Associations of proton pump inhibitors with susceptibility to influenza, pneumonia, and COVID-19: Evidence from a large population-based cohort study
Figures
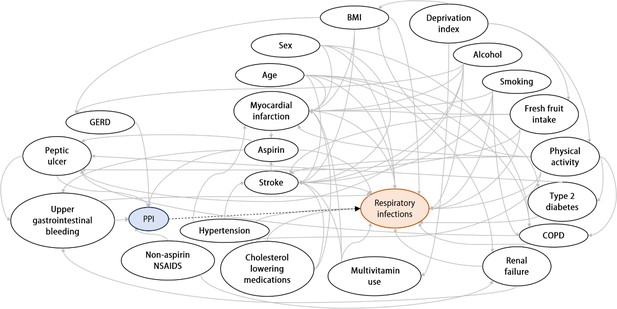
Directed acyclic graph (DAG) for evaluation of covariates in the logistic regression model.
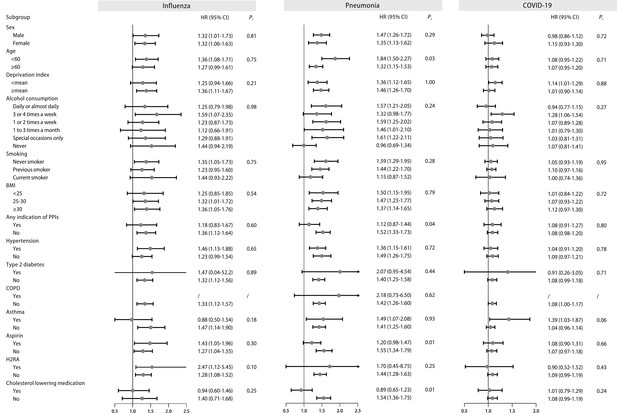
Stratified analysis of regular proton pump inhibitor (PPI) users and the risk of influenza, pneumonia, and COVID-19 infection.
Effect estimates were based on age, sex, deprivation index, alcohol consumption, smoking, body mass index (BMI), indications of PPIs, hypertension, type 2 diabetes, chronic obstructive pulmonary disease (COPD), asthma, aspirin, histamine 2 receptor antagonist (H2RA), and cholesterol-lowering medication, using the fully adjusted model. CI: confidence interval; HR: hazard ratio; Pi: P value for interaction.
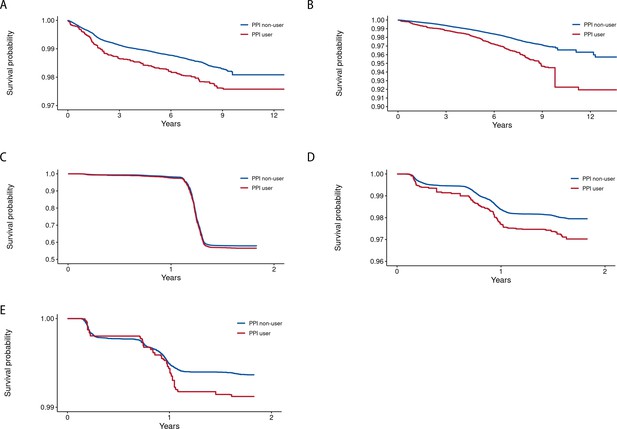
Kaplan-Meier curves illustrating the event-free probability for the outcomes among users and non-users of proton pump inhibitors (PPIs).
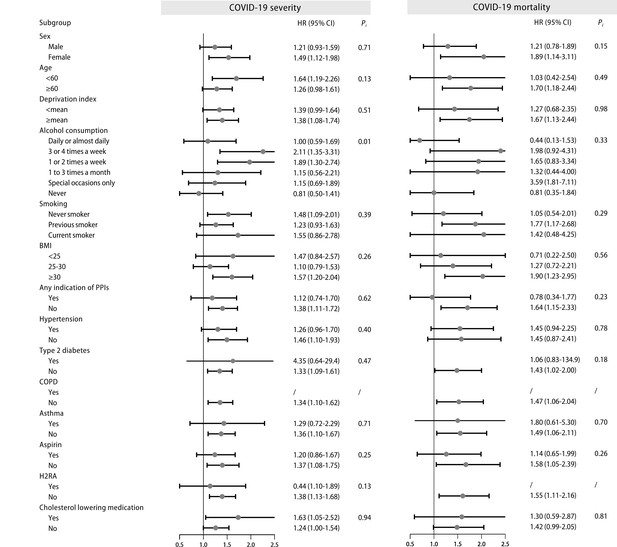
Stratified analysis of proton pump inhibitor (PPI) users and the risk of COVID-19 severity and mortality.
