Beyond Haldane’s rule: Sex-biased hybrid dysfunction for all modes of sex determination
Figures
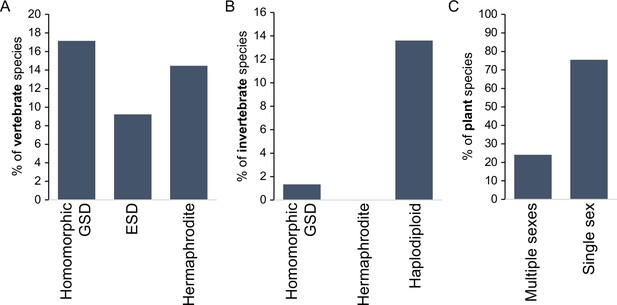
Incidence of different sexual modes in vertebrates (A), invertebrates (B), and plants (C).
Vertebrate values out of 1475 species with information on the karyotype (homomorphic genetic sex determination, GSD) or 2145 species with information on sexual system Environmental sex determination (ESD), hermaphrodite. Invertebrate values out of 11914 species, with homomorphic value including any incidence of homomorphism and haplodiploidy excluding cases of paternal genome elimination; only 2 cases (0.02%) of hermaphroditism are indicated. Species with heteromorphic sex chromosomes are reported for 57% of species in both vertebrates and insects. Plant values out of 11038 species with information on the sexual system. Plant cases with single sex include hermaphrodite and monoecy (excludes apomixis); multiple sexes include dioecy, androdioecy, gynodioecy, andromonoecy, etc. Data was redrawn from Ashman et al., 2014; Bachtrog et al., 2014.
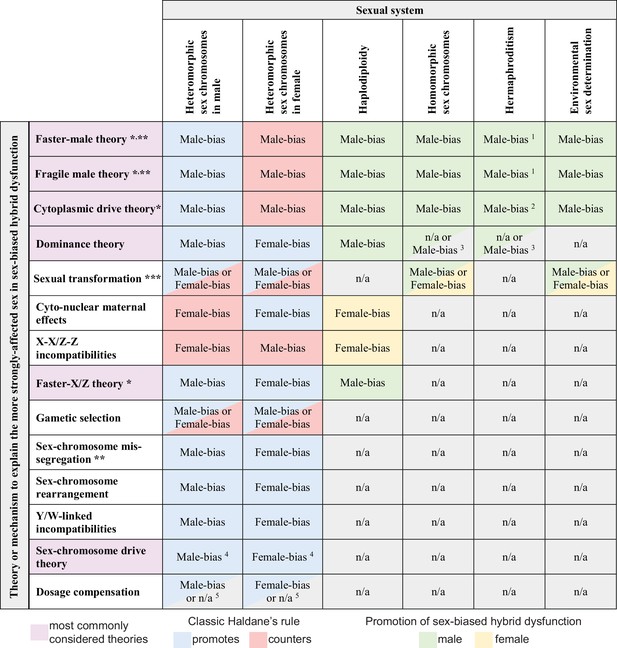
Predicted contributions to sex-biased hybrid dysfunction in different sexual systems for alternative hypotheses that aim to explain the classic Haldane’s rule pattern.
Predictions for haplodiploidy applies to comparisons involving F1 females with males from F2 and later-generation hybrids (Laurie, 1997; Koevoets and Beukeboom, 2009; Bendall et al., 2023). * mechanisms sometimes subsumed under the umbrella of ‘faster heterogametic sex theory’ (Kulathinal and Singh, 2008); ** primarily or only expected to affect hybrid sterility; *** details of sex determination pathway disruption may predispose taxa of a given sexual system to a particular direction of sex bias in absence or rarity; 1 hybrid dysfunction biased toward male gametes (sperm, pollen) and accessory structures; 2 cytoplasmic male sterility in F1 hybrids may not serve as a reproductive isolating barrier (Rieseberg and Blackman, 2010); 3 applies to haploid gametophytic phase (e.g. following pollen germination) for taxa like plants with active haploid stages of male gametes; 4 does not apply in XO or ZO systems (Coyne et al., 1991); 5 does not apply to systems lacking global dosage compensation mediated by downregulation of both sex chromosome copies in the homogametic sex.
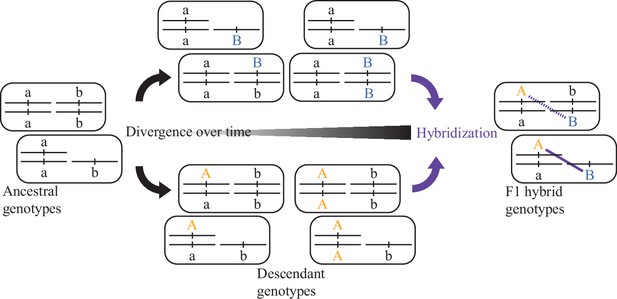
Evolution of a two-locus DMI.
