Mechanical force of uterine occupation enables large vesicle extrusion from proteostressed maternal neurons
Figures
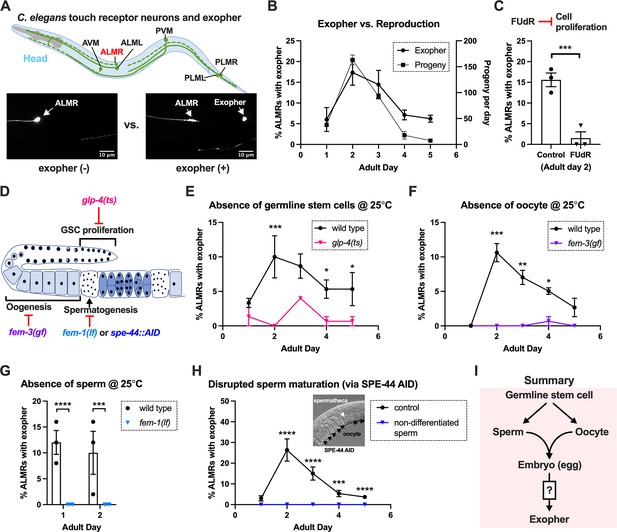
Exophergenesis is dependent on the presence of the germline, ooyctes, and sperm.
(A) Exophers produced by ALMR are readily visualized in living C. elegans. Top, positions of the six C. elegans touch receptor neurons: AVM (Anterior Ventral Microtubule cell), ALMR (Anterior Lateral Microtubule cell, Right), ALML (Anterior Lateral Microtubule cell, Left), PVM (Posterior Ventral Microtubule cell, Right), PLMR (Posterior Lateral Microtubule cell, Right), and PLML (Posterior Lateral Microtubule cell, Left). Bottom panels are representative pictures (n > 100, scale bar = 10 μm) of an ALMR neuron without (lower left) or with (lower right) exopher production from strain ZB4065 bzIs166[Pmec-4::mCherry], which expresses elevated mCherry in the touch receptor neurons. Over-expression of mCherry in bzIs166 is associated with enlargement of lysosomes and formation of large mCherry foci that often correspond to LAMP::GFP-positive structures; ultrastructure studies reveal considerable organelle morphological change not seen in low reporter-expression neurons; polyQ74, polyQ128, Aβ1-42 over-expression all increase exophers (Melentijevic et al., 2017; Arnold et al., 2023). Most genetic compromise of different proteostasis branches--heat shock chaperones, proteasome, and autophagy--enhance exophergenesis, supporting exophergenesis as a response to proteostress. (B) Both exopher production and reproduction typically peak around Ad2 in the Pmec-4::mCherry strain. Left axis: the frequency of exopher events in the adult hermaphrodite C. elegans strain ZB4065 at 20 °C; Mean ± SEM of nine independent trials (~50 animals in each trial). Right axis: daily progeny count (Mean ± SEM) from 10 wild-type N2 hermaphrodites. (C) 5-fluoro-2'-deoxyuridine (FUdR), which inhibits progeny production, suppresses early adult exopher production. Data are the percentage of ALMR exopher events among >50 Ad2 hermaphrodites in each trial (total of 3 independent trials) for strain ZB4065 at 20 °C in absence (control) or presence of 51 μM FUDR. ***p<0.001 in Cochran–Mantel–Haenszel test. (D) Illustration of the roles of germline development genes tested for impact on exophers. The C. elegans reproductive system comprises a bilobed gonad in which germ cells (light blue, dark nuclei) develop into mature oocytes, which are fertilized in the spermatheca (sperm indicated as dark dots) and held in the uterus until about the 30-cell gastrulation stage, at which point eggs (dark blue) are laid. Indicated are the steps impaired by germline developmental mutations we tested. (E) Germline stem cells are required for efficient exopher production. Data show the percentage of ALMR exopher events (Mean ± SEM) among 50 adult hermaphrodite C. elegans that express the wild-type GLP-4 protein or the GLP-4(ts) protein encoded by glp-4(bn2) at 25 °C. All animals express Pmec-4mCherry, and the glp-4(ts) mutants lack a germline when reared at the restrictive temperature (25 °C). Eggs collected from both wild-type and the glp-4(ts) mutants were grown at 15 °C for 24 hr before being shifted to 25 °C (at L1 stage) to enable development under restrictive conditions; three independent trials of 50 animals represented by each dot; ***p<0.001 or *p<0.05 in Cochran–Mantel–Haenszel test. Note that in wild-type (WT), temperature shift normally induces a modest elevation in exopher numbers (typically a few % increase, supplemental data in Cooper et al., 2021) and is thus not itself a factor in exopher production levels. (F) Oogenesis is required for efficient exopher production. Spermatogenesis occurs but oogenesis is blocked when fem-3(gf) mutant hermaphrodites are cultured at 25°C. Data show the percentage of ALMR exopher events (Mean ± SEM) in hermaphrodite C. elegans that express the wild-type FEM-3 protein or the temperature-dependent gain-of-function (gf) FEM-3 protein (25 °C; three independent trials, n=50/trial), ***p<0.001; **p<0.01; or *p<0.05 in the Cochran–Mantel–Haenszel test. (G) Spermatogenesis is required for efficient exopher production. There is no spermatogenesis in fem-1(lf) at the restrictive temperature of 25 °C, while oogenesis is normal in the hermaphrodite. Shown is the percentage of ALMR exopher events (Mean ± SEM) in adult hermaphrodites that express the wild-type FEM-1 protein or the temperature-dependent loss of function (lf) FEM-1 protein (25 °C; three independent trials, 50 animals/trial). ****p<0.0001 or ***p<0.001 in Cochran–Mantel–Haenszel test. (H) Spermatogenesis is required for efficient exopher production. Data show the percentage of ALMR exopher events (Mean ± SEM) in hermaphrodite C. elegans that express the SPE-44::degron fusion. SPE-44 is a critical transcription factor for spermatogenesis (Kulkarni et al., 2012), and is tagged with a degron sequence that enables targeted degradation in the presence of auxin in line ZB4749. In the auxin-inducible degron (AID) system, auxin is added to the plates in 0.25% ethanol, so ‘control’ is treated with 0.25% ethanol and ‘no sperm’ is treated with 1 mM auxin applied to plates in 0.25% ethanol from egg to adult day 1; 4–6 independent trials of 50 animals per trial. ****p<0.0001 or ***p<0.001 in Cochran–Mantel–Haenszel test. Note that under no sperm or non-functional sperm production, oocytes still transit through the spermatheca and enter the uterus (as shown by the DIC picture); unfertilized oocytes can be laid if the egg-laying apparatus is intact. (I) Summary: Genetic interventions that block major steps of germ cell development strongly block ALMR exophergenesis in the adult hermaphrodite. The dual requirement for sperm and oocytes suggests that fertilization and embryogenesis are required events for inducing ALMR exophergenesis.