Tables
Baseline characteristics of the included participants.
| Characteristics | Regular PPI use | Overall | |
|---|---|---|---|
| Yes | No | ||
| Number of participants, n (%) | 9 997 (6.2) | 150 926 (93.8) | 160 923 (100.0) |
| Age, years, mean (SD) | 59.4 (7.4) | 56.3 (8.2) | 56.5 (8.1) |
| Sex, female, n (%) | 5 533 (55.4) | 79 709 (52.8) | 85 242 (53.0) |
| Ethnicity, white, n (%) | 9 571 (95.7) | 144 295 (95.6) | 153 866 (95.6) |
| Deprivation index, mean (SD) | –0.9 (3.3) | –1.4 (3.0) | –1.4 (3.0) |
| Alcohol consumption, n (%) | |||
| Daily or almost daily | 1 805 (18.1) | 28 846 (20.5) | 32 874 (20.4) |
| 3 or 4 times a week | 1 923 (19.2) | 33 533 (23.8) | 37 570 (23.4) |
| 1 or 2 times a week | 2 396 (24.0) | 37 443 (26.6) | 42 228 (26.2) |
| 1–3 times a month | 1 179 (11.8) | 15 669 (11.1) | 17 988 (11.2) |
| Special occasions only | 1 522 (15.2) | 14 965 (10.6) | 17 616 (11.0) |
| Never | 1 161 (11.6) | 10 414 (7.4) | 12 570 (7.8) |
| Smoking, n (%) | |||
| Never smoker | 4 572 (45.7) | 82 998 (55.0) | 87 570 (54.4) |
| Previous smoker | 4 289 (42.9) | 52 013 (34.5) | 56 302 (35.0) |
| Current smoker | 1 136 (11.4) | 15 915 (10.6) | 17 051 (10.6) |
| Physical activity, MET minutes/week, median (IQR) | 1 525.5 (2 722.0) | 1 815.0 (2 848.5) | 1 794.0 (2 838.5) |
| Fresh fruit intake, pieces, mean (SD) | 2.0 (2.6) | 1.9 (2.6) | 1.9 (2.6) |
| BMI, kg/m2, mean (SD) | 29.2 (5.1) | 27.4 (4.7) | 27.5 (4.8) |
| Indication of PPIs, n (%) | |||
| GERD | 3 235 (32.4) | 4 015 (2.7) | 7 250 (4.5) |
| Peptic ulcer | 561 (5.6) | 1 303 (0.9) | 1 864 (1.2) |
| Upper gastrointestinal bleeding | 18 (0.2) | 38 (0.03) | 56 (0.03) |
| Comorbidities, n (%) | |||
| Hypertension | 4 116 (41.2) | 38 162 (25.3) | 42 278 (26.3) |
| Type 2 diabetes | 124 (1.2) | 890 (0.6) | 1 014 (0.6) |
| Renal failure | 60 (0.6) | 243 (0.2) | 303 (0.2) |
| Myocardial infarction | 331 (3.3) | 1 632 (1.1) | 1 963 (1.2) |
| Stoke | 140 (1.4) | 943 (0.6) | 1 083 (0.7) |
| COPD | 46 (0.5) | 200 (0.1) | 246 (0.2) |
| Asthma | 841 (8.4) | 8 471 (5.6) | 9 312 (5.8) |
| Medication use, n (%) | |||
| Aspirin | 2 457 (24.6) | 21 108 (14.0) | 23 565 (14.6) |
| Non-aspirin NSAIDS | 1 224 (12.2) | 22 568 (15.0) | 23 792 (14.8) |
| H2RA | 297 (3.0) | 2 956 (2.0) | 3 253 (2.02) |
| Cholesterol lowering medications | 1 537 (15.4) | 9 241 (6.1) | 10 778 (6.70) |
| Multivitamin use, n (%) | 2 227 (22.3) | 33 201 (22.0) | 35 428 (22.0) |
-
BMI: body mass index; COPD: chronic obstructive pulmonary disease; GERD: gastroesophageal reflux disease; H2RA: histamine 2 receptor antagonist; IQR: interquartile range; MET: metabolic equivalent of task; PPI: proton pump inhibitor; NSAIDS: non-steroidal anti-inflammatory drugs; SD: standard deviation.
Associations of PPI use with the susceptibility to pneumonia, influenza, COVID-19 positivity, and other respiratory infections.
| Case/person-years | Non-adjusted model | Age/sex-adjusted model | Fully adjusted model* | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| Influenza | |||||||
| Non-regular PPI use | 2 009/6 011 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Regular PPI use | 183/539 | 1.38 (1.19–1.62) | <0.001 | 1.49 (1.28–1.74) | <0.001 | 1.32 (1,12–1.56) | 0.001 |
| Pneumonia | |||||||
| Non-regular PPI use | 2 904/12 867 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Regular PPI use | 378/1 702 | 2.04 (1.83–2.27) | <0.001 | 1.74 (1.56–1.94) | <0.001 | 1.42 (1.26–1.59) | <0.001 |
| COVID-19 positivity | |||||||
| Non-regular PPI use | 23 989/29 080 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Regular PPI use | 1 440/1 702 | 1.18 (1.09–1.26) | <0.001 | 1.07 (0.99–1.15) | 0.058 | 1.08 (0.99–1.17) | 0.101 |
| Other upper respiratory infections | |||||||
| Non-regular PPI use | 14 449/52 499 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Regular PPI use | 1 118/3 988 | 1.30 (1.22–1.38) | <0.001 | 1.31 (1.23–1.39) | <0.001 | 1.19 (1.11–1.27) | <0.001 |
| Other lower respiratory infections | |||||||
| Non-regular PPI use | 14 494/55 384 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Regular PPI use | 1 486/5 598 | 1.78 (1.67–1.88) | <0.001 | 1.65 (1.56–1.74) | <0.001 | 1.37 (1.29–1.46) | <0.001 |
-
CI: confidence interval; COVID-19: coronavirus disease 2019; HR: hazard ratio; PPI: proton pump inhibitor.
