Human induced pluripotent stem cell-derived cardiomyocytes to study inflammation-induced aberrant calcium transient
Figures
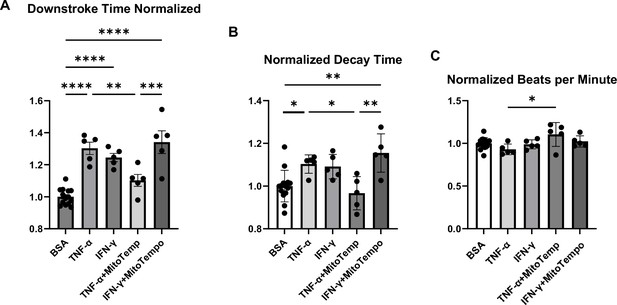
Analyses of calcium transient in hiPSC-CMs treated with cytokines and/or MitoTempo.
Normalized downstroke time (A), decay time (B), and beating rate as assessed by beats per minute (C) in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) treated with bovine serum albumin (BSA), (control), TNF-α, IFN-γ, TNF-α plus mito-Tempo, and IFN-γ plus mito-Tempo. N = 5–14. Data were analyzed by ordinary one-way ANOVA and post hoc Tukey’s multiple-comparison test. Bars represent group mean, and error bars indicate standard error of the mean.
-
Figure 1—source data 1
Analyses of calcium transient in hiPSC-CMs treated with cytokines and/or MitoTempo.
- https://cdn.elifesciences.org/articles/95867/elife-95867-fig1-data1-v1.xlsx
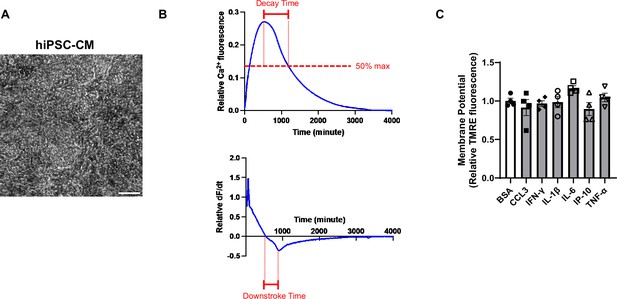
A representative calcium transient curve in hiPSC-CMs and their mitochondrial potential.
(A) Representative image of human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM). Scale bar represents 100 μm. (B) Representative curves of calcium transient and its derivative in hiPSC-CM. (C) Mitochondrial membrane potential (MMP) as assessed by tetramethylrhodamine ethyl ester (TMRE) in hiPSC-CM treated with various cytokines. Concentration of cytokines used is as follows: CCL3 1 nM, TNF-α 300 pg/ml, IL-6 1 ng/ml, IL-1β 200 pg/ml, IP-10 (or CXCL10) 500 pg/ml, IFN-γ 1 ng/ml. N = 4, Data were analyzed by ordinary one-way ANOVA and post hoc Tukey’s multiple-comparison test. Bars represent group mean , and error bars indicate standard error of the mean.
-
Figure 1—figure supplement 1—source data 1
A epresentative image of hiPSC-CMs, a calcium transient curve, and a relative dF/dt curve.
- https://cdn.elifesciences.org/articles/95867/elife-95867-fig1-figsupp1-data1-v1.xlsx
-
Figure 1—figure supplement 1—source data 2
Relative TMRE fluorescence in hiPSC-CMs with cytokine treatments.
- https://cdn.elifesciences.org/articles/95867/elife-95867-fig1-figsupp1-data2-v1.xlsx
Representative video of human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) contracting spontaneously in a culture dish.
Scale bar represents 100 μm.