-
Figure 1—source data 1
Daily progeny count for panel B, and exopher score for panels B, C, E, F, G, H.
- https://cdn.elifesciences.org/articles/95443/elife-95443-fig1-data1-v1.docx
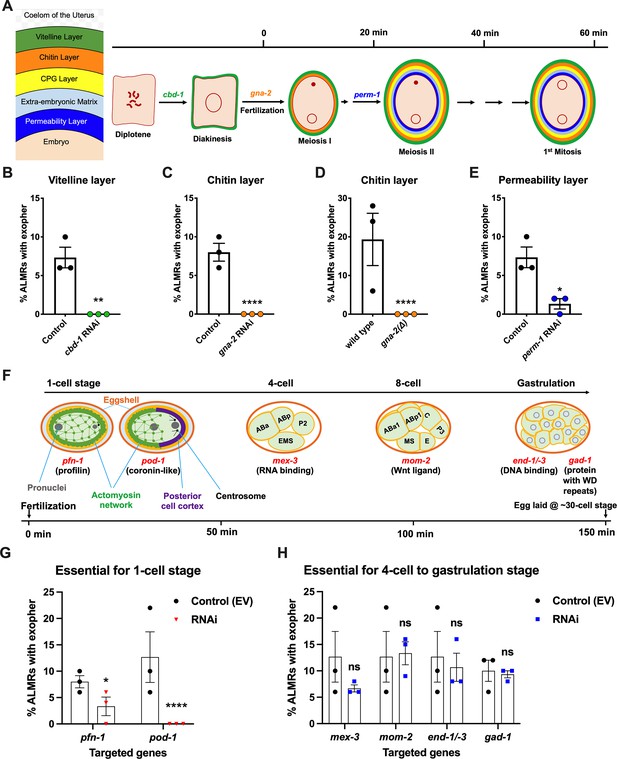
Events required for adult day 2 (Ad2) elevation of exopher production occur during the earliest stages consequent to fertilization and are largely completed by the 4-cell stage.
(A) Diagram of eggshell layers, post-fertilization timeline for layer formation, and indication of steps at which RNAi disrupts eggshell biogenesis. The formation of the multilayered eggshell is accomplished via a hierarchical assembly process from outside to inside, with outer layers required for later formation of inner layers. The outmost vitelline layer assembles in part prior to fertilization, dependent on chitin-binding domain protein (CBD-1) González et al., 2018. The next eggshell layer is made up of chitin, which confers eggshell stiffness and requires the gna-2-encoded enzyme glucosamine-6-phosphate N-acetyltransferase (GNPNAT1) for precursor biosynthesis (Johnston et al., 2006). The innermost lipid layer of eggshell is called the permeability layer, which is lipid-rich and is needed to maintain osmotic integrity of the embryo. PERM-1 (Olson et al., 2012) (among others, including FASN-1 [Rappleye et al., 2003], POD-1 [Rappleye et al., 1999], and EMB-8 [Benenati et al., 2009]) is required for permeability barrier formation (Stein and Golden, 2018; Johnston and Dennis, 2012). Note that eggshell biogenesis is critical for polyspermy barrier, spermathecal exit, meiotic chromosome segregation, polar body extrusion, AP polarization and internalization of membrane and cytoplasmic proteins, and correct first cell divisions (Johnston and Dennis, 2012), so genetic separation of eggshell malformation from the earliest embryonic formation is not possible. (B) cbd-1, a gene encoding an essential component of the eggshell vitelline layer, is critical for Ad2 exopher elevation. Exopher scores in Ad2 animals (strain ZB4757: bzIs166[Pmec-4::mCherry] II) that were treated with RNAi against cbd-1, total of three trials (50 hermaphrodites per trial), **p<0.01 in Cochran–Mantel–Haenszel test, as compared to the empty vector (EV) control. (C) gna-2, a gene required for chitin precursor biosynthesis and chitin layer formation, is critical for Ad2 exopher elevation. (C) Exopher scores in Ad2 animals treated from the L1 stage with RNAi against gna-2. The gna-2 gene encodes enzyme glucosamine-6-phosphate N-acetyltransferase (GNPNAT1) required for chitin precursor biosynthesis. (D) Exopher scores in Ad2 animals harboring a null mutation in the essential gna-2 gene. gna(∆) homozygous null worms are GFP negative progeny of stain ZB4941: bzIs166[Pmec-4::mCherry]; gna-2(gk308) I/hT2 [bli-4(e937) let-?(q782)] qIs48[Pmyo-2::GFP; Ppes-10::GFP; Pges-1::GFP] (I;III). Data represent a total of three trials (50 hermaphrodites per trial), **** p<0.0001 in Cochran–Mantel–Haenszel test, as compared to wild-type animals. (E) perm-1, a gene required for permeability barrier synthesis, is critical for Ad2 exopher elevation. Exopher scores in Ad2 animals treated with RNAi against perm-1, which encodes a sugar modification enzyme that acts in the synthesis of CDP-ascarylose. Data represent a total of 3 trials (50 hermaphrodites per trial), *p<0.05 in Cochran–Mantel–Haenszel test, as compared to the EV