Amoeboid cells undergo durotaxis with soft end polarized NMIIA
Figures
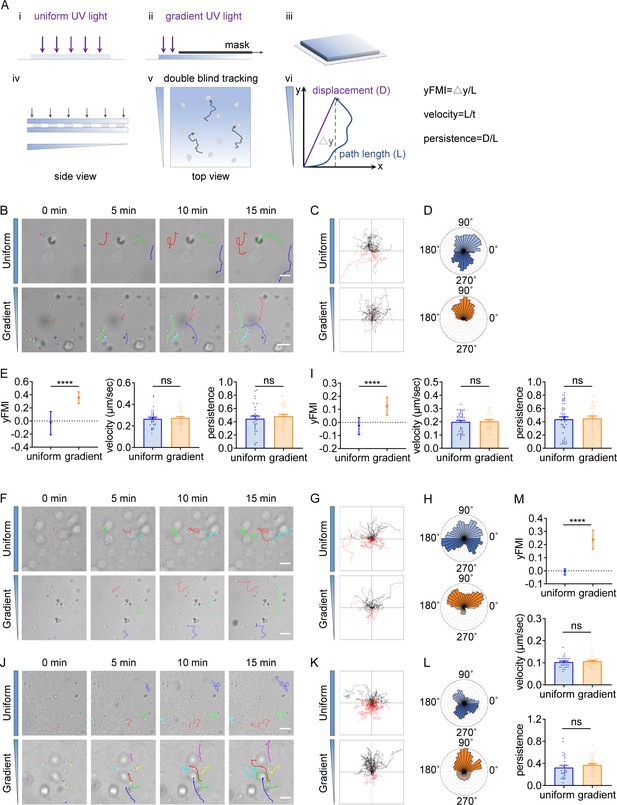
T cells and neutrophils undergo durotactic migration.
(A) Illustration of the imaging-based cell migration device and yFMI value calculation. (B) Representative time-lapse images of single CD4+ Naïve T cells migrating on Intercellular Adhesion Molecule 1 (ICAM-1) coated polyacrylamide gels with uniform stiffness (top, 30 kPa) and stiffness gradient (bottom, with greater stiffness towards the top, 20.77 kPa/mm). Color lines indicated the migration trajectories of a single cell. Scale bar: 50 μm. (C) Representative tracks of migrating CD4+ Naïve T cells on the uniform stiffness gels (top) or stiffness gradient gels (bottom, with greater stiffness towards the top). Note that a higher proportion of cells migrate toward the stiffer end of the gradient substrate (black tracks) compared to cells migrating opposite to the gradient (red tracks). Tracks on the uniform substrate showed equal proportions for each part. (D) Angular displacement of CD4+ Naïve T cells on the uniform stiffness gels (top) or stiffness gradient gels (bottom, with greater stiffness towards the top). (E) y-FMI (Forward Migration Index), velocity and migration persistence of CD4+ Naïve T cells cultured on uniform substrate or gradient substrate (n≥30 tracks were analyzed for each experiment, N=3 independent experiments for each condition, replicates are biological). All error bars are SEM. ****p<0.0001, ns, not significant, by Student’s t-test. (F) Representative time-lapse images of single neutrophils derived from mouse bone marrow migrating on fibronectin-coated polyacrylamide gels with uniform stiffness (top) or stiffness gradient (bottom). Color lines indicated the migration trajectories of a single cell. Scale bar: 50 μm. (G) Representative tracks of migrating neutrophils on the uniform stiffness gels (top) or stiffness gradient gels (bottom, with greater stiffness towards the top). (H) Angular displacement of neutrophils on the uniform stiffness gels (top) or stiffness gradient gels (bottom, with greater stiffness towards the top). (I) y-FMI, velocity, and migration persistence of neutrophils cultured on uniform substrate or gradient substrate (n≥30 tracks were analyzed for each experiment, N=3 independent experiments for each condition, replicates are biological). All error bars are SEM. ****p<0.0001, ns, not significant, by Student’s t-test. (J) Representative time-lapse images of differentiated HL-60 (dHL-60) cells migrating on fibronectin-coated polyacrylamide gels with uniform stiffness (top) or stiffness gradient (bottom). Color lines indicated the migration trajectories of a single cell. Scale bar: 50 μm. (K) Representative tracks of migrating dHL-60 cells on the uniform stiffness gels (top) or stiffness gradient gels (bottom, with greater stiffness towards the top). (L) Angular displacement of dHL-60 cells on the uniform stiffness gels (top) or stiffness gradient gels (bottom, with greater stiffness towards the top). (M) y-FMI, velocity, and migration persistence of dHL-60 cells cultured on uniform substrate or gradient substrate (n≥30 tracks were analyzed for each experiment, N=3 independent experiments for each condition, replicates are biological). All error bars are SEM. ****p<0.0001, ns, not significant, by Student’s t-test.