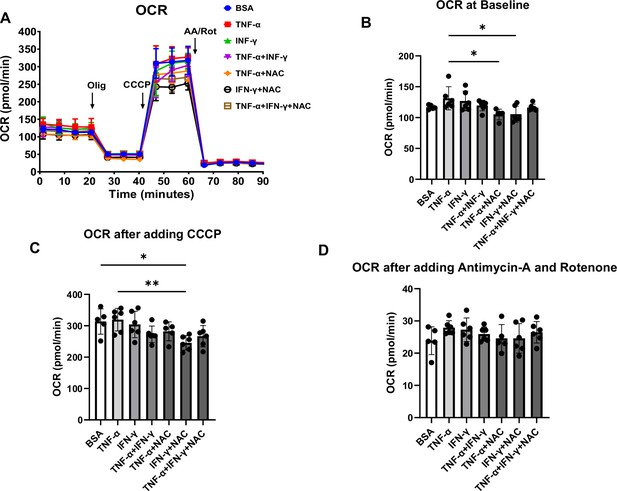
Oxygen consumption rate (OCR) in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) treated with cytokines and antioxidant agent N-acetylcysteine (NAC).
(A) OCR trace of hiPSC-CM treated with bovine serum albumin (BSA), TNF-α, IFN-γ, TNF-α + IFN-γ, TNF-α + NAC, IFN-γ + NAC, and TNF-α + IFN-γ + NAC. (B–D) Bar graph summary of data in (A) with OCR at baseline (B), after adding carbonyl cyanide m-chlorophenyl hydrazone (CCCP) (C), and after adding antimycin A and rotenone (D). N = 5–6. Data were analyzed by ordinary one-way ANOVA and post hoc Tukey’s multiple-comparison test. Bars represent group mean, and error bars indicate standard error of the mean.
-
Figure 2—source data 1
OCR curve in hiPSCs treated with cytokines and NAC.
- https://cdn.elifesciences.org/articles/95867/elife-95867-fig2-data1-v1.xlsx
-
Figure 2—source data 2
OCR at baseline, after CCCP treatment and after rotenone and antimycin A treatment.
- https://cdn.elifesciences.org/articles/95867/elife-95867-fig2-data2-v1.xlsx
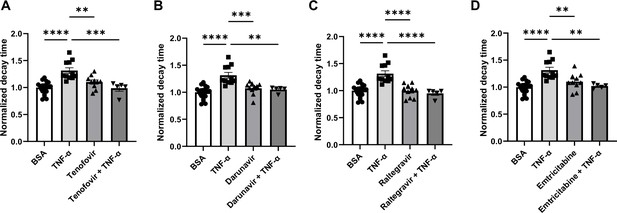
Decay time in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) after treatment with TNF-α and various antiretroviral therapy (ART) drugs.
(A) Decay time after treatment with TNF-α, tenofovir, and TNF-α+tenofovir. (B) Decay time after treatment with TNF-α, darunavir, and TNF-α+darunavir. (C) Decay time after treatment with TNF-α, raltegravir, and TNF-α + raltegravir. (D) Decay time after treatment with TNF-α, emtricitabine, and TNF-α + emtricitabine. N = 5–21. Data were analyzed by ordinary one-way ANOVA and post hoc Tukey’s multiple-comparison test. Bars represent group mean, and error bars indicate standard error of the mean.
-
Figure 3—source data 1
Normalized decay times in hiPSC-CMs treated with TNF-α and/or anti-HIV drugs.
- https://cdn.elifesciences.org/articles/95867/elife-95867-fig3-data1-v1.xlsx
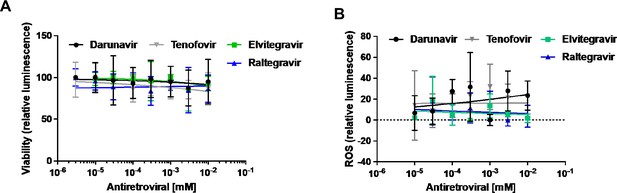
Cell viability and ROS levels in hiPSC-CMs treated with anti-HIV drugs.
Cell viability (A) and cellular reactive oxygen species (ROS) levels (B) in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) exposed to concentrations ranging from between 3 nM and 10 μM of tenofovir (a nucleotide-analog reverse transcriptase inhibitor), darunavir (a protease inhibitor), raltegravir, and elvitegravir (integrase inhibitors). N=1-6.