control. Note that previously characterized strong exopher suppressors pod-1, emb-8, and fasn-1 (Melentijevic et al., 2017; Cooper et al., 2021) are also needed for egg shell permeability barrier layer formation (Rappleye et al., 1999; Benenati et al., 2009). (F) Diagram of select genes required for specific stages of embryonic development. pfn-1 RNAi (arrest at the one-cell stage Schonegg et al., 2014; pod-1 RNAi (arrest at the two-cell stage Luke-Glaser et al., 2005; arrest stage phenotype for other genes is not precisely documented, but these genes play significant roles at the indicated stages; images annotated according to WormAtlas (https://doi.org/10.390/wormatlas.4.1). (G) RNAi targeting of early acting embryonic development genes lowers exopher production. Exopher scores from Ad2 animals that were treated with RNAi against genes characterized to be essential for 1-cell to 2-cell stage embryonic development. Total of three trials (50 hermaphrodites per trial). *p<0.05 or ****p<0.0001 in Cochran–Mantel–Haenszel test, as compared to the empty vector control. Strong exopher suppressor pod-1 has been previously reported (Melentijevic et al., 2017). We found RNAi directed against gene emb-8 (as early as 2 cell arrest, but arrest at the 1- to 50 cell stage reported [Schierenberg et al., 1980]) to be more variable in outcome (not shown). (H) RNAi targeting of genes that disrupt 4 cell stage and later embryonic development does not alter exopher production levels. Exopher scores in Ad2 animals that were treated with RNAi against genes that are essential for 4 cell to gastrulation stages of embryonic development. Total of three trials (50 hermaphrodites per trial). ns, not significant in Cochran–Mantel–Haenszel test, as compared to the empty vector control.
-
Figure 2—source data 1
Exopher score for panels B, C, D, E, G, H.
- https://cdn.elifesciences.org/articles/95443/elife-95443-fig2-data1-v1.docx
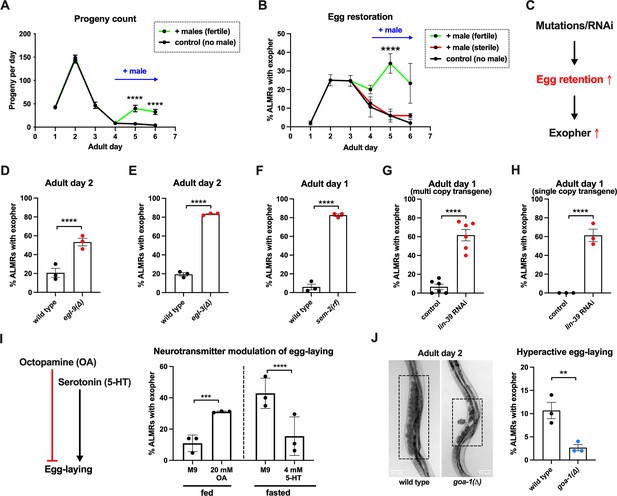
Anterior Lateral Microtubule cell (ALMR) exophergenesis levels are markedly influenced by the number of fertilized eggs retained in the uterus.
(A) Later life mating extends the C. elegans reproductive period. Progeny count for each wild-type hermaphrodite in the presence (green) or absence (black) of males (1 hermaphrodite +/-5 males) from Ad1 to Ad6. Males are present from Ad4 to Ad6. Total of 12 wild-type hermaphrodites scored for each condition. Data shown are mean ± SEM. ****p<0.0001 in two-way ANOVA with Šídák’s multiple comparisons test. (B) Introducing fertilized eggs in late adulthood extends the period of elevated exophergenesis. Males carrying functional (green) or nonfunctional (red) sperm (spe-45(tm3715)) were added to plates housing hermaphrodites as endogenous stores of sperm are depleted at Ad3. Data shown are mean ± SEM of percentage hermaphrodite ALMR neurons exhibiting an exopher event on days indicated. Total of three trials, and 50 hermaphrodites for each treatment at a single time point. ****p<0.0001 in Cochran–Mantel–Haenszel test, as compared to the sterile male group or no male control. (C) Hypothesis: Genetic interventions (mutations/RNAi) that increase egg retention increase ALMR exophergenesis. (D) Genetic interventions that induce egg retention elevate exopher levels. egl-9(sa307). ALMR exopher scores Ad2. Strain ZB4757: bzIs166[Pmec-4::mCherry] II vs. strain ZB4772: bzIs166[Pmec-4::mCherry] II; egl-9(sa307) V. Total