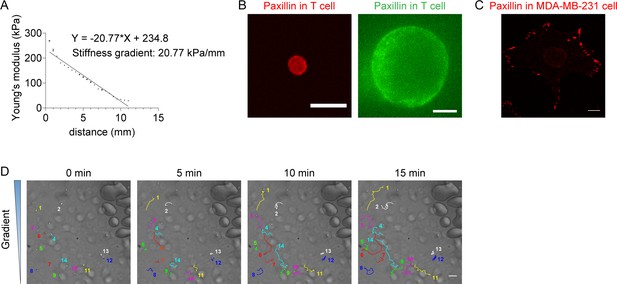
Stiffness gradient of PA-gels, distribution of Paxillin in T cells and MDA-MB-231 cells, and trajectories of migrating neutrophils on gradient gel.
(A) Representative stiffness gradient of polyacrylamide gels measured by atomic force microscope was plotted (20.77 kPa/mm). All error bars are SEM. (n=3 regions, replicates are biological). (B) Left: Representative immunofluorescence images (obtained by confocal imaging) of CD4+ naïve T cells stained with Paxillin antibody and Alexa Fluor 555-conjugated secondary antibody. Right: Representative immunofluorescence images (obtained by expansion microscopy) of CD4+ naïve T cells stained with Paxillin antibody and Alexa Fluor 488-conjugated secondary antibody. Scale bar: 10 μm. (C) Representative immunofluorescence images (obtained by confocal imaging) of MDA-MB-231 cells stained with Paxillin antibody and Alexa Fluor 555-conjugated secondary antibody. Scale bar: 10 μm. (D) Representative tracks of migrating neutrophils on stiffness gradient gel with greater stiffness towards the top. Color lines and numbers indicated the migration trajectories of a single cell. Scale bar: 50 μm.
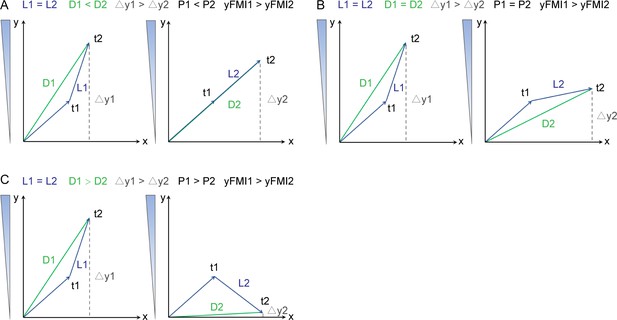
Representative trajectories of three different conditions of persistence value when the yFMI1 >yFMI2.
In all conditions, the single cell migrated the same locomotion, which means L1=L2. t1 and t2 can be regarded as the two consecutive time points during cell migration. Each graph showed one condition for a migrating single cell. In all three conditions, yFMI on the left was greater than on the right. But the persistence on the left was less (A), equal (B), or greater (C) than the right.
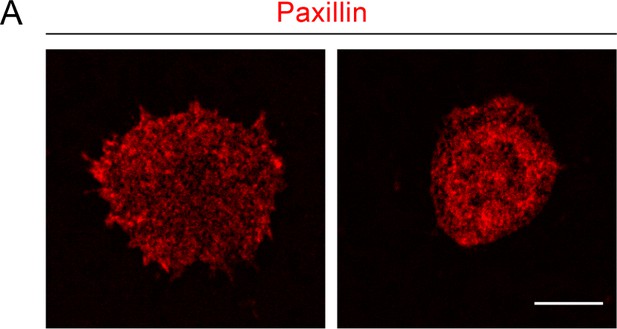
Distribution of Paxillin in dHL-60 cells.
(A) Representative immunofluorescence images (obtained by confocal imaging) of dHL-60 cells stained with Paxillin antibody and Alexa Fluor 555-conjugated secondary antibody. Scale bar: 10 μm.