-
*
Adjusted for age, sex, ethnicity, deprivation index, smoking, alcohol consumption, physical activity, fresh fruit intake, body mass index, any indication of PPIs (gastroesophageal reflux disease [GERD], peptic ulcer, upper gastrointestinal bleeding), comorbidities (hypertension, type 2 diabetes, renal failure, myocardial infarction, stroke, chronic obstructive pulmonary disease [COPD], asthma), medications (aspirin, non-aspirin non-steroidal anti-inflammatory drugs [NSAIDs, ibuprofen], histamine 2 receptor antagonists (H2RAs), cholesterol lowering medications), multivitamin use, and influenza vaccination (for influenza) or COVID-19 vaccination (for COVID-19-related outcomes).
Comparisons of the risks of influenza, pneumonia, and COVID-19 between proton pump inhibitor (PPI) and histamine-2 receptor antagonist (H2RA) users.
| Cases / Person-years | HR (95% Cl)* | p | |
|---|---|---|---|
| Influenza | |||
| Regular H2RA use | 32/102 | 1.00 (Reference) | |
| Regular PPI use | 175/524 | 1.74 (1.19–2.54) | 0.004 |
| Pneumonia | |||
| Regular H2RA use | 86/385 | 1.00 (Reference) | |
| Regular PPI use | 368/1653 | 1.22 (0.96–1.54) | 0.104 |
| COVID-19 positivity | |||
| Regular H2RA use | 425/506 | 1.00 (Reference) | |
| Regular PPI use | 1 409/1 665 | 1.06 (0.90–1.24) | 0.509 |
| Other upper respiratory infection | |||
| Regular H2RA use | 146/522 | 1.00 (Reference) | |
| Regular PPI use | 602/2099 | 1.28 (1.07–1.54) | 0.008 |
| Other lower respiratory infection | |||
| Regular H2RA use | 339/1350 | 1.00 (Reference) | |
| Regular PPI use | 1438/5398 | 1.33 (1.18–1.50) | <0.001 |
-
CI: confidence interval; COVID-19: coronavirus disease 2019; H2RA: histamine-2 receptor antagonist; HR: hazard ratio; PPI:585 proton pump inhibitor.
-
*
Adjusted for age, sex, ethnicity, deprivation index, smoking, alcohol consumption, physical activity, fresh fruit intake, body mass index, any indication of PPIs (gastroesophageal reflux disease [GERD], peptic ulcer, upper gastrointestinal bleeding), comorbidities (hypertension, type 2 diabetes, renal failure, myocardial infarction, stroke, chronic obstructive pulmonary disease [COPD], asthma), medications (aspirin, non-aspirin non-steroidal anti-inflammatory drugs [NSAIDs, ibuprofen], cholesterol lowering medications), multivitamin use, and influenza vaccination (for influenza) or COVID-19 vaccination (for COVID-19-related outcomes).
Additional files
-
Supplementary file 1
Supplementary information for the analyses.
(a) Generic name and examples of trade name of proton pump inhibitors. (b) Definitions of outcomes in the UK Biobank cohort. (c) The proportional hazards assumption tested by Schoenfeld residuals tests. (d) Associations of PPI use with the risk of influenza, pneumonia, and other respiratory infections (with inclusion of self-reported cases). (e). Associations of PPI use with COVID-19 severity and mortality. (f) Associations of PPI use with the risk of influenza, pneumonia, COVID-19, and other respiratory infections by different types of PPIs. (g) Associations of PPI use with the risk of influenza, pneumonia, COVID-19, and other respiratory infections by CYP2C19 phenotypes (h) Associations of PPI use with COVID-19 severity and mortality by CYP2C19 phenotypes. (i) Associations of PPI use with the risk of influenza, pneumonia, COVID-19, and other respiratory infections with multiple imputation. (j) Analysis of associations of PPI use with COVID-19 severity and mortality with multiple imputation. (k) Clinical characteristics of included participants after propensity score-matching. (l) Propensity score-matched analysis of associations of PPI use with the risk of influenza, pneumonia, COVID-19, and other respiratory infections. (m) Propensity score-matched analysis of associations of PPI use with COVID-19 severity and mortality. (n) Comparisons between proton pump inhibitor (PPI) and histamine-2 receptor antagonist (H2RA) users for COVID-19 severity and mortality.
- https://cdn.elifesciences.org/articles/94973/elife-94973-supp1-v2.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/94973/elife-94973-mdarchecklist1-v2.pdf