Decoupling of the onset of anharmonicity between a protein and its surface water around 200 K
Figures
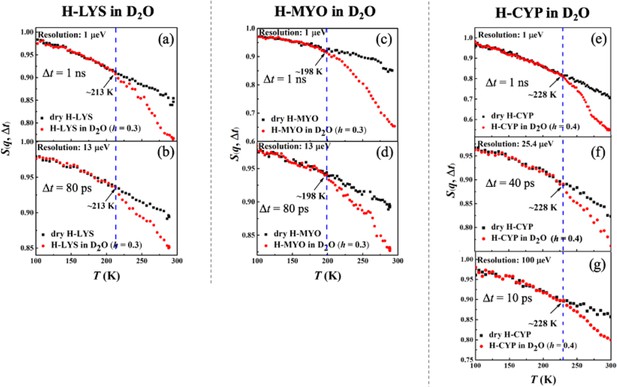
Resolution dependence of the onset of protein dynamical transition.
Neutron spectrometers with different resolutions (1, 13, 25.4, and 100 μeV) were applied. Elatic intensity S(q, Δt) of (a, b) dry H-LYS and H-LYS in D2O at h = 0.3, (c, d) dry H-MYO and H-MYO in D2O at h = 0.3, and (e–g) dry H-CYP and H-CYP in D2O at h = 0.4. All the experimental S(q, Δt) are normalized to data measured at ~10 K and summed over values of q ranging from 0.45 to 1.75 Å−1. The dashed lines in each figure identify the onset temperatures of the transition, Ton, where the neutron data of the hydrated system deviate from the dry form.
Structures of proteins studied in this work.
Structures of (a) lysozyme (LYS), (b) myoglobin (MYO), (c) cytochrome P450 (CYP), and (d) green fluorescent protein (GFP).
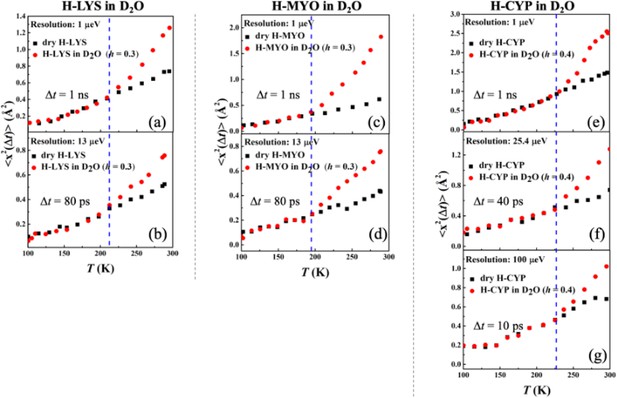
Resolution dependence of the onset of protein dynamical transition.
Mean-squared atomic displacements <x2(Δt)> derived from Figure 1 using Gaussian approximation, of (a, b) dry H-LYS and H-LYS in D2O at h = 0.3, (c, d) dry H-MYO and H-MYO in D2O at h = 0.3, and (e–g) dry H-CYP and H-CYP in D2O at h = 0.4.
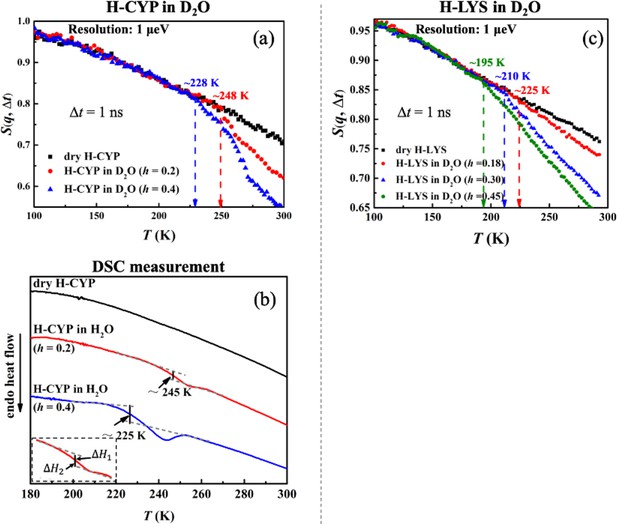
Hydration dependence of the onset of protein dynamical transition.
S(q, Δt) of (a) dry H-CYP and H-CYP in D2O at h = 0.2 and 0.4 and (c) dry H-LYS and H-LYS in D2O at h = 0.18, 0.30, and 0.45, all measured using HFBS with the instrumental resolution of 1 μeV. All the data in (c) were replotted from Roh et al., 2006. (b) Differential scanning calorimetry (DSC) curves obtained for dry H-CYP and H-CYP in water at h = 0.2 and 0.4. TDSC is defined as the midpoint between two heat flow baselines, where ΔH1 = ΔH2 (Bassi et al., 2003; Stolwijk et al., 2013; ASTM International, 2014).
