Drosophila HCN mediates gustatory homeostasis by preserving sensillar transepithelial potential in sweet environments
Figures
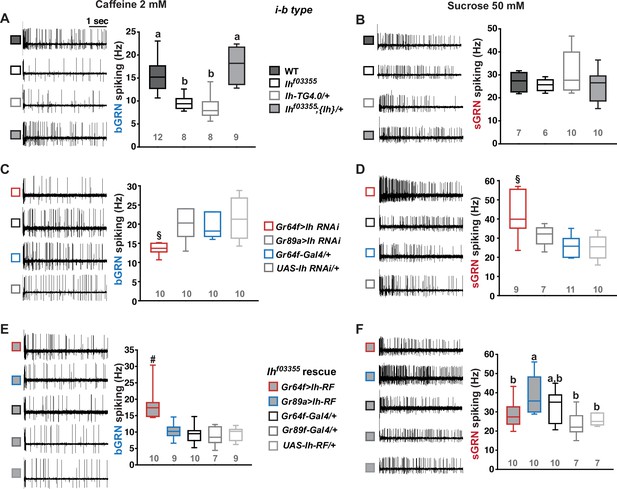
Hyperpolarization-activated cyclic nucleotide-gated (HCN) channel is necessary for the normal activity of bitter-sensing GRNs (bGRNs), although expressed in sweet-sensing GRNs (sGRNs).
Representative 5 s-long traces of sensillum recording with either caffeine or sucrose at the indicated concentrations, shown along with box plots of spiking frequencies. (A) Caffeine-evoked bitter spiking responses of wild-type (WT), the Ih-deficient mutants, Ihf03355 and Ih-TG4.0/+, and the genomic rescue, Ihf03355;{Ih}/+. (B) Sucrose responses were similar among the genotypes tested in (A). (C) Ih RNAi knockdown in sGRNs, but not bGRNs, reduced the bGRN responses to 2 mM caffeine. (D) Ih RNAi knockdown in sGRNs increased the sGRN responses to 50 mM sucrose. (E) Introduction of the Ih-RF cDNA in sGRNs, but not bGRNs, of Ihf03355 restored the bGRN response to 2 mM caffeine. (F) For sucrose responses, the introduction of Ih-RF to bGRNs increased the spiking frequency. Letters indicate statistically distinct groups (a and b): Tukey’s test, p<0.05 (A), Dunn’s, p<0.05 (F). §: Welch’s ANOVA, Games-Howell test, p<0.05. #: Dunn’s test, p<0.05. Numbers in gray indicate the number of tested naïve bristles, which are from at least three individuals.
-
Figure 1—source data 1
Spiking frequencies from the first 5-sec bin following the contact with indicated tastants, which are for box plots in the Figure 1.
- https://cdn.elifesciences.org/articles/96602/elife-96602-fig1-data1-v1.xlsx
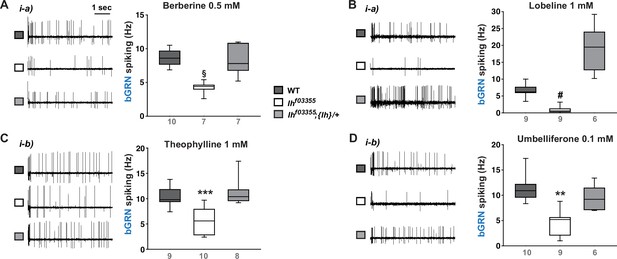
Ih is required for spiking responses to various bitter chemical compounds.
Representative 5 s-long traces of sensillum recording in wild-type (WT), Ihf03355 and a genomic rescue are shown along with box plots of spiking frequencies for indicated bitters, such as berberine (A), lobeline (B), theophylline (C), and umbelliferone (D). §: Welch’s ANOVA, Games-Howell test, p<0.05. #: Dunn’s test, p<0.05. ** and ***: Tukey’s, p<0.01 and p<0.001, respectively. Numbers in gray indicate the number of naïve bristles tested in at least three animals.
-
Figure 1—figure supplement 1—source data 1
The first 5-sec spiking frequencies in response to the indicated bitter compounds, which were used to draw the box plots.