-
Figure 3—figure supplement 1—source data 1
Cell viability and ROS levels in hiPSC-CMs treated with anti-HIV drugs.
- https://cdn.elifesciences.org/articles/95867/elife-95867-fig3-figsupp1-data1-v1.xlsx
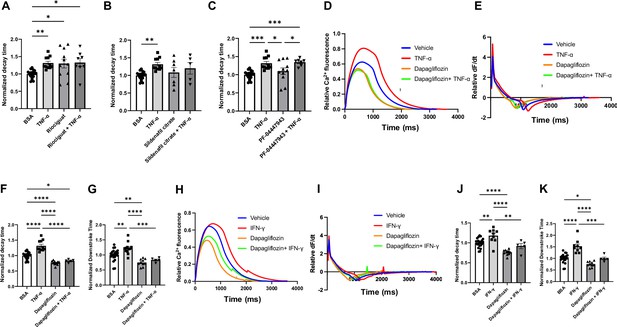
Decay time in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) after treatment with TNF-α and riociguat, sildenafil citrate, PF-04447943, and dapagliflozin.
(A) Decay time after treatment with TNF-α, sGS agonist riociguat, and TNF-α + riociguat (N = 8–21). (B) Decay time after treatment with TNF-α, sildenafil citrate, and TNF-α + sildenafil citrate (N = 5–21). (C) Decay time after treatment with TNF-α, PF-04447943, and TNF-α+PF-04447943 (N = 7–21). (D) Representative curve of calcium transient in hiPSC-CM treated with TNF-α, dapagliflozin, and TNF-α + dapagliflozin. (E) Curve of derivative calculated with data in (D). (F) Decay time after treatment with TNF-α, dapagliflozin, and TNF-α+dapagliflozin (N = 5–21). (G) Downstroke time after treatment with TNF-α, dapagliflozin, and TNF-α + dapagliflozin(N = 5–21). (H) Representative curve of calcium transient in hiPSC-CM treated with IFN-γ, dapagliflozin, and IFN-γ + dapagliflozin. (I) Curve of derivative calculated with data in (H). (J) Decay time after treatment with INF-γ, dapagliflozin, and IFN-γ + dapagliflozin (N = 5–21). (K) Downstroke time after treatment with INF-γ, dapagliflozin, and IFN-γ + dapagliflozin (N = 5–21). Data were analyzed by ordinary one-way ANOVA and post hoc Tukey’s multiple-comparison test. Bars represent group mean, and error bars indicate standard error of the mean.
-
Figure 4—source data 1
Normalizaed decay times in hiPSC-CMs treated with TNFα and Riociguat, Sildenafil citrate and PF-04447943.
- https://cdn.elifesciences.org/articles/95867/elife-95867-fig4-data1-v1.xlsx
-
Figure 4—source data 2
Analyses of calcium transient in hiPSC-CMs treated with TNFα, INFγ and 10 μM Dapagliflozin.
- https://cdn.elifesciences.org/articles/95867/elife-95867-fig4-data2-v1.xlsx
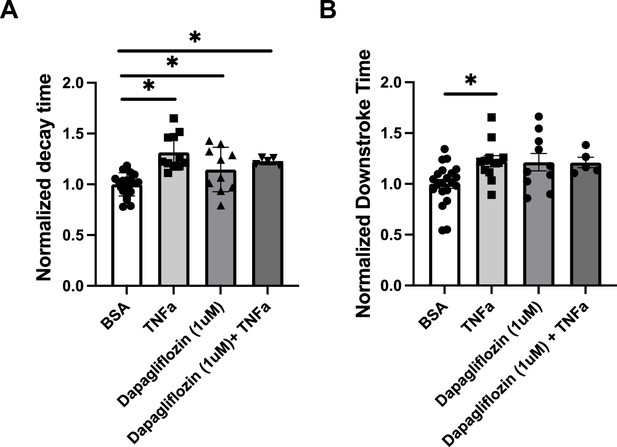
Analyses of calcium transient in hiPSC-CMs treated with TNFa and/or 1 μM Dapagliflozin.