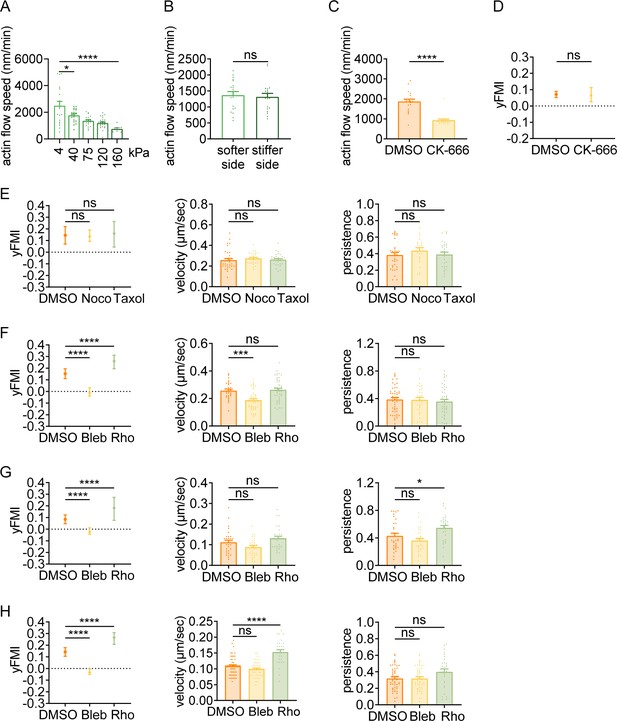
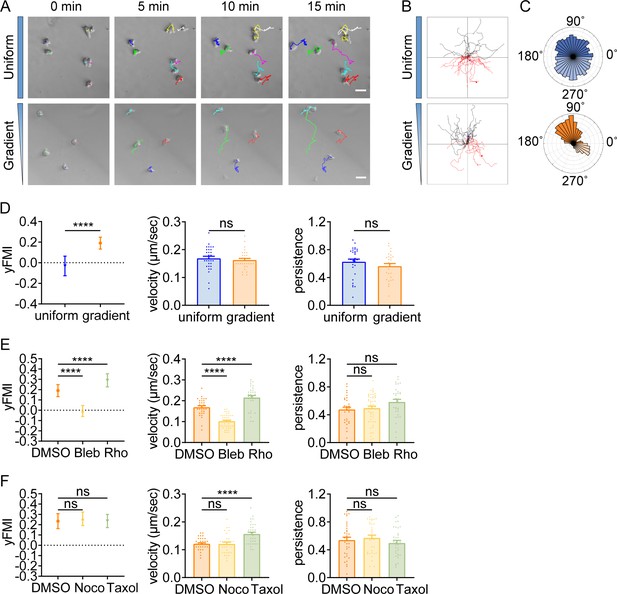
Amoeboid durotaxis may not be propelled by differential actin flow.
(A) Actin flow speed of dHL-60 cells adhered to different substrate stiffness (4 kPa, 40 kPa, 75 kPa, 120 kPa, and 160 kPa). Each plot indicated the average actin flow speed of every individual cell adhered to a polyacrylamide gel. All error bars are SEM. *p<0.05, ****p<0.0001, by one-way ANOVA. (B) Actin flow speed of the softer side and stiffer side of the dHL-60 cells adhered on gradient substrate. Each plot indicated the average actin flow speed of the softer or stiffer part of a cell adhered on stiffness gradient gel. All error bars are SEM. ns, not significant, by Student’s t-test. (C) Actin flow speed of control (DMSO) and CK-666 (100 μM, pre-treated for 5 hr) treated CD4+ Naïve T cells moving on stiffness gradient gel (n=20 tracks were analyzed for each experiment, N=3 independent experiments for each condition, replicates are biological). All error bars are SEM. ****p<0.0001, by Student’s t-test. (D) y-FMI of control (DMSO) and CK-666 (100 μM, pre-treated for 5 hr) treated CD4+ Naïve T cells moving on stiffness gradient gel (n≥30 tracks were analyzed for each experiment, N=3 independent experiments for each condition, replicates are biological). All error bars are SEM. ns, not significant, by Student’s t-test. (E) y-FMI, velocity, and migration persistence of control (DMSO), nocodazole (Noco, 32 μM, pre-treated for 10 min) treated and taxol (70 nM, pre-treated for 10 min) treated CD4+ Naïve T cells moving on stiffness gradient gel (n≥30 tracks were analyzed for each experiment, N=3 independent experiments for each condition, replicates are biological). All error bars are SEM. ns, not significant, by one-way ANOVA. (F) y-FMI, velocity and migration persistence of control (DMSO), blebbistatin (Bleb, 10 μM, pre-treated for 10 min) treated and Rho activator II (Rho, 0.25 μg/mL, pre-treated for 2 hr) treated CD4+ Naïve T cells moving on stiffness gradient gel (n≥30 tracks were analyzed for each experiment, N=3 independent experiments for each condition, replicates are biological). All error bars are SEM. ***p<0.001, ****p<0.0001, ns, not significant, by one-way ANOVA. All drugs were incubated for the whole experiment. (G) y-FMI, velocity and migration persistence of control (DMSO), blebbistatin (Bleb, 10 μM, pre-treated for 10 min) treated and Rho activator II (Rho, 0.25 μg/mL, pre-treated for 2 hr) treated neutrophils moving on stiffness gradient gel (n≥30 tracks were analyzed for each experiment, N=3 independent experiments for each condition, replicates are biological). All error bars are SEM. *p<0.05, ****p<0.0001, ns, not significant, by one-way ANOVA. All drugs were