of three trials (50 worms per trial, each trial one dot); **** p<0.0001 in Cochran–Mantel–Haenszel test, as compared to the wild-type control. (E) Genetic interventions that induce egg retention elevate exopher levels. egl-3(gk238). ALMR exopher scores Ad1. Strain ZB4757: bzIs166[Pmec-4::mCherry] II vs. strain ZB4904: bzIs166[Pmec-4::mCherry] II; egl-3(gk238) V. Total of three trials (50 worms per trial, each trial one dot); ****p<0.0001 in Cochran–Mantel–Haenszel test, as compared to the wild-type control. (F) Genetic interventions that induce egg retention elevate exopher levels. sem-2(n1343). ALMR exopher scores Ad1. Strain ZB4757: bzIs166[Pmec-4::mCherry] II vs. strain ZB4902: sem-2(n1343) I; bzIs166[Pmec-4::::mCherry] II. Exophers were scored on Ad1 because of the damaging excessive bagging that ensues in this background. Total of three trials (50 worms per trial, each trial one dot); ****p<0.0001 in Cochran–Mantel–Haenszel test, as compared to the wild-type control. (G) Genetic interventions that induce egg retention elevate exopher levels. lin-39 RNAi on a strain expressing Pmec-4::mCherry from a multi-copy transgene. ALMR exopher scores Ad2. Strain ZB4757: bzIs166[Pmec-4::mCherry] II treated with RNAi against lin-39. Total of six trials (50 worms per trial, each trial one dot); ****p<0.0001 in Cochran–Mantel–Haenszel test, as compared to the empty vector control. (H) Genetic interventions that induce egg retention elevate exopher levels. lin-39 RNAi on a strain expressing Pmec-18::mKate from a single copy transgene. ALMR exopher scores Ad2. Strain OD2984: ltSi953 [Pmec-18::vhhGFP4::zif-1::operon-linker::mKate::tbb-2 ’'UTR +Cbr-unc-119(+)] II; unc-119(et3) III treated with RNAi against lin-39. Total of three trials (50 worms per trial, each trial one dot); ****p<0.0001 in Cochran–Mantel–Haenszel test, as compared to the empty vector control. (I) Egg-laying modulating neurotransmitters octopamine (OA) and serotonin (5-HT) influence ALMR exophergenesis levels. Data show the mean ± SEM of percentage hermaphrodite ALMR neurons exhibiting an exopher event at Ad2 after 48 hr of treatment with 20 mM octopamine (OA) or 4 mM serotonin (5-HT) on OP50 bacteria seeded NGM plates. Because 5-HT (which increases egg laying) was hypothesized to suppress exopher production, we assayed under conditions of 6 hr food limitation, which markedly raises the exopher production baseline, enabling easier quantification of suppression effects (Cooper et al., 2021). Total of three trials and 50 hermaphrodites per trial for each condition. ***p<0.001 or ****p<0.0001 in Cochran–Mantel–Haenszel test, as compared to the control group treated with M9 buffer (solvent for OA or 5-HT). (J) Mutant goa-1(n1134), with hyperactive egg-laying and low egg retention in the uterus (pictures on the left, representative of 20, and scale bar = 50 μm), has low exopher scores at Ad2. Strain ZB4757: bzIs166[Pmec-4::mCherry] II vs. strain ZB5352: goa-1(n1134) I; bzIs166[Pmec-4::mCherry] II. Boxes highlight egg zone. **p<0.01 in Cochran–Mantel–Haenszel test.
-
Figure 3—source data 1
Daily progeny count for panel A, and exopher score for panels B, D, E, F, G, H, I, J.
- https://cdn.elifesciences.org/articles/95443/elife-95443-fig3-data1-v1.docx
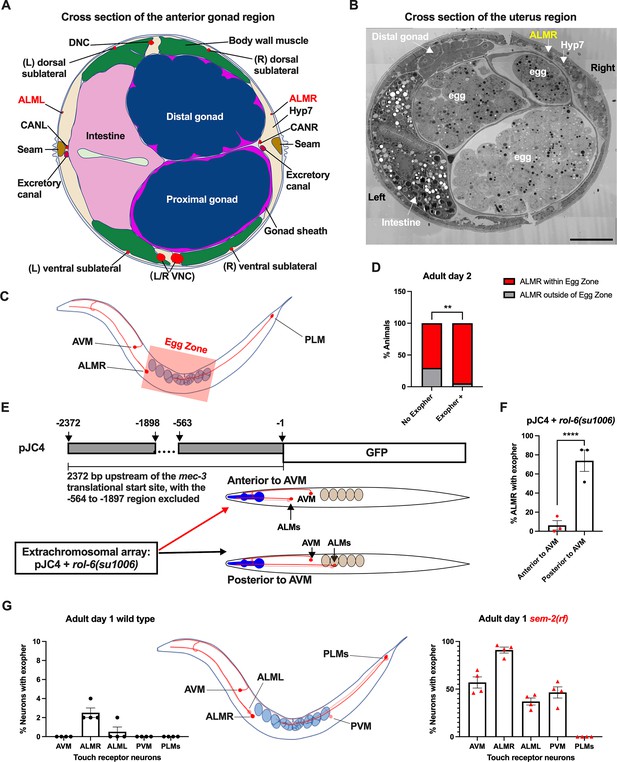
Anterior Lateral Microtubule cell (ALMR) exophergenesis levels correlate with proximity to the egg zone.