Decay time (A) and downstroke time (B) in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) after treatment with TNF-α and 1 μM of dapagliflozin.
N = 5–21, Data were analyzed by ordinary one-way ANOVA and post hoc Tukey’s multiple-comparison test. Bars represent group mean, and error bars indicate standard error of the mean.
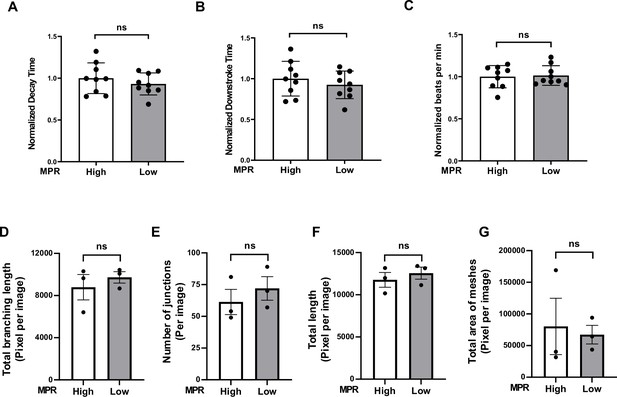
Ca2+-decay time of human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) and angiogenic function of ECs after treatment with serum from group 1 HIV+ patients with diastolic dysfunction (DD).
(A–C) Pooled normalized decay time (A), downstroke time (B), and beating rate (C) in hiPSC treated with 10% serum for 48 hr from group 1 HIV+ patients with DD (3 values from cells treated with each serum). (D–G) Angiogenic parameters, including total length of branching (D) (N = 3), number of junctions (E) (N = 3), total length (F) (N = 3), and number of meshes (G) (N = 3) in human umbilical vein endothelial cells (HUVEC) after treatment with 2% serum from group 1 patients for 20 hr. Data were analyzed by unpaired Student’s t-test. Bars represent group mean, and error bars indicate standard error of the mean.
-
Figure 5—source data 1
Analyses of calcium transient in hiPSCs treated with serum from HIV patients.
- https://cdn.elifesciences.org/articles/95867/elife-95867-fig5-data1-v1.xlsx
-
Figure 5—source data 2
Quantitative analyses of tube formation assay in HUVECs treated with serum from HIV patients.
- https://cdn.elifesciences.org/articles/95867/elife-95867-fig5-data2-v1.xlsx
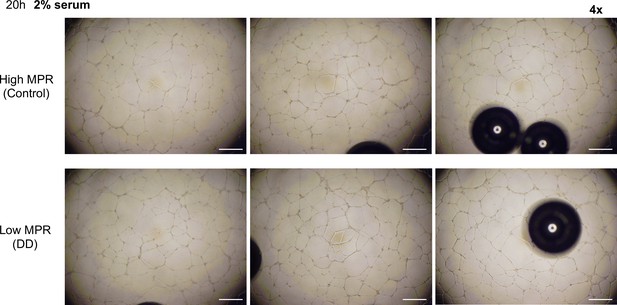
Representative images of formed tube structure with human umbilical vein endothelial cells (HUVEC) treated with 2% serum from HIV+ patients with high or low myocardial perfusion reserve (MPR) for 20 hr.
Scale bars represent 500 μm.