- https://cdn.elifesciences.org/articles/96602/elife-96602-fig1-figsupp1-data1-v1.xlsx
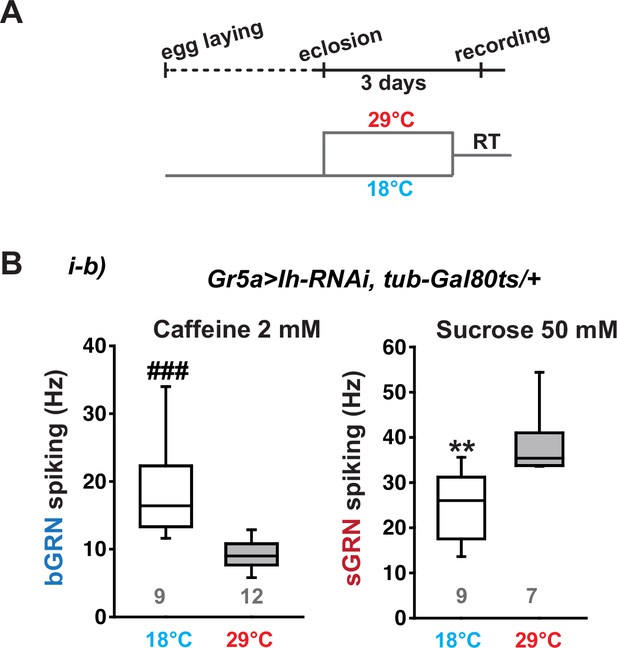
Ih RNAi knockdown in adulthood reduces spiking frequencies in response to 2 mM caffeine but increases spiking frequencies to 50 mM sucrose.
(A) Schematic diagram depicting the design of temporal control of the RNAi. (B) Box plots of spiking frequencies obtained with indicated bitter and sweet chemical compounds at temperatures permissive and non-permissive for Gal80ts. ###: Dunn’s, p<0.001. **: Tukey’s, p<0.01. Numbers in gray indicate the number of naïve bristles tested in at least three animals.
-
Figure 1—figure supplement 2—source data 1
The first 5-sec firing frequencies in response to the indicated tastants in Figure 1—figure supplement 2B.
- https://cdn.elifesciences.org/articles/96602/elife-96602-fig1-figsupp2-data1-v1.xlsx
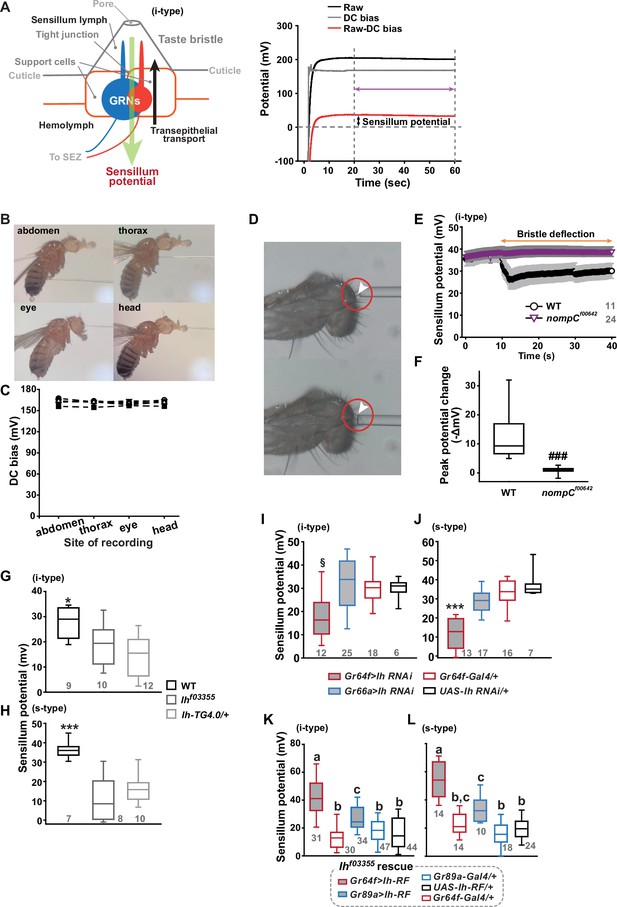
Sensillum potential (SP) is reduced in hyperpolarization-activated cyclic nucleotide-gated (HCN) channel-deficient animals.