Independent accumulation of derived alleles (autosomal orange A, sex-chromosomal blue B) in different populations for distinct loci can lead to incompatible genetic interactions between them upon hybridization (purple). When one of the loci is linked to a sex-chromosome, then recessive-acting incompatibility loci can reveal their fitness effects in the F1 generation of hybrid individuals hemizygous for that sex chromosome.
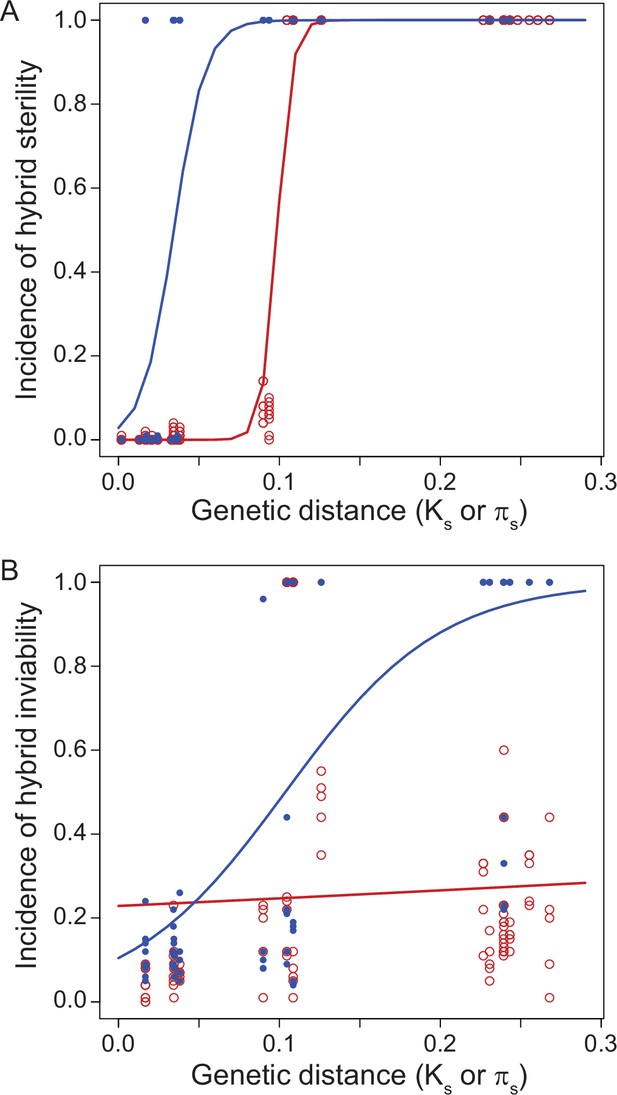
Reproductive isolation clocks for male (blue) and female (red) post-zygotic reproductive isolation documents the accumulation of sex-specific hybrid dysfunction over population divergence.
Hybrid sterility (A) and hybrid inviability (B) evolve sooner for males than for females, on average, in Drosophila. Figure is redrawn from Turissini et al., 2018.
Tables
Exemplar taxa and exceptional examples with experimental evidence that is informative about sex biases in hybrid dysfunction for different sexual systems.
| Sexual system | Exemplar taxa * | Exceptional examples ** | Evidence informative to sex biases in hybrid dysfunction *** | Review references |
|---|---|---|---|---|
| Heteromorphic sex chromosomes in male | Many insects (e.g. Drosophila, Lepidoptera), mammals, nematodes; Silene plants | Teleogryllus (field crickets; XX/XO) | Pervasive male-biased hybrid sterility & inviability; support for multiple mechanisms affecting Haldane’s rule | Delph and Demuth, 2016; Fontdevila, 2016; Moran et al., 2017 |
| Heteromorphic sex chromosomes in female | Many insects, birds, nematodes | Xenopus (clawed frogs; ZW/ZZ) | Pervasive female-biased hybrid sterility & inviability; support for multiple mechanisms affecting Haldane’s rule | Malone and Michalak, 2008b; Delph and Demuth, 2016; Fontdevila, 2016 |
| Haplodiploidy | Nasonia (wasps), Formica (ants), Tetramorium (ants), Tetranychus (mites) | Neodiprion (sawflies) | Sex-biased hybrid inviability more common than hybrid sterility; support for cytoplasmic drive | Koevoets and Beukeboom, 2009; Bendall et al., 2023 |
| Homomorphic sex chromosomes | Aedes (mosquitoes), Tigriopus (copepods), many amphibians, and fish | Bufo (toads) | Heterogeneous support for sex-biases across taxa; higher female inviability and higher male sterility in Bufo; support for faster male and/or fragile male theories (Aedes, Bufo) | Presgraves and Orr, 1998; Malone and Fontenot, 2008a; Lima, 2014; Dufresnes and Crochet, 2022 |
| Hermaphroditism | Many plants (e.g. Mimulus, Solanum, Helianthus, Arabidopsis), molluscs, nematodes | Argopecten (scallops) | Pervasive sterility of male function (pollen, sperm); support for cytonuclear incompatibilities | Rieseberg and Blackman, 2010; Yu et al., 2023 |
| Environmental sex determination | Many turtles and fish, some lizards | Lepomis (sunfish) | Male-biased sex ratios | Bolnick, 2009 |
-
*
taxonomic scale varies across study systems used in speciation research; ** recent or rare well-developed example system; *** the commonness or rarity of sex-biased hybrid dysfunction across taxa remains to be determined for most sexual systems that lack heteromorphic sex chromosomes.