incubated for the whole experiment. (H) y-FMI, velocity and migration persistence of control (DMSO), blebbistatin (Bleb, 10 μM, pre-treated for 10 min) treated and Rho activator II (Rho, 0.25 μg/mL, pre-treated for 2 hr) treated dHL-60 cells moving on stiffness gradient gel (n≥30 tracks were analyzed for each experiment, N=3 independent experiments for each condition, replicates are biological). All error bars are SEM. ****p<0.0001, ns, not significant by one-way ANOVA.
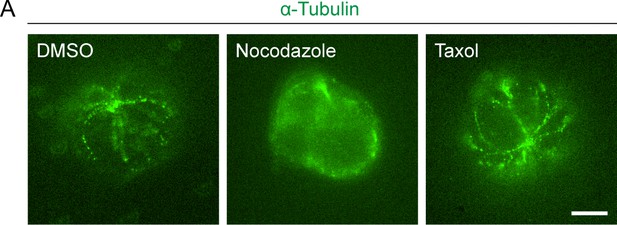
Effect of Nocodazole and Taxol treatment on microtubule network.
(A) Representative immunofluorescence images (obtained by expansion microscopy) of CD4+ naïve T cells stained with α-Tubulin antibody and Alexa Fluor 488-conjugated secondary antibody. Left: control (DMSO) cells. Middle: Nocodazole (32 μM, pre-treated for 10 min) treated cells. Right: Taxol (70 nM, pre-treated for 10 min) treated cells. Scale bar: 10 μm.

Durotaxis of T cells and neutrophils is regulated by polarized actomyosin activity.
(A) Western blot showing the protein level of non-muscle myosin IIA (NMIIA) (left) and NMIIB (right) in the negative control (Ctrl), NMIIA knocked down (NMIIAKD) and NMIIB knocked down (NMIIBKD) HL-60 cells. α-tubulin was used as the loading control. Numbers below the ladders were the relative intensity versus α-tubulin. (B) Representative time-lapse images of NMIIAKD (top) and NMIIBKD (bottom) dHL-60 cells migrating on polyacrylamide gels with stiffness gradient. Color lines indicated the migration trajectories of a single cell. Scale bar: 50 μm (C) Representative tracks of migrating NMIIAKD (top) and NMIIBKD (bottom) dHL-60 cells on stiffness gradient gels. (D) Angular displacement of NMIIAKD (top) and NMIIBKD (bottom) dHL-60 cells on stiffness gradient gels (with greater stiffness towards the top). (E) y-FMI, velocity and migration persistence of control, NMIIAKD and NMIIBKD dHL-60 cells migrating on stiffness gradient gel (n≥30 tracks were analyzed for each experiment, N=3 independent experiments for each condition, replicates are biological). All error bars are SEM. ****p<0.0001, ns, not significant, by one-way ANOVA. (F) Representative immunofluorescent staining of NMIIA (left) and NMIIB (right) of dHL-60 cells on stiffness gradient gel (with greater stiffness towards the top). The yellow triangles indicated the location of NMIIA and NMIIB. Scale bar: 10 μm. (G) Fluorescent intensity ratio of NMIIA or NMIIB in cell softer part to stiffer part in F. All error bars are SEM. ****p<0.0001, by Student’s t-test. (H) Fluorescent intensity ration of NMIIA in softer part to stiffer part of neutrophils and CD4+ Naïve T cells in Figure 3—figure supplement 1D and E. All error bars are SEM. ns, not significant, by Student’s t-test.