(A) Schematic diagram illustrating the sensillum potential in the taste bristle sensilla (Left). Black upward arrow indicates ion transport by pumps and transporters in support cells from the hemolymph to the sensillum lymph. These body fluids are physiologically separated by tight junctions between support cells. The resulting transcellular disparity of ions leads to a positive sensillum potential (greed downward arrow). Representative traces of potentials measured to evaluate SP (Right). Raw: the potential reading upon the contact of the recording electrode with the sensillum bristle tip (black). DC (direct current) bias: the potential reading upon impalement of the head by the recording electrode (gray). Red line indicates the difference between raw and DC bias, which represents the sensillum potential. The values resulting from the subtraction of the data between 20–60 s after the initial contact (time indicated by the purple double-headed arrow) were averaged to determine SP. (B) Photographs of impaled flies for DC bias determination at indicated sites. (C) DC bias values were obtained from indicated body parts. There is no statistical significance between the body sites (ANOVA Repeated Measures). (D) Photos before (top) and after (bottom) deflection of an i-type bristle. (E) Sensillum potential traces as a function of time from wild-type (WT) and nompCf00642. Bristle bending started at 10 s, and the duration is marked by an orange double-headed arrow. (F) The peak SP changes of WT and nompCf00642 were compared. (G, H) SP was reduced in i- (G) and s-type (H) bristles of the indicated Ih-deficient mutants, relative to WT. (I, J) Ih RNAi in sweet-sensing GRNs (sGRNs) reduced SPs of the i- and s-type bristles. (K, L) The SP of Ihf03355 was restored by Ih-RF expression in gustatory receptor neurons (GRNs) (red for sGRNs, blue for bGRNs). ###: Dunn’s, p<0.001. * and ***: Tukey’s, p<0.05 and p<0.001, respectively. §: Welch’s ANOVA, Games-Howell test, p<0.05. Letters indicate statistically distinct groups: Tukey’s test, p<0.05. Numbers in gray indicate the number of naive bristles tested in at least three animals.
-
Figure 2—source data 1
Acquired potential values in indicated experiments in Figure 2.
- https://cdn.elifesciences.org/articles/96602/elife-96602-fig2-data1-v1.xlsx
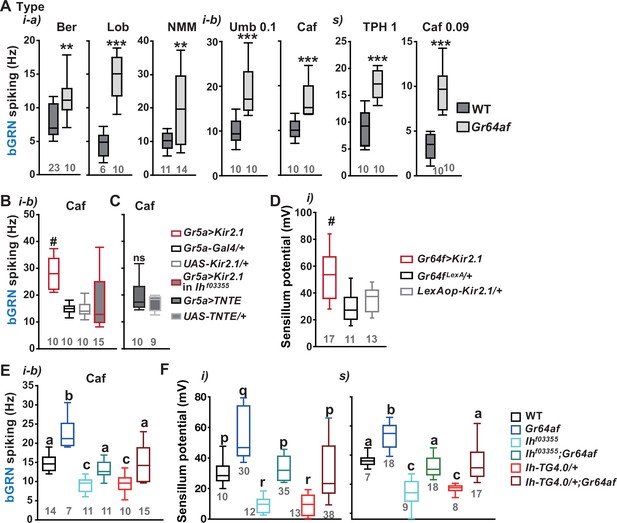
Inactivation of sweet-sensing GRNs (sGRNs) raises bitter-sensing gustatory receptor neurons (bGRN) activity and sensillum potential (SP), both of which are reversed by Ih deficiency.
(A) The bGRN spiking was increased in response to the indicated bitters in Gr64af mutants impaired in sucrose and glucose sensing. Ber: 0.5, Lob: 0.5, NMM: 2, Caf: 2 (i-type), and 0.09 (s-type), Umb: 0.1, TPH: 1 mM. ** and ***: Student’s t-test, p<0.01 and p<0.001, respectively. (B, C) Silencing by Kir2.1 (B), but not blocking chemical synaptic transmission (C), in sGRNs increased the spiking of bGRNs stimulated by 2 mM caffeine, which was reversed in Ihf03355 (B). #: Dunn’s, p<0.05. (D) Silencing sGRNs by Kir2.1 increased SP. #: Dunn’s, p<0.05. (E) The increased bGRN spiking in Gr64af was restored to wild-type (WT) levels by Ih deficiencies. Letters indicate significantly different groups (Tukey’s, p<0.05). Caffeine 2 mM was used (B, C, E). (F) Regardless of bristle type, SP was increased upon sGRN inactivation, which was reduced by Ih deficiencies. (p–r): Dunn’s test, p<0.05. (a–c): Welch’s ANOVA, Games-Howell test, p<0.05. Numbers in gray indicate the number of naïve tested bristles in at least three animals.