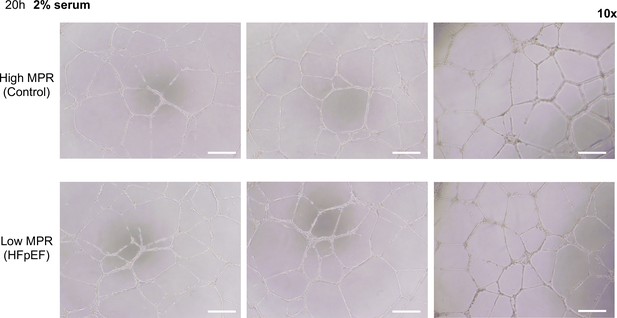
Representative magnified images of formed tube structure with human umbilical vein endothelial cells (HUVEC) treated with 2% serum from HIV+ patients with high or low myocardial perfusion reserve (MPR) for 20 hr.
Scale bars indicate 250 μm.
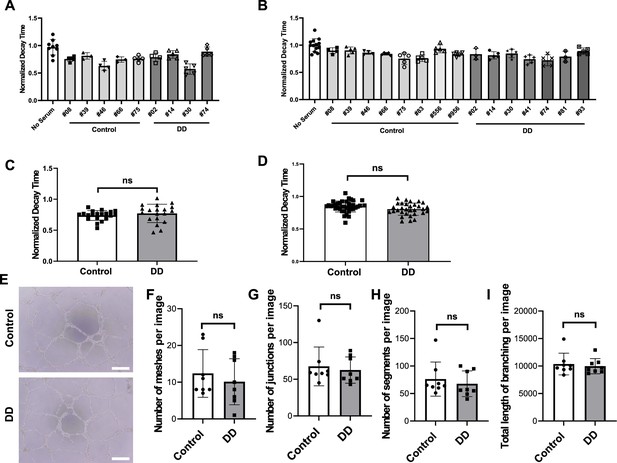
Diastolic function of human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) and angiogenic function of ECs after treatment with serum from HIV+ patients with diastolic dysfunction (DD) (group 2).
(A, B) Individual normalized decay time 3 hr (N = 3–9) (A) or 24 hr (N = 3–13) (B) after treatment of hiPSC-CM with 2% serum from patients. (C, D) Pooled decay time data from patients in (A) and (B) with 3 hr (C) and 24 hr (D) after treatment with serum (N = 21–22 for (C) and N = 31-38 for (D)). (E) Representative image of formed tube structure with cultured human umbilical vein endothelial cells (HUVEC) treated with serum from HIV+ patients with DD. Scale bars indicate 250 μm. (F–I) Assessment of tube formation of HUVECs 20 hr after treatment with serum of patients, including number of meshes (F) (N = 8), number of junctions (G) (N = 8), number of segments (H) (N = 8), and total length of branching (I) (N = 8). Data were analyzed by unpaired Student’s t-test for (C, D, F, G, H, I). Bars represent group mean, and error bars indicate standard error of the mean.
-
Figure 6—source data 1
Normalized decay time in iPS-CMs treated with HIV patient serum with or without diastolic dysfunction.
- https://cdn.elifesciences.org/articles/95867/elife-95867-fig6-data1-v1.xlsx
-
Figure 6—source data 2
Quantitative analyses of tube formation assay in HUVECs treated with serum from HIV patients.
- https://cdn.elifesciences.org/articles/95867/elife-95867-fig6-data2-v1.xlsx
Tables
Clinical characteristics of study population for group 1.
| Participant | Age | High or low myocardial perfusion reserve group | Sex | CD4 count | Diabetes mellitus? | Active cigarette smoking? | Hypertension? | Coronary artery disease diagnosis? | Left ventricular ejection fraction | Myocardial perfusion reserve |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | High | Male | 487 | No | Yes | Yes | No | 64 | 2.74 |
| 2 | 62 | Low | Male | 703 | No | No | Yes | Yes | 59 | 1.03 |
| 3 | 64 | High | Male | 321 | No | No | No | Yes | 65 | 3.43 |
| 4 | 55 | High | Male | 749 | No | No | Yes | Yes | 59 | 1.90 |
| 5 | 71 | Low | Male | 1743 | No | No | Yes | Yes | 55 | 1.03 |
| 6 | 59 | Low | Male | 678 | No | No | Yes | No | 52 | 1.13 |