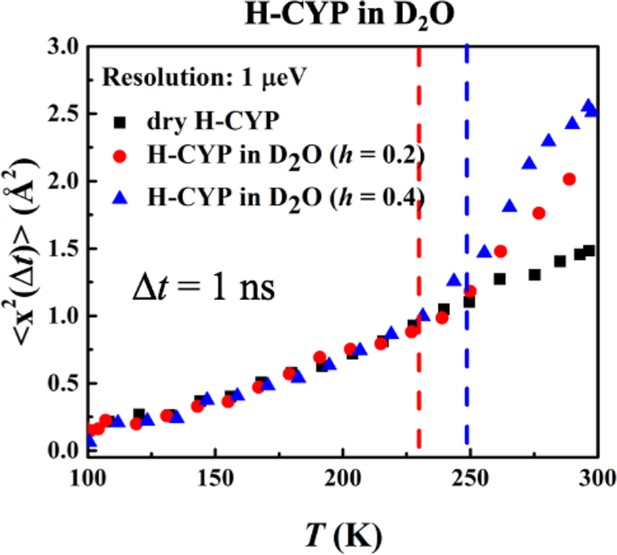
Resolution dependence of the onset of protein dynamical transition.
, derived from Figure 2c using Gaussian approximation, of dry H-CYP and H-CYP in D2O at h = 0.2 and 0.4.
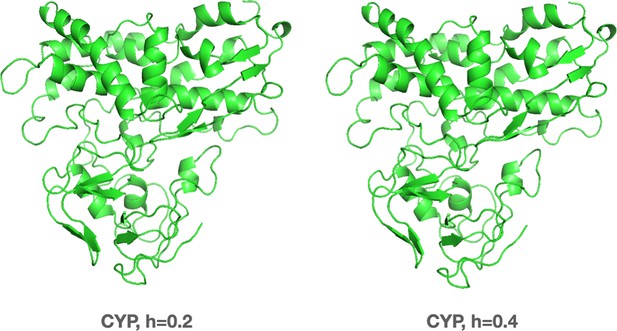
The three-dimensional (3D) structure of cytochrome P450 (CYP) protein at different hydration levels obtained from molecular dynamics (MD) simulations (PDB ID: 2ZAX).
The potential energy as a function of MD trajectory time of cytochrome P450 (CYP).
Resolution dependence of the anharmonic onset of hydration water.
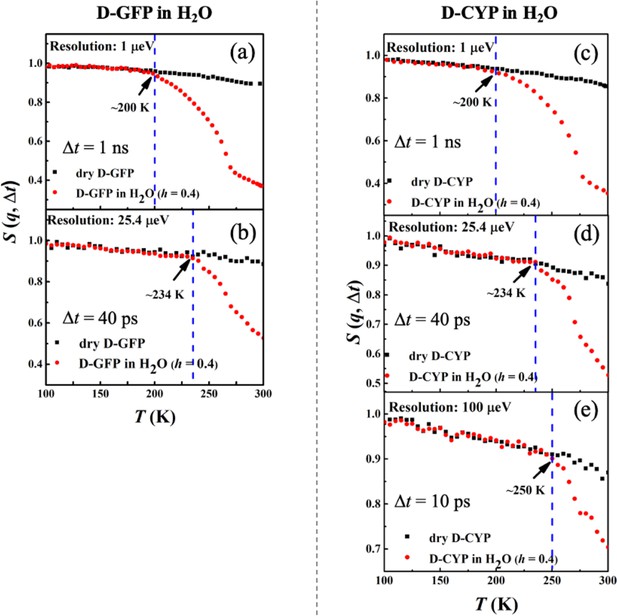
Neutron spectrometers with different resolutions (1, 25.4, and 100 μeV) were applied. S(q, Δt) of (a, b) dry D-GFP and D-GFP in H2O at h = 0.4, and (c–e) dry D-CYP and D-CYP in H2O at h = 0.4.
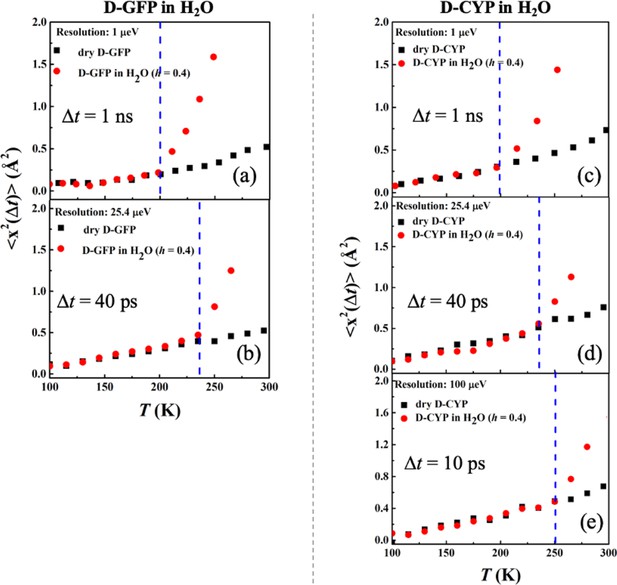
Resolution dependence of the anharmonic onset of hydration water.
Mean-squared atomic displacements <x2(Δt)>, derived from Figure 3 using Gaussian approximation, of (a, b) dry D-GFP and D-GFP in H2O at h = 0.4, (c–e) dry D-CYP and D-CYP in H2O at h = 0.4.