-
Figure 3—source data 1
Spiking frequencies and sensillum potentials obtained in the experiments of Figure 3.
- https://cdn.elifesciences.org/articles/96602/elife-96602-fig3-data1-v1.xlsx
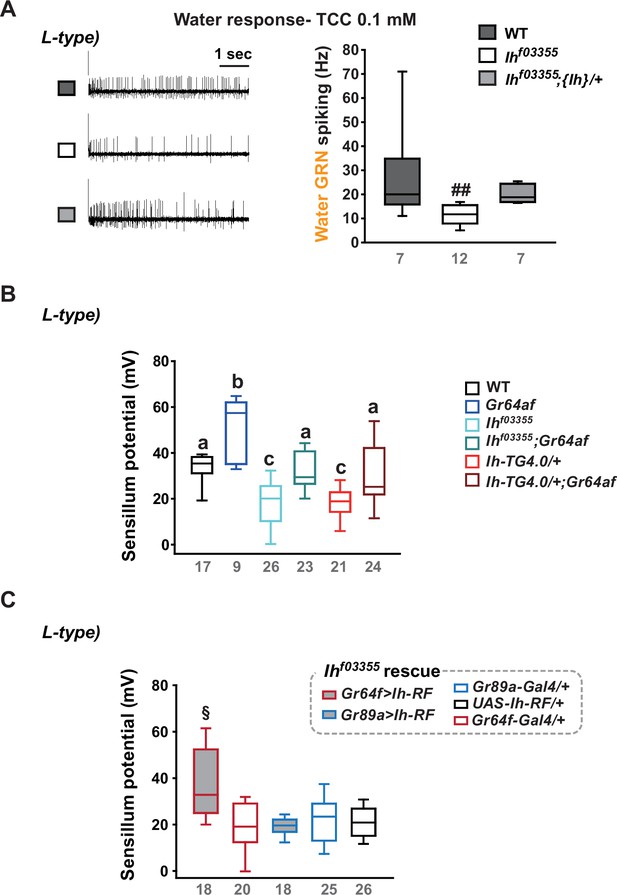
Water gustatory receptor neurons (GRNs) rely on the sensillum potential (SP) guarded by hyperpolarization-activated cyclic nucleotide-gated (HCN) channel in the L-type bristles.
(A) Water GRN activity evoked by 0.1 mM tricholine citrate (TCC) was appraised in wild-type (WT), Ihf03355 and a genomic rescue. The representative traces (Left) and box plots of spiking frequencies (Right) are shown. ##: Dunn’s, p<0.01. (B) SP in L-type bristles is reduced in Ih-deficient mutants but increased in Gr64af. Combination of Ih and Gr64af deficiencies cancels the respective effects, moving SPs towards the level observed in WT in i- and s-type bristles. Letters, a to c, indicate statistically distinct groups: Tukey’s, p<0.05. (C) Introduction of the Ih-RF cDNA in sGRNs, but not in bGRNs, restored SP in Ihf03355. §: Welch’s ANOVA, Games-Howell test, p<0.05. Numbers in gray indicate the number of naïve bristles tested in at least three animals.
-
Figure 3—figure supplement 1—source data 1
Water cell spiking frequencies and L-type bristle sensillum potential data.
- https://cdn.elifesciences.org/articles/96602/elife-96602-fig3-figsupp1-data1-v1.xlsx
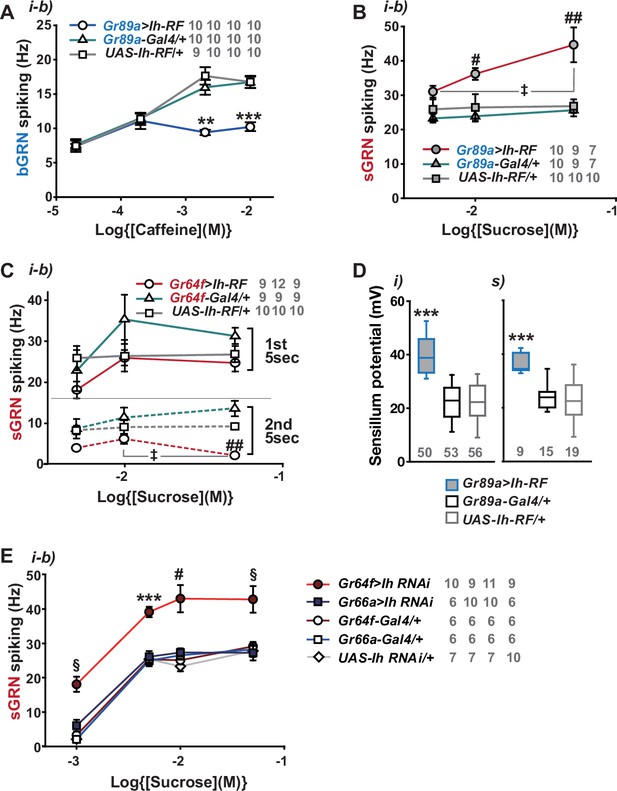
Hyperpolarization-activated cyclic nucleotide-gated (HCN) channel suppresses HCN-expressing gustatory receptor neurons (GRNs) and increases sensillum potential (SP).