-
Figure 3—source data 1
Original files for Western blot in Figure 3A.
- https://cdn.elifesciences.org/articles/96821/elife-96821-fig3-data1-v1.zip
-
Figure 3—source data 2
Western blot with labeled bands in Figure 3A.
- https://cdn.elifesciences.org/articles/96821/elife-96821-fig3-data2-v1.zip
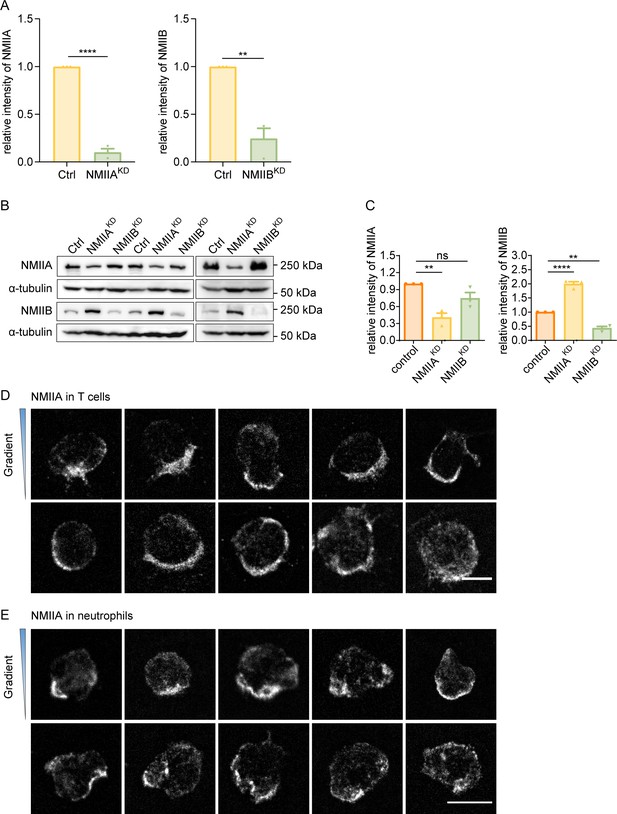
Protein level of NMIIA and NMIIB in NMIIAKD and NMIIBKD HL-60 cells and distribution of NMIIA in T cells and neutrophils.
(A) Quantification of protein level in Figure 3A. α-tubulin was used as the loading control. Bar chart shows the quantification of protein levels normalized to α-tubulin in each condition (n=3 independent experiments, replicates are biological). All error bars are SEM. **p<0.01, ****p<0.0001, by Student’s t-test. (B) Western blot showing the protein level of non-muscle myosin IIA (NMIIA) and NMIIB in the negative control (Ctrl), NMIIA knocked down (NMIIAKD) and NMIIB knocked down (NMIIBKD) HL-60 cells. α-tubulin was used as the loading control. (C) Quantification of protein level in B. α-tubulin was used as the loading control. Bar chart shows the quantification of protein levels normalized to α-tubulin in each condition (n=3 independent experiments, replicates are biological). All error bars are SEM. **p<0.01, ****p<0.0001, ns, not significant, by one-way ANOVA. (D) Representative immunofluorescent staining of NMIIA of CD4+ Naïve T cells on stiffness gradient gel (with greater stiffness towards the top). Scale bar: 5 μm. (E) Representative immunofluorescent staining of NMIIA of neutrophils on stiffness gradient gel (with greater stiffness towards the top). Scale bar: 10 μm.
-
Figure 3—figure supplement 1—source data 1
Original files for Western blot in Figure 3—figure supplement 1B.
- https://cdn.elifesciences.org/articles/96821/elife-96821-fig3-figsupp1-data1-v1.zip
-
Figure 3—figure supplement 1—source data 2
Western blot with labeled bands in Figure 3—figure supplement 1B.