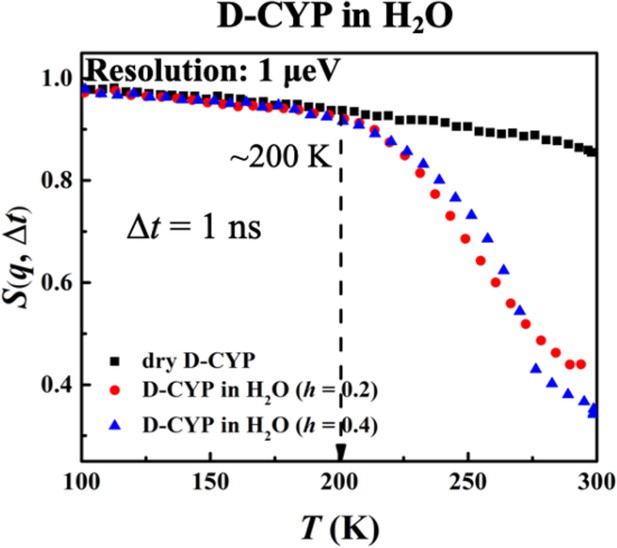
Hydration dependence of the anharmonic onset of hydration water.
S(q, Δt), for dry D-CYP and D-CYP in H2O at h = 0.2 and 0.4, measured using HFBS neutron instrument with an energy resolution of 1 μeV.
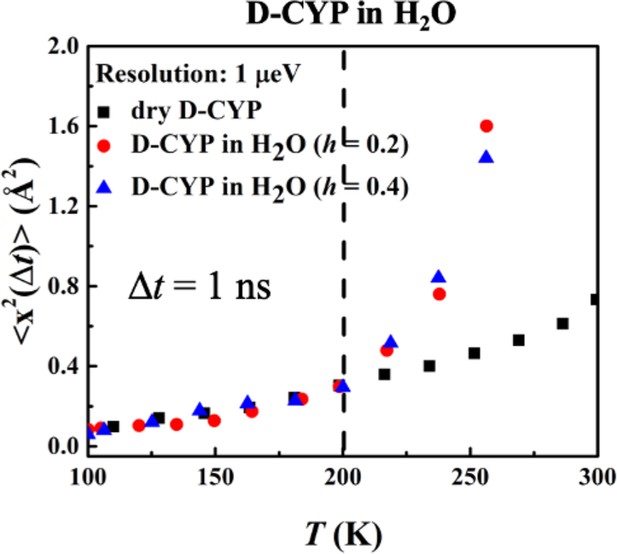
Hydration dependence of the anharmonic onset of hydration water.
<x2(Δt)>, derived from Figure 4 using Gaussian approximation, of dry D-CYP and D-CYP in H2O at h = 0.2 and 0.4.
Tables
Relative content of each secondary structure in the proteins.
The secondary structure content of cytochrome P450 (CYP) protein at different hydration levels.
| Alpha-helix | Beta-sheet | Loop and turn | |
|---|---|---|---|
| CYP (h = 0.2) | 52% | 11% | 37% |
| CYP (h = 0.4) | 52% | 11% | 37% |
Ton of protein in q-ranges from q = 0.45–0.9 Å−1.
| 1 ns | 80 ps | 40 ps | 10 ps | |
|---|---|---|---|---|
| LYS | 213 K | 213 K | - | - |
| MYO | 198 K | 198 K | - | - |
| CYP | 228 K | - | 228 K | 228 K |
Ton of protein in q-ranges from q = 1.1–1.75 Å−1.
| 1 ns | 80 ps | 40 ps | 10 ps | |
|---|---|---|---|---|
| LYS | 212 K | 213 K | - | - |
| MYO | 197 K | 199 K | - | - |
| CYP | 228 K | - | 227 K | 228 K |
Ton of protein at different time resolution.
| 1 ns | 80 ps | 40 ps | 10 ps | |
|---|---|---|---|---|
| LYS (h = 0.3) | 213 K | 213 K | - | - |
| MYO (h = 0.3) | 198 K | 198 K | - | - |
| CYP (h = 0.4) | 228 K | - | 228 K | 228 K |
Ton of protein at different hydration level.
| 0.18 | 0.2 | 0.3 | 0.4 | 0.45 | |
|---|---|---|---|---|---|
| LYS (1 ns) | 225 K | - | 213 K | - | 195 K |
| CYP (1 ns) | - | 248 K | - | 228 K | - |
| CYP (TDSC) | - | 245 K | - | 225 K | - |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Escherichia coli) | Escherichia coli, BL21(DE3) | Sigma-Aldrich | CMC0016 | |
| Peptide, Recombinant protein | Lysozyme, chicken egg white | Sigma-Aldrich | CAS: 12650-88-3 | |
| Peptide, Recombinant protein | Myoglobin, equine skeletal muscle | Sigma-Aldrich | CAS: 100684-32-0 | |
| Chemical compound, drug | H2O | Millipore | ||
| Chemical compound, drug | D2O | Sigma-Aldrich | CAS: 7789-20-0 |