(A) HCN misexpressed in bitter-sensing gustatory receptor neurons (bGRNs) flattened the dose dependence to caffeine. (B) HCN ectopically expressed in bGRNs elevates sweet-sensing GRN (sGRN) responses to sucrose. (C) Overexpression of HCN in sGRNs reduced the sGRN responses to sucrose 5 s after the initial contact. (D) Ih misexpression in bGRNs increased SP in i- and s-type bristles, which correlates with laterally increased sGRN activity (B). (E) Ih RNAi knockdown in sGRNs (Gr64f-Gal4 cells) dramatically elevates spiking frequencies in response to 1-, 5-, 10-, and 50 mM sucrose. *, **, and ***: Tukey’s, p<0.05, p<0.01, and p<0.001, respectively (A, D, E). # and ##: Dunn’s, p<0.05 and p<0.01 between genotypes, respectively (B, C, E). ‡: Dunn’s, p<0.05 between responses to different sucrose concentrations (B, C). §: Welch’s ANOVA, Games-Howell test, p<0.05 (E). The numbers in gray indicate the number of tested naïve bristles in at least three animals.
-
Figure 4—source data 1
Spiking frequencies and sensillum potential data from Figure 4.
- https://cdn.elifesciences.org/articles/96602/elife-96602-fig4-data1-v1.xlsx
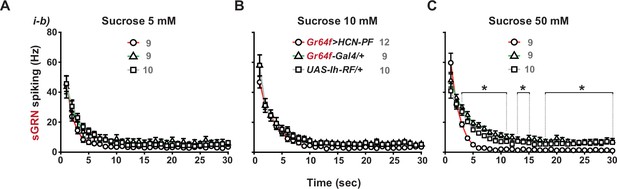
Overexpression of Ih-RF in WT sGRNs suppresses their spiking responses to 50 mM sucrose in a delayed manner.
Post-stimulus spiking frequencies binned every second are shown for sucrose concentrations, 5, 10, and 50 mM (A, B and C, respectively). *: p<0.05, Tukey’s, Dunn’s or Games-Howell test, depending on the data distribution and variance. The data from i-b type bristles of Gr64f>Ih RF are significantly different from those of the genetic controls within the three indicated ranges. Numbers in gray indicate the number of naïve bristles tested in at least three animals. See Figure 4C for a different style of data presentation.
-
Figure 4—figure supplement 1—source data 1
Post-stimulus spiking frequenceis in 1-sec bins.
- https://cdn.elifesciences.org/articles/96602/elife-96602-fig4-figsupp1-data1-v1.xlsx
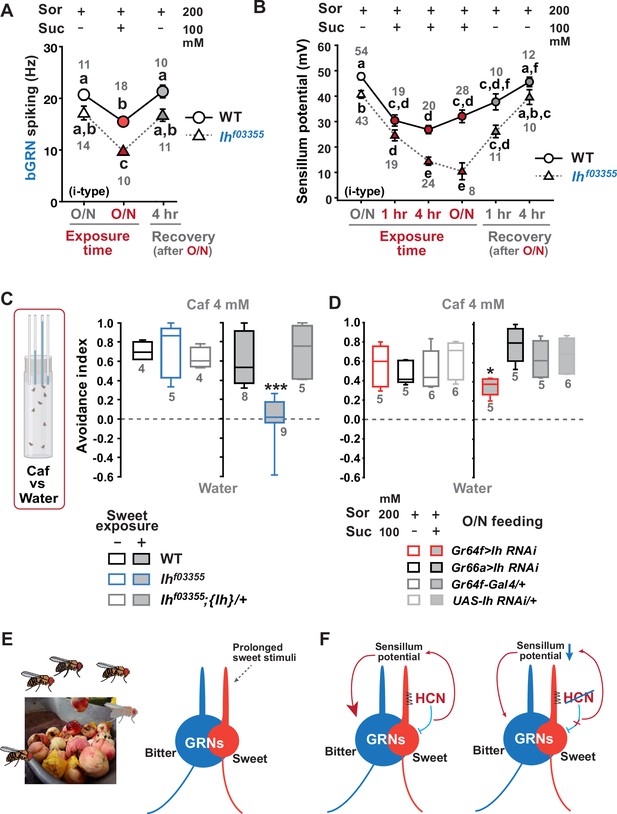
Sweetness in the diet decreases sensillum potential (SP), bitter-sensing gustatory receptor neuron (bGRN) activity, and bitter avoidance.
