mg/kg three times at 12 hr intervals. Twenty-eight hours after the first treatment, the cecum mucosa was collected for immunoblotting. (C) Immunoblotting analysis of lysates from HT-29 cells treated with DMSO or 10 µM MEK inhibitor for 24 hr. (D) HT-29 cells were transfected with HA-Ubiquitin. One day later, the cells were treated with DMSO or 10 µM MEK inhibitor for 24 hr. Then all the cells were incubated with 10 µM MG132 for additional 4 hr. The cell lysates were collected for immunoprecipitation and immunoblotting with the indicated antibodies.
-
Figure 6—source data 1
Uncropped and labelled gels for (Figure 6).
- https://cdn.elifesciences.org/articles/94605/elife-94605-fig6-data1-v1.zip
-
Figure 6—source data 2
Raw unedited gels for (Figure 6).
- https://cdn.elifesciences.org/articles/94605/elife-94605-fig6-data2-v1.zip
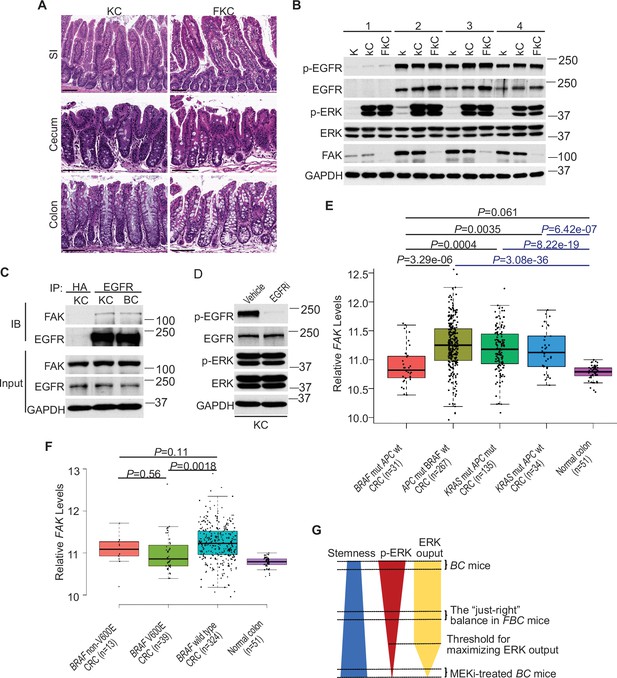
ERK activation is FAK/EGFR-independent in KC mice.
(A) Representative hematoxylin and eosin (H&E) staining of the small intestine, cecum, and colon from indicated 9-month-old mice. (B) Immunoblotting analysis of intestinal mucosa lysates from indicated bowel subsites in indicated 6-week-old mice. (C) The cecal mucosa lysates from 6-week-old KC and BC mice were used for immunoprecipitation and immunoblotting with the indicated antibodies. (D) Immunoblotting analysis of cecum lysates from 6-week-old KC mice treated with vehicle or EGFR inhibitor erlotinib for 4 hr. (E and F) Comparison of FAK expression levels between CRCs with indicated mutations by analysis of TCGA RNA-sequencing dataset. Data were analyzed for statistical significance using a Student t-test. (G) Diagram of the ‘just-right’ MAPK signaling model in the serrated pathway.
-
Figure 7—source data 1
Uncropped and labelled gels for (Figure 7).
- https://cdn.elifesciences.org/articles/94605/elife-94605-fig7-data1-v1.zip
-
Figure 7—source data 2
Raw unedited gels for (Figure 7).
- https://cdn.elifesciences.org/articles/94605/elife-94605-fig7-data2-v1.zip
Additional files
-
Supplementary file 1
The results of whole-exome sequencing on paired tumors (n=2) and neighboring mucosa show no additional driver mutations were detected in the cecal tumors.
- https://cdn.elifesciences.org/articles/94605/elife-94605-supp1-v1.xlsx
-
Supplementary file 2
List of the antibodies used in this study.
- https://cdn.elifesciences.org/articles/94605/elife-94605-supp2-v1.docx
-
Supplementary file 3
List of the PCR primers used in this study.
- https://cdn.elifesciences.org/articles/94605/elife-94605-supp3-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/94605/elife-94605-mdarchecklist1-v1.docx