(A) ALMR is positioned close to the uterus and Anterior Lateral Microtubule cell (ALML) is situated on the opposite side, close to the intestine. A diagram of ALMR and ALML positions relative to major body organs in cross-section that is anterior to the ‘egg zone.’ Image drawing based on the EM pictures of adult hermaphrodite slice #273 of WormAtlas. DNC: dorsal nerve cord; VNC: ventral nerve cord; Hyp7: hypodermal cell 7; CAN(L/R): canal-associated neurons (left/right); neurons in red. (B) Electron microscopy cross-section image of the uterus region indicating ALMR soma and eggs within the adult uterus. Note ALMR is close to the egg-filled uterus, ALML is on the opposite side closer to the intestine. The ALML soma is not evident in this cross section. Scale bar = 10 μm. (C) Illustration of the egg zone definition, distance between outermost eggs. The measure of this distance corresponds to uterine length. (D) ALMRs positioned close to the filled gonad produce exophers more frequently than ALMRs that are positioned a bit more distally. We selected Ad2 mCherry animals at random, then identified whether or not ALMR had produced an exopher, and subsequently determined whether ALMR was positioned within the egg zone or outside the egg zone as indicated (neuronal soma positioning differences are a consequence of developmental variation). Neurons with somas positioned further from the egg area produced fewer exophers than neurons within the egg zone indicated; total of 91 worms for the ‘No Exopher’ group and 37 for the ‘Exopher +’ group; **p<0.01 in Chi-Square test. (E) The Aamodt group (Toms et al., 2001) previously reported that high copy numbers of plasmid pJC4 containing the mec-3 promoter region (−1 to –563, and –1898 to –2372 of the mec-3 translational start) exhibited increased abnormal positioning of ALM neurons anterior to AVM. We introduced plasmid pJC4 along with transformation reporter pRF4 rol-6(su1006) in the background of mCherryAg2 (note this revealed that rol-6(su1006) is a strong exopher enhancer) and identified neurons that were positioned posterior to AVM (normal, close to the uterus) and those that were positioned anterior to AVM (further away from the uterus). (F) ALMR neurons genetically induced to adopt positions further away from the uterus generate fewer exophers then those close to the uterus. We counted numbers of exophers produced in Ad2 Rol hermaphrodites for each position type. Strain ZB5046: Ex [(pJC4) Pmec-3::GFP +pRF4]; bzIs166[Pmec-4::mCherry] II. Total of three trials (61(34a + 27p); 39(19a + 20p); 51(20a + 31p) animals per trial); ****p<0.0001 in Cochran–Mantel–Haenszel test. (G) When eggs cannot be laid in the sem-2(rf) mutant, the eggs that accumulate in the body are brought in closer proximity to ALML, AVM, and PVM touch neurons with a resulting increase in their exopher production. Strain ZB4757: bzIs166[Pmec-4::mCherry] II vs. strain ZB4902: sem-2(n1343) I; bzIs166[Pmec-4::mCherry] II. Left, Exopher scoring (Mean ± SEM) of all six touch receptor neurons in Ad1 wild-type hermaphrodite; Right, Exopher scoring (Mean ± SEM) of all six touch receptor neurons in Ad1 sem-2(rf) hermaphrodite. Total of 4 trials (50 worms per trial) for each. Wild-type, egg laying proficieint animals on Ad1 exhibit low exopher levels, but when eggs accumulate early in the sem-2 mutant, exophers markedly increase in ALMR and other touch neurons that are in the vicinity of an expanded uterus. PLM neurons are situated posterior to the anus and are not subject to uterine squeezing effects.
-
Figure 4—source data 1
Contingency data for panel D, and exopher score for panels F, G.
- https://cdn.elifesciences.org/articles/95443/elife-95443-fig4-data1-v1.docx
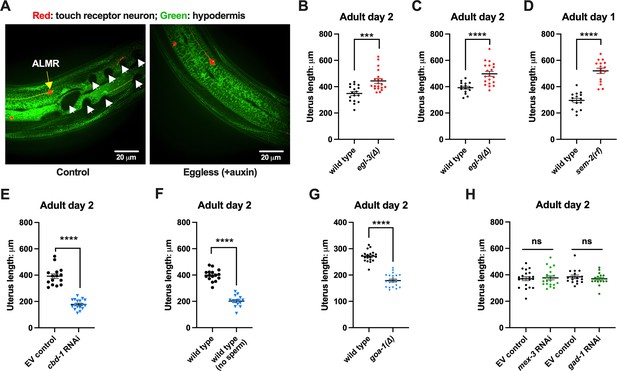
Uterine length measures for egg-laying defective and gastrulation defective mutants support the correlation of high exopher production and uterine expansion.
(A) Eggs can distort tissues in their vicinity. Shown (representative of 10, scale bar = 20 μm) is strain ZB4942: fxIs1[Ppie-1::TIR1::mRuby] I; bzIs166[Pmec-4::mCherry] II; spe-44(fx110[spe-44::degron]) IV; pwSi93[Phyp7::oxGFP::lgg-1], with touch neurons expressing mCherry (red); and the hypodermis expressing GFP. Dark round areas (white arrows) are eggs that press into the hypodermis when viewed in this focal plane. On the right, slit-like dark regions correspond to hypodermal seam cells. (B) Uterus length of WT vs. egl-3(∆); strain ZB4757: bzIs166[Pmec-4::mCherry] II vs. ZB4904: bzIs166[Pmec-4::mCherry] II; egl-3(gk238) V. n = ~20 hermaphrodites from one trial. ***p<0.001 in two-tailed t-test. Note that we did not normalize uterine length to body length in B-E. (C) Uterus length of wild-type (WT) vs. egl-9(∆) ZB4757: bzIs166[Pmec-4::mCherry] II vs. ZB4772: bzIs166[Pmec-4::mCherry] II; egl-9(sa307) V. n = ~20 hermaphrodites from one trial. ****p<0.0001 in two-tailed t-test. (D) Uterus length of WT vs. sem-2(rf); strain ZB4757: bzIs166[Pmec-4::mCherry] II vs. ZB4902: sem-2(n1343) I; bzIs166[Pmec-4::mCherry] II. n = ~20 hermaphrodites from one trial. ****p<0.0001 in two-tailed t-test. (E) Uterine length is short under cbd-1 RNAi compared to WT + empty vector RNAi. Uterus length of strain ZB4757: bzIs166[Pmec-4::mCherry] treated with RNAi against cbd-1 or control empty vector feeding RNAi. n = ~20 hermaphrodites from one trial. ****p<0.0001 in two-tailed t-test. (F) When sperm maturation is blocked in egg-laying competent animals, leaving oocytes to occupy reproductive structures, the uterus length is short. Uterus length of strain ZB4749: fxIs1[Ppie-1::TIR1::mRuby] zdIs5[Pmec-4::GFP] I; bzIs166[Pmec-4::mCherry] II; spe-44(fx110[spe-44::degron]) IV. 1 mM auxin treatment induces the no sperm status. n = ~20 hermaphrodites from one trial. ****p<0.0001 in two-tailed t-test. (G) Uterine length is short in the hyperactive egg-laying mutant which has low occupancy of eggs in the uterus. Uterus length of strain N2: wild-type vs. strain MT2426: goa-1(n1134) I. n=20 hermaphrodites from one trial. ****p<0.0001 in two-tailed t-test. (H) Knocking down the 4 cell stage gene mex-3 or the gastrulation gene gad-1 has normal uterine length. Uterus length of strain ZB4757: bzIs166[Pmec-4::mCherry] treated with RNAi against the mex-3 or gad-1 gene. mex-3 RNAi disrupts embryonic deveopment at the 4 cell stage, while gad-1 RNAi disrupts gastrulation at the stage at which eggs are normally laid and perturbs later development but not egg shell formation and egg laying. n = ~20 hermaphrodites from one trial. Not significant (ns) in two-tailed t-test as compared to the empty vector control.