(A) Sweetness in the media reduced the 2 mM caffeine-evoked bGRN spiking, which was fully recovered in 4 hr incubation with sorbitol only food. Ihf03355 was affected by the type of the media more severely than wild-type (WT). O/N: overnight incubation with sorbitol only (gray) or sucrose food (red). (B) The SP of Ihf03355 bristle sensilla showed dysregulated reduction after 4 hr and overnight incubation on sweet media. These reductions started to be recovered in 1 hr feeding and were nearly fully recovered in 4 hr feeding on the indicated sorbitol only food. (C) Caffeine (Caf) avoidance was assessed with capillary feeder assay (CAFE). Ih is required for robust caffeine avoidance for flies maintained on sweet cornmeal food (sweet exposure +: filled boxes). Ihf03355 flies avoided 4 mM caffeine like WT flies when separated from sweet food for 20 hr (blank boxes). (D) Ih RNAi knockdown in sGRNs (Gr64f-Gal4) but not bGRNs (Gr66a-Gal4) led to relatively poor avoidance to caffeine after feeding on the sweet diet with sucrose. Suc: sucrose, and Sor: sorbitol. Letters indicate statistically distinct groups: a-f, Dunn’s, p<0.05 (A, B). * and ***: Tukey’s, p<0.05 and<0.001, respectively. (E) Illustration depicting the flies’ sweet feeding niche in overripe fruit (Left), leading to prolonged exposure of sGRNs to the sweetness (Right). (F) A schematic model of gustatory homeostasis in Drosophila bristle sensilla. Despite the prolonged sweetness in the environment robustly and frequently stimulating sweet-sensing GRNs (sGRNs), the sGRN activity is moderated by hyperpolarization-activated cyclic nucleotide-gated (HCN) channel to preserve the sensillum potential, which is required for normal bGRN responsiveness (Left). When HCN in sGRNs is incapacitated, sGRNs can become overly excited by sweetness of overripe fruit and deplete the sensillum potential, resulting in decreased bGRN activity and bitter avoidance (Right).
-
Figure 5—source data 1
Electrophysiology data and avoidance indices.
- https://cdn.elifesciences.org/articles/96602/elife-96602-fig5-data1-v1.xlsx
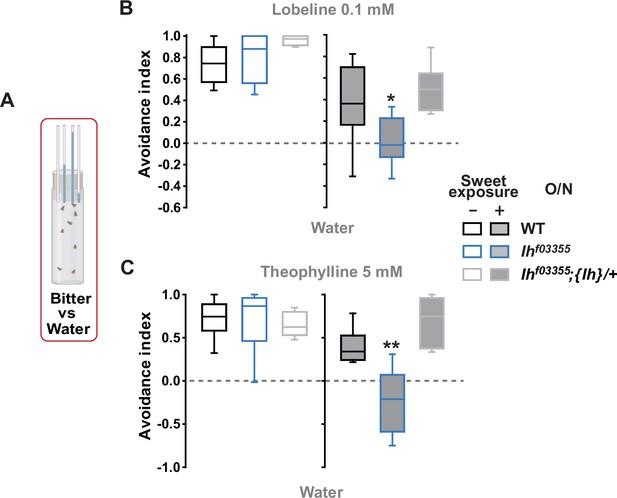
Feeding avoidance to lobeline and theophylline is reduced in Ihf03355 following prior exposure to sweetness.