- https://cdn.elifesciences.org/articles/96821/elife-96821-fig3-figsupp1-data2-v1.zip
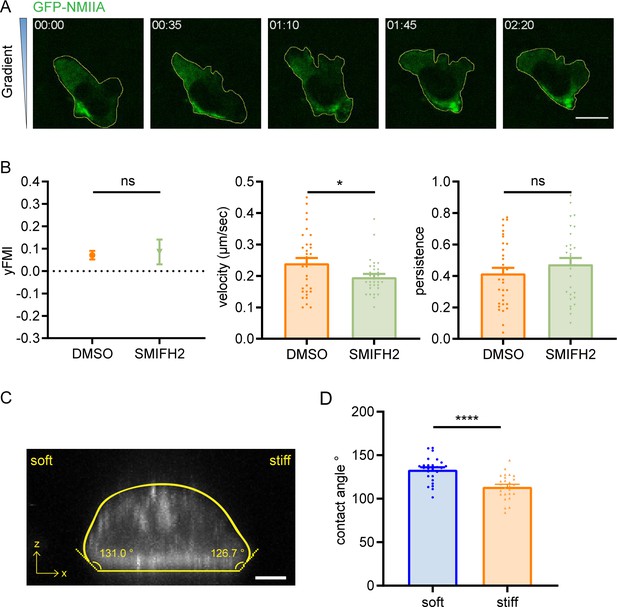
Distribution of GFP-NMIIA in dHL-60 cells, effect of SMIFH2 treatment on amoeboid durotaxis, and contact angles of dHL-60 cells.
(A) Representative time-lapse images of GFP-non-muscle myosin IIA (NMIIA) dHL-60 cells migrating on gradient gel with greater stiffness towards the top. Scale bar: 10 μm. (B) y-FMI, velocity and migration persistence of control (DMSO) and SMIFH2 (15 μM, pre-treated for 5 hr) treated CD4+ Naïve T cells migrating on stiffness gradient gel (n≥30 tracks were analyzed for each experiment, N=3 independent experiments for each condition, replicates are biological). All error bars are SEM. *p<0.05, ns, not significant, by Student’s t-test. (C) Representative confocal image of dHL-60 cells stained with 0.1 μM SiR-Actin on gradient gel. Scale bar: 5 μm. (D) Quantification of contact angle of cell soft end and stiff end (n=26). Error bars are SEM. ****p<0.0001, by Student’s t-test.
Amoeboid durotaxis is evolutionarily conserved.
(A) Representative time-lapse images of Dictysotelium on polyacrylamide gels with uniform stiffness (top) or stiffness gradient (bottom). Color lines indicated the migration trajectories of single cell. Scale bar: 50 μm. (B) Representative tracks of migrating Dictysotelium on the uniform stiffness gels (top) or stiffness gradient gels (bottom, with greater stiffness towards the top). (C) Angular displacement of Dictysotelium on the uniform stiffness gels (top) or stiffness gradient gels (bottom, with greater stiffness towards the top). (D) y-FMI, velocity and migration persistence for Dictysotelium cultured on uniform substrate or gradient substrate (n≥30 tracks were analyzed for each experiment, N=3 independent experiments for each condition, replicates are biological). All error bars are SEM. ****p<0.0001, ns, not significant, by Student’s t-test. (E) y-FMI, velocity, and migration persistence of control (DMSO), blebbistatin (Bleb,10 μM, pre-treated for 10 min) treated and Rho activator II (Rho, 0.25 μg/mL, pre-treated for 2 hr) treated Dictysotelium moving on stiffness gradient gel (n≥30 tracks were analyzed for each experiment, N=3 independent experiments for each condition, replicates are biological). All error bars are SEM. ****p<0.0001, ns, not significant, by one-way ANOVA. (F) y-FMI, velocity and migration persistence of control (DMSO), nocodazole (Noco, 32 μM, pre-treated for 10 min) treated and taxol (70 nM, pre-treated for 10 min) treated Dictysotelium moving on stiffness gradient gel (n≥30 tracks were analyzed for each experiment, N=3 independent experiments for each condition, replicates are biological). All error bars are SEM. ****p<0.0001, ns, not significant, by one-way ANOVA.