Outstanding questions for Haldane’s rule and sex-biased hybrid dysfunction.
| Consideration | Questions in need of addressing |
|---|---|
| Model development in exploring sex-biased hybrid dysfunction | How can models of classic Haldane’s rule be extended to provide predictions for sex-biased hybrid dysfunction in taxa lacking heteromorphic sex chromosomes? |
| What new models can be developed to explain sex-biased hybrid dysfunction in taxa with homomorphic GSD, ESD, haplodiploidy, and hermaphroditism? | |
| Relative influence of factors causing Haldane’s rule | What is the balance across sexual systems of forces that reinforce and oppose hybrid dysfunction biased toward a particular sex? |
| Which mechanisms most commonly explain exceptions to classic Haldane’s rule, and how do they link to phylogeny? | |
| Associating sex-biased hybrid dysfunction with developmental timing over life history | Under what circumstances do alternative mechanisms for sex-biased hybrid dysfunction affect predictions differently for sterility than for inviability? |
| Is sex-biased hybrid dysfunction truly more prevalent for sterility than for inviability, and if so, why? | |
| To what extent does sex bias in pre-zygotic reproductive isolation associate with sex bias in post-zygotic hybrid dysfunction? | |
| Connecting to other features of reproductive isolation | How do predictions for sex-biased hybrid dysfunction intersect with parent-of-origin asymmetries (Darwin’s corollary to Haldane’s rule)? |
| What is the extent and biological significance of within-species genetic variation for sex-biased hybrid dysfunction? | |
| Approaches and methodologies to investigate sex-biased hybrid dysfunction | What is the relative contribution of divergence in regulatory and coding sequence to sex-biased hybrid dysfunction, and how do they conform to alternate theoretical expectations? |
| How might inference of different rates of accumulation of sex-biased hybrid dysfunction from ‘speciation clock’ analysis inform alternate explanatory models? | |
| How might taxa with multiple sex chromosomes, neo-sex chromosomes, and autosomal paleo-sex chromosomes be exploited to inform alternate models for sex-biased hybrid dysfunction? | |
| How can analysis of hybrid zones and hybrid populations differentiate among alternative explanations for sex-biased hybrid dysfunction? | |
| What do experimental manipulations of sex using hormone treatments, transgenic alterations, and karyotype perturbations indicate for general causes of sex-biased hybrid dysfunction? | |
| How can alternative causes of sex-biased hybrid dysfunction be distinguished by testing for differences in the ontogenetic timing at which dysfunctional development manifests? |
Recommended research directions to establish a generalized view of Haldane’s rule and sex-biased hybrid dysfunction.
| Recommendation | Status, prospect, or approach |
|---|---|
| Compile sexual system information across taxa | Tree of Sex database in progress (Ashman et al., 2014; Bachtrog et al., 2014) |
| Compile sex-biased reproductive isolation information across taxa | Speciation database proposed (Stankowski et al., 2024) |
| Integrate speciation modeling predictions for sex bias explicitly with distinct sexual systems | Feasible for DMI, fitness landscape, systems theory, and other paradigms (Orr, 1993a; Orr and Turelli, 1996; Simon et al., 2018; Schiffman and Ralph, 2022) |
| Test for presence/absence of distinct sources of sex-biased hybrid dysfunction across taxa | Well-studied in Diptera and Lepidoptera, but requires further empirical study and integration more broadly |
| Quantify the relative contribution of distinct sources of sex-biased hybrid dysfunction | Most feasible in genetic model organisms, but diverse experimental (e.g. backcross analysis, hormone treatment) and genomic (e.g. transcriptomes, molecular evolution) techniques empower study in many taxa |
| Conduct developmental analyses of genetic complexity for hybrid sterility and inviability for each sex | Experimentally feasible with interspecies QTL mapping, hybrid allele-specific expression analysis, or other approaches |
| Characterize ‘speciation clocks’ separately for each sex in different sets of taxa | Available for some Drosophila (Turissini et al., 2018), feasible to test in any focal group with partial reproductive isolation between many species pairs |