(A) Bitter avoidance was evaluated by capillary feeder assay (CAFE). (B, C) Ih is required for avoidance to indicated bitters for flies maintained on sweet cornmeal food (sweet exposure +: filled boxes) but not for flies separated from sweetness for 20 hr (sweet exposure -: blank boxes). * and **: Tukey’s, p<0.05 and 0.01, respectively. Numbers in gray indicate the number of naïve bristles tested in at least three animals.
-
Figure 5—figure supplement 1—source data 1
Avoidance indices obtained with indicated bitters.
- https://cdn.elifesciences.org/articles/96602/elife-96602-fig5-figsupp1-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Drosophila melanogaster) | Cantonized w1118 | NA | NA | NA |
| Genetic reagent (D. melanogaster) | Gr64af | Dr. Moon at Yonsei U. | NA | NA |
| Genetic reagent (D. melanogaster) | Ihf03355 | Bloomington Drosophila Stock Center | BDSC: 85660; Flybase: FBti0051182 | NA |
| Genetic reagent (D. melanogaster) | Mi{Trojan-GAL4.0}IhMI03196-TG4.0 (Ih-TG4.0) | Bloomington Drosophila Stock Center | BDSC: 76162; Flybase: FBti0187533 | NA |
| Genetic reagent (D. melanogaster) | Duplicate of Dp(2;3)GV-CH321-22I11 | Bloomington Drosophila Stock Center | BDSC: 89744; Flybase: FBab0048672 | NA |
| Genetic reagent (D. melanogaster) | Gr5a-Gal4 | Dr. Scott at UC Berkeley | NA | NA |
| Genetic reagent (D. melanogaster) | Gr64fLexA | Dr. Amrein at TAMU | NA | NA |
| Genetic reagent (D. melanogaster) | Gr64f-Gal4 | Dr. Amrein at TAMU | NA | NA |
| Genetic reagent (D. melanogaster) | Gr89a-Gal4 | Dr. Carlson at Yale | NA | NA |
| Genetic reagent (D. melanogaster) | Gr66a-Gal4 | Dr. Amrein at TAMU | NA | NA |
| Genetic reagent (D. melanogaster) | UAS-Kir2.1 | Bloomington Drosophila Stock Center | BDSC: 6595 | NA |
| Genetic reagent (D. melanogaster) | LexAop-Kir2.1 | Dr. Dickson at Janellia | NA | NA |
| Genetic reagent (D. melanogaster) | UAS-TNTE | Bloomington Drosophila Stock Center | BDSC: 28837; Flybase: FBst0028837 | NA |
| Genetic reagent (D. melanogaster) | tub-Gal80ts | Bloomington Drosophila Stock Center | NA | NA |
| Genetic reagent (D. melanogaster) | UAS-Ih-RF | This study or doi: 10.1101/2023.08.04.551918 | Flybase: FBtr0290109 | NA |
| Genetic reagent (D. melanogaster) | UAS-Ih RNAi | Bloomington Drosophila Stock Center | BDSC: 58089; Flybase: FBst0058089 | NA |
| Genetic reagent (D. melanogaster) | nompCf00642 | Korea Drosophila Resource Center | KDRC: K3137; Flybase: FBt0041920 | NA |
| Chemical compound, drug | Tricholine citrate | Sigma-Aldrich | Cat. #T0252 | |
| Chemical compound, drug | Caffeine | Sigma-Aldrich | Cat. #C0750 | |
| Chemical compound, drug | Berberine chloride form | Sigma-Aldrich | Cat. #B3251 | |
| Chemical compound, drug | Lobeline hydrochloride | Sigma-Aldrich | Cat. #141879 | |
| Chemical compound, drug | Umbelliferone | Sigma-Aldrich | Cat. #H24003 | |
| Chemical compound, drug | Theophylline anhydrous | Sigma-Aldrich | Cat. #T1633 | |
| Chemical compound, drug | Sucrose | Georgia Chem | Cat. #57-50-1 | |
| Chemical compound, drug | D-Sorbitol | Sigma-Aldrich | Cat. #S1876 | |
| Chemical compound, drug | N-methyl maleimide | Sigma-Aldrich | Cat. #389412 | |
| Software, algorithm | LabChart 8 | AD Instrument | https://www.adinstruments.com | NA |
| Software, algorithm | SigmaPlot 14.0 | Systat Software Inc | https://systatsoftware.com/ | NA |