-
Figure 5—source data 1
Uterus length data for panels B, C, D, E, F, G, H.
- https://cdn.elifesciences.org/articles/95443/elife-95443-fig5-data1-v1.docx
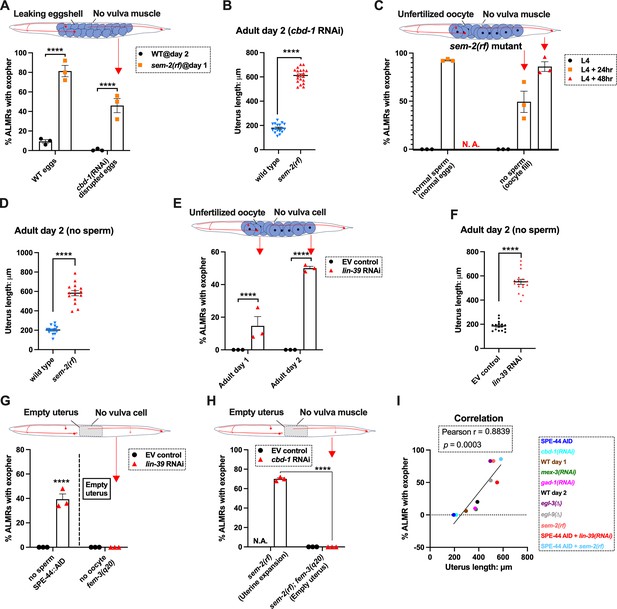
Uterine expansion correleates strongly with Anterior Lateral Microtubule cell (ALMR) exophergenesis regardless of whether eggs, oocytes, or debris are retained.
(A) Despite cbd-1 RNAi mediated disruption of eggshell formation and earliest embryonic cell divisions, exopher levels are high in the sem-2(rf) egg retention background. The percentage of ALMR exopher events among 50 Ad2 wild-type (left) or Ad1 sem-2(rf) hermaphrodite C. elegans that are treated with either empty vector control RNAi or RNAi targeting cbd-1 in each trial (total of 3 independent trials). sem-2 mutants bag extensively at Ad2 and cannot be tested. Diagram indicates uterine filling status of test sem-2(rf) + cbd-1 RNAi. Strain ZB4757: bzIs166[Pmec-4::mCherry] II vs. strain ZB4902: sem-2(n1343) I; bzIs166[Pmec-4::mCherry] II. ****p<0.0001 in Cochran–Mantel–Haenszel test. (B) Uterine length remains long in the egg-laying defective sem-2(rf) + cbd-1 RNAi. Uterus length of strain ZB4757: bzIs166[Pmec-4::mCherry] II vs. ZB4902: sem-2(n1343) I; bzIs166[Pmec-4::mCherry] II treated with RNAi against cbd-1. n = ~20 hermaphrodites from one trial, Ad2, ****p<0.0001 in two-tail unpaired t-test. (C) Blocking sperm maturation in a sem-2(rf) mutant, which fills the uterine space with oocytes, induces exophers in the absence of eggs. The percentage of ALMR exopher events among 50 sem-2(rf) hermaphrodite C. elegans that express the SPE-44 AID system (‘control’ is treated with 0.25% ethanol vehicle and ‘no sperm’ is treated with 1 mM auxin in 0.25% ethanol from egg to adult day 2) in each trial (total of 3 independent trials). L4 stage is the last larval stage before adult. Diagram indicates uterine oocyte filling status of test sem-2(rf) + SPE-44 AID. Strain ZB4953: sem-2(n1343) fxIs1[Ppie-1::TIR1::mRuby] I; bzIs166[Pmec-4::mCherry] II; spe-44(fx110[spe-44::degron]) IV. (D) When sperm maturation is disrupted in mutants blocked for egg laying, leaving oocytes to occupy reproductive structures, the uterus expands as oocytes accumulate. Uterus length of strain ZB4749: fxIs1[Ppie-1::TIR1::mRuby] zdIs5[Pmec-4::GFP] I; bzIs166[Pmec-4::mCherry] II; spe-44(fx110[spe-44::degron]) IV vs. ZB4953: sem-2(n1343) fxIs1[Ppie-1::TIR1::mRuby] I; bzIs166[Pmec-4::mCherry] II; spe-44(fx110[spe-44::degron]) IV. 1 mM auxin treatment induces the no sperm status to both strains. n = ~20 hermaphrodites from one trial, Ad2. ****p<0.0001 in two-tail unpaired t-test. (E) Disrupting sperm maturation in lin-39 RNAi animals blocked for egg-laying fills the uterine space with oocytes, and induces exophers in the absence of eggs. The percentage of ALMR exopher events among 50 adult day 1 or 2 SPE-44 AID no sperm hermaphrodite C. elegans treated with either control RNAi or lin-39 RNAi in each trial (total of 3 independent trials). Diagram indicates uterine oocyte filling status of test lin-39 RNAi + SPE-44 AID. ZB4749: fxIs1[Ppie-1::TIR1::mRuby] zdIs5[Pmec-4::GFP] I; bzIs166[Pmec-4::mCherry] II; spe-44(fx110[spe-44::degron]) IV. ****p<0.0001 in Cochran–Mantel–Haenszel test. (F) When sperm maturation is blocked, leaving oocytes to occupy reproductive structures, the uterus