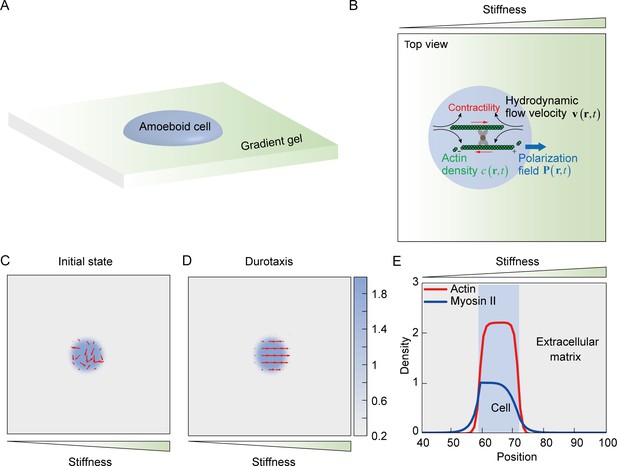
Mechanosensing active gel model of amoeboid durotaxis.
(A) Illustration of an amoeboid cell crawling on the polyacrylamide (PA) gel with stiffness gradient. (B) Details of the mechanosensing active gel model. The blue circle represented an amoeboid cell. The position of the blue circle was defined by actin density (green) with a given polarization field. To recapitulate cell migration, myosin contractility (red), and hydrodynamic flow velocity (black) were illustrated in the model. (C) Initial density and polarization field of actin. The background color indicated the density of actin both inside and outside of the cell border. The red arrows indicated the polarization field of actin. (D) Density and polarization field of actin during amoeboid durotaxis. The background color indicated the density of actin both inside and outside of the cell border. The red arrows indicated the polarization field of actin. (E) Spatial distribution of NMIIA (blue line) and actin (red line) along the direction of stiffness gradient. The cell position was marked with a blue rectangle.
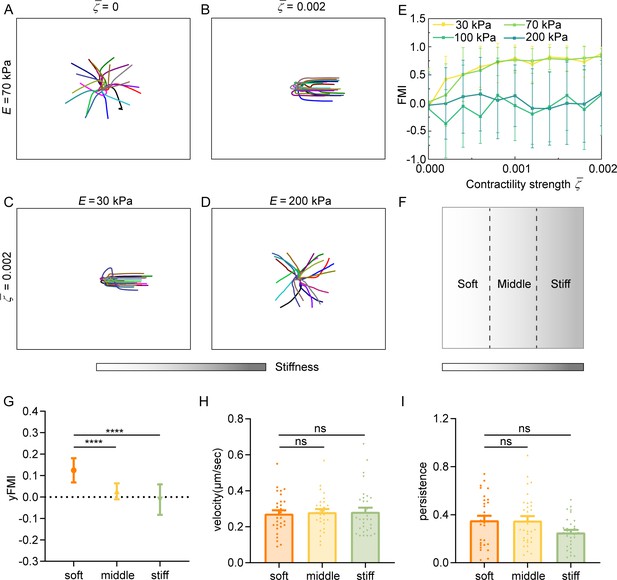
Regulation of amoeboid durotaxis by cell contractility and substrate stiffness.
(A) Trajectories of migrating amoeboid cells with no contractility (=0) on the middle region of gradient substrates, and the substrate stiffness of the initial cell centroid was set as E=70 kPa. (B) Trajectories of migrating amoeboid cells with normal contractility (=0.002) on middle region of gradient substrates, and the substrate stiffness of the initial cell centroid was set as E=70 kPa. (C) Trajectories of migrating amoeboid cells with normal contractility (=0.002) on soft region of gradient substrates, and the substrate stiffness of the initial cell centroid was set as E=30 kPa. (D) Trajectories of migrating amoeboid cells with normal contractility (=0.002) on stiff region of gradient substrates, and the substrate stiffness of the initial cell centroid was set as E=200 kPa. (E) The forward migration index (FMI) with different contractility strength and different initial substrate stiffness of cell centroid. (F) Illustration of different regions (soft, middle, and stiff) on gradient substrates with the greater stiffness towards the right. (G–I) yFMI, velocity, and migration persistence of CD4+ Naïve T cells migrating on different regions illustrated in F (n≥30 tracks were analyzed for each experiment, N=3 independent experiments for each condition, replicates are biological). All error bars are SEM. ****p<0.0001, ns, not significant, by one-way ANOVA.
Videos
Representative video of fast crawling CD4+ Naïve T cells on uniform gel in Figure 1B.
Color lines indicated the migration trajectories of a single cell. Scale bar: 50 μm.
Representative video of fast crawling CD4+ Naïve T cells on gradient gel in Figure 1B.