length is short; but if oocytes cannot be laid in the lin-39 RNAi background, the uterus expands as oocytes accumulate. Uterus length of strain ZB4749: fxIs1[Ppie-1::TIR1::mRuby] zdIs5[Pmec-4::GFP] I; bzIs166[Pmec-4::mCherry] II; spe-44(fx110[spe-44::degron]) IV +1 mM auxin treatment to eliminate sperm maturation. n = ~20 hermaphrodites. ****p<0.0001 in two-tail unpaired t-test. (G) Blocking oocyte production in the background of lin-39 RNAi-mediated disruption of the egg-laying apparatus eliminates early adult exophergenesis. We used SPE-44 AID to block sperm maturation and fem-3(q20) to prevent oocyte production; lin-39 RNAi to disrupt egg-laying capacity. Diagram indicates empty uterus status of test fem-3(q20); spe-44 AID; lin-39(RNAi) strain. Exopher scoring of Ad2 ZB4749: fxIs1[Ppie-1::TIR1::mRuby] zdIs5[Pmec-4::GFP] I; bzIs166[Pmec-4::mCherry] II; spe-44(fx110[spe-44::degron]) IV +1 mM auxin or ZB5042: bzIs166[Pmec-4::mCherry] II; fem-3(q20)ts IV, treated with either control empty vector (EV) RNAi or lin-39 RNAi at 25 °C. Total of three trials (50 worms per trial). ****p<0.0001 in Cochran–Mantel–Haenszel test. (H) Blocking oocyte production in the background of sem-2(rf)-mediated disruption of the egg-laying apparatus eliminates early adult exophergenesis. We used cbd-1 RNAi to disrupt eggshell and fem-3(q20) to prevent oocyte production; sem-2(n1343) to disrupt egg-laying capacity. Exopher scoring of adult day 2 hermaphrodites, treated with either control empty vector (EV) RNAi or cbd-1 RNAi at 25 °C. Total of three trials (50 worms per trial). Diagram indicates empty uterus status of test fem-3(q20); cbd-1 RNAi; sem-2(rf) strain. (I) The uterus length is correlated with ALMR exophergenesis. Data shown are the mean of uterus length (X-aixs) and percentage ALMRs with exopher (Y-axis) for different genotypes/treatments measured in this study. The correlation line is based on a linear fit model and the Pearson r and p value is based on the correlation assay. Uterus length from short to long: SPE-44 AID; cbd-1 RNAi; wild-type (adult day 1); mex-3 RNAi; gad-1 RNAi; wild-type (adult day 2); egl-3(Δ); egl-9(Δ); sem-2(rf); SPE-44 AID + lin-39 RNAi; SPE-44 AID + sem-2(rf).
-
Figure 6—source data 1
Exopher score for panels A, C, E, G, H, I, and uterus length data for panels B, D, F, I.
- https://cdn.elifesciences.org/articles/95443/elife-95443-fig6-data1-v1.docx
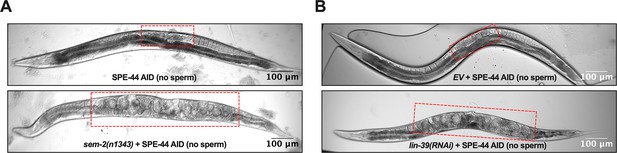
Representative pictures of oocytes retention (red rectangle) in the uterus of Adult day 2 hermaphrodite.
(A) Strain ZB4749 (top): fxIs1[Ppie-1::TIR1::mRuby] zdIs5[Pmec-4::GFP] I; bzIs166[Pmec-4::mCherry] II; spe-44(fx110[spe-44::degron]) IV vs. ZB4953 (bottom): sem-2(n1343) fxIs1[Ppie-1::TIR1::mRuby] I; bzIs166[Pmec-4::mCherry] II; spe-44(fx110[spe-44::degron]) IV. 1mM auxin treatment induces no sperm status to both strains. (B) Strain ZB4749: fxIs1[Ppie-1::TIR1::mRuby] zdIs5[Pmec-4::GFP] I; bzIs166[Pmec-4::mCherry] II; spe-44(fx110[spe-44::degron]) IV treated with either control empty vector (EV) (top) or lin-39 RNAi (bottom). 1mM auxin treatment induces no sperm status. Representative of 10, and scale bar = 100 μm.
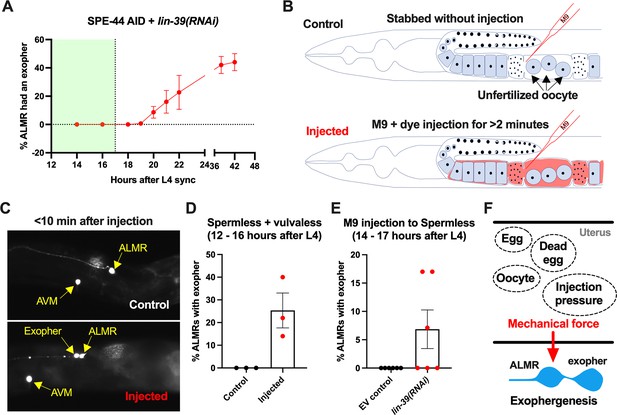
Anterior lateral microtubule cell (ALMR) exophergenesis can be induced by uterine compartment distortion that accompanies fluid injection.