Color lines indicated the migration trajectories of single cell. Scale bar: 50 μm.
Representative video of fast crawling neutrophils on both uniform (first 60 frames) and gradient gel (last 60 frames) in Figure 1F.
Color lines indicated the migration trajectories of a single cell. Scale bar: 50 μm.
Representative video of fast crawling neutrophils on gradient gel in Figure 1—figure supplement 1D.
Color lines and numbers indicated the migration trajectories of a single cell. Scale bar: 50 μm.
Representative video of fast crawling dHL-60 cells on both uniform (first 60 frames) and gradient gel (last 60 frames) in Figure 1J.
Color lines indicated the migration trajectories of a single cell. Scale bar: 50 μm.
Representative video of dHL-60 cell stained with 0.1 μM SiR-Actin on 160 kPa substrate.
Left: live cell video of dHL-60 cell. Middle: raw video of left panel integrated with actin flow vectors. Right: heat map of actin flow distribution for the left panel. The video is captured at 15- s intervals for 10 min. Scale bar: 10 μm.
Representative video of dHL-60 cell stained with 0.1 μM SiR-Actin on 4 kPa substrate.
Left: live cell video of dHL-60 cell. Middle: raw video of left panel integrated with actin flow vectors. Right: heat map of actin flow distribution for left panel. The video is captured at 15- s intervals for 10 min. Scale bar: 10 μm.
Representative video of dHL-60 cell stained with 0.1 μM SiR-Actin on 40 kPa substrate.
Left: live cell video of dHL-60 cell. Middle: raw video of left panel integrated with actin flow vectors. Right: heat map of actin flow distribution for the left panel. The video is captured at 15- s intervals for 10 min. Scale bar: 10 μm.
Representative video of dHL-60 cell stained with 0.1 μM SiR-Actin on 75 kPa substrate.
Left: live cell video of dHL-60 cell. Middle: raw video of left panel integrated with actin flow vectors. Right: heat map of actin flow distribution for the left panel. The video is captured at 15- s intervals for 10 min. Scale bar: 10 μm.
Representative video of dHL-60 cell stained with 0.1 μM SiR-Actin on 120 kPa substrate.
Left: live cell video of dHL-60 cell. Middle: raw video of left panel integrated with actin flow vectors. Right: heat map of actin flow distribution for the left panel. The video is captured at 15- s intervals for 10 min. Scale bar: 10 μm.
Representative video of both soft (first 40 frames) and stiff parts (last 40 frames) of dHL-60 cell stained with 0.1 μM SiR-Actin on gradient gel in Figure 2B.
Left: live cell video of dHL-60 cell. Right: raw video of left panel integrated with actin flow vectors. The video is captured at 2- s intervals for 80 sec. Scale bar: 10 μm.
Representative video of fast crawling CD4+ Naïve T cells on gradient gel with 32 μM Nocodazole treatment in Figure 2E.
Scale bar: 50 μm.
Representative video of fast crawling CD4+ Naïve T cells on gradient gel with 70 nM Taxol treatment in Figure 2E.
Scale bar: 50 μm.
Representative video of fast crawling neutrophils on gradient gel with 10 μM blebbistatin treatment (first 60 frames) and 0.25 μg/mL Rho activator II treatment (last 60 frames) in Figure 2G.
Scale bar: 50 μm.
Representative video of both NMIIAKD (first 60 frames) and NMIIBKD dHL-60 cells (last 60 frames) migrating on gradient gel in Figure 3B.
Color lines indicated the migration trajectories of a single cell. Scale bar: 50 μm.
Representative video of a GFP-NMIIA dHL-60 cell migrating on gradient gel in Figure 3—figure supplement 2A.
Scale bar: 10 μm.
Representative video of fast crawling Dictyostelium cells on both uniform (first 60 frames) and gradient gel (last 60 frames) in Figure 4A.
Color lines indicated the migration trajectories of a single cell. Scale bar: 50 μm.
Representative video of amoeboid durotaxis active gel model.
The green arrows indicated the polarization field of actin. The background color indicated the density of myosin both inside and outside of the cell border. The white track indicated the migration trajectory of migrating amoeboid cells.
Additional files
-
Supplementary file 1
Parameters used in simulations.
- https://cdn.elifesciences.org/articles/96821/elife-96821-supp1-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/96821/elife-96821-mdarchecklist1-v1.docx