(A) Summary of timing of ALMR exophergenesis from age synchronized spermless + vulvaless hermaphrodite. Total of three trials and 50 hermaphrodites in each trial. Strain ZB4749: fxIs1[Ppie-1::TIR1::mRuby] zdIs5[Pmec-4::GFP] I; bzIs166[Pmec-4::mCherry] II; spe-44(fx110[spe-44::degron]) IV, cultured on the 1 mM auxin treated nematode growth media (NGM) agar plates seeded with HT115 E. coli expressing dsRNA against the C. elegans lin-39 gene. Eventually, oocytes accumulate in this strain, but at worst very few are evident in the timeframe in which we performed injections. Data shown are mean ± SEM at each time point. The data demonstrate that there is no ALMR exophergenesis in this background before the 18th hr after L4 sync. Testing the impact of injection on ALMR exophergenesis before the 17th hr after L4 thus monitors injection consequences during a timeframe in which no exophers are normally produced. (B) Illustrated experimental design for testing the ALMR exophergenesis response to physically expanding the gonad via 2 min continuous fluid injection. We performed 2+ min duration injections of M9 buffer mixed with food color dye 1:10 or 1.5:10 dye/M9 ratio (to verify successful injection; dye contains water, propylene glycol, FD&C reds 40 and 3, propylparaben) into the uteri of sperm-less only (EV control) or sperm-less + vulvaless (treated with lin-39 RNAi) animals. Strain: ZB4749 as in panel A, treated with either empty vector (EV) or RNAi against lin-39 in the presence of 1 mM auxin. lin-39 RNAi disrupts vulval development such that injected fluids are retained to expand the uterus. In injections with animals that have functioning egg-laying apparatus, fluids can exit the animal and do not expand the uterus. (C) 2 min sustained injection into egg-laying blocked, reproduction blocked animals can induce rapid exophergenesis: representative picture of an ALMR exophergenesis event consequent to injection. (D) 2 min sustained injection into egg-laying blocked, reproduction blocked animals can induce rapid exophergenesis: exopher scoring of the control mock injected and 2 min injected animals. Strain ZB4749: fxIs1[Ppie-1::TIR1::mRuby] zdIs5[Pmec-4::GFP] I; bzIs166[Pmec-4::mCherry] II; spe-44(fx110[spe-44::degron]) IV treated with auxin and lin-39 RNAi to induce the sperm-less and vulva-less status, respectively. Data represent a total of three trials (6–10 worms in each trial). p<0.05 in Cochran–Mantel–Haenszel test, as compared to the no-injection fluid control. (E) 2 min sustained injection induces rapid ALMR exophergenesis only from vulvaless animals. Data showing the exopher scoring of the sperm-less EV control (with functional vulva) or sperm-less +vulvaless (treated with lin-39 RNAi) worms. Strain: ZB4749 (genotype in panel A legend) treated with either EV or RNAi against lin-39 in the presence of 1 mM auxin to disrupt sperm maturation. Data represent a total of 6 trials (6–10 worms in each trial). 3 out of 6 trials showed ALMR exopher induction by M9 injection to the vulvaless worms; while not a single trial produced ALMR exopher induction by M9 injection to animals with WT vulvae. If the vulva is intact, injected fluids are observed to leak out, consistent with the assumption that the gonads of egg-laying proficient animals will not sustain required expansion. (F) Summary. Eggs, dead egg accumulation, oocyte accumulation, or injection pressure all lead to ALMR exophergenesis. These varied interventions have a similar impact on the uterus, which is the uterine distortion by mechanical forces. We propose that early adult ALMR exophergenesis requires mechanical or stretch-associated force generated by the uterine cargos.
-
Figure 7—source data 1
Exopher score for panels A, D, E.
- https://cdn.elifesciences.org/articles/95443/elife-95443-fig7-data1-v1.docx
Tables
Strain list.
| Strain Name | Genotype | Index |
|---|---|---|
| N2 | wild-type | wild-type |
| ZB4065 | bzIs166[Pmec-4::mCherry] II | wild-type |
| ZB4757 | bzIs166[Pmec-4::mCherry] II (ZB4065 outcrossed to N2 six times) | wild-type |
| ZB4768 | glp-4(bn2)ts I; bzIs166[Pmec-4::mCherry] II | glp-4(ts) |
| ZB5042 | bzIs166[Pmec-4::mcherry] II; fem-3(q20) IV | fem-3(gf) |
| ZB4915 | bzIs166[Pmec-4::mCherry] II; fem-1(hc17) IV | fem-3(lf) |
| ZB4749 | fxIs1[Ppie-1::TIR1::mRuby] zdIs5[Pmec-4::GFP] I; bzIs166[Pmec-4::mCherry] II; spe-44(fx110[spe-44::degron]) IV. | SPE-44 |
| ZB4941 | bzIs166[Pmec-4::mCherry]; gna-2(gk308) I/hT2 [bli-4(e937) let-?(q782)] qIs48[Pmyo-2::GFP; Ppes-10::GFP; Pges-1::GFP] (I;III) | gna-2(∆) |
| AD295 | spe-45(tm3715); him-5(e1490); asEx89 [spe-45 ‘fosmid 1’ mixture +Pmyo::gfp] | spe-45(tm3715) |
| ZB4772 | bzIs166[Pmec-4::mCherry] II; egl-9(sa307) V | egl-9(∆) |
| ZB4904 | bzIs166[Pmec-4::mCherry] II; egl-3(gk238) V | egl-3(∆) |
| ZB4902 | sem-2(n1343) I; bzIs166[Pmec-4::::mCherry] II | sem-2(rf) |
| ZB5352 | goa-1(n1134) I; bzIs166[Pmec-4::mCherry] II | goa-1(∆) |
| ZB5046 | Ex [(pJC4) Pmec-3::gfp +pRF4]; bzIs166[Pmec-4::mCherry] II | pJC4 +rol-6(su1006) |
| ZB4942 | fxIs1[Ppie-1::TIR1::mRuby] I; bzIs166[Pmec-4::mCherry] II; spe-44(fx110[spe-44::degron]) IV; pwSi93[Phyp7::oxGFP::lgg-1] | Figure 5A |
| ZB4953 | sem-2(n1343) fxIs1[Ppie-1::TIR1::mRuby] I; bzIs166[Pmec-4::mCherry] II; spe-44(fx110[spe-44::degron]) IV | sem-2(rf) |
| ZB5709 | sem-2(n1343) I; bzIs166[Pmec-4::mCherry] II; fem-3(q20) IV. | sem-2(rf); fem-3(q20) |
| OD2984 | ltSi953 [Pmec-18::vhhGFP4::zif-1::operon-linker::mKate::tbb-2 3'UTR +Cbr-unc-119(+)] II; unc-119(ed3) III | Single-copy transgene |





