Cortical beta oscillations map to shared brain networks modulated by dopamine
Figures

Beta activity is a widely distributed resting brain rhythm in invasive cortical recordings.
(A) Exemplar stereoelectroencephalography (sEEG) electrodes with dominant beta (teal; 20 Hz) or dominant alpha (purple; 9 Hz) rhythms, alongside the respective raw signal and parametrised power spectrum. (B) 1005/1772 channels showed higher maximum peak power in beta (13–30 Hz) than theta (4–8 Hz), alpha (8–12 Hz), and gamma (30–100 Hz). 397/1772 were alpha-dominant (theta and gamma not shown). (C) Beta-dominant channels were distributed across the entire cortex, from occipital to frontal regions, with highest density in sensorimotor areas like precentral gyrus, frontal middle gyrus, frontal inferior gyrus, and supplementary motor area. Alpha channels were largely concentrated posteriorly in temporoparietal and occipital areas based on the automatic anatomical labelling atlas (Rolls et al., 2020). Neither alpha nor beta activity showed systematic hemispheric differences (p>0.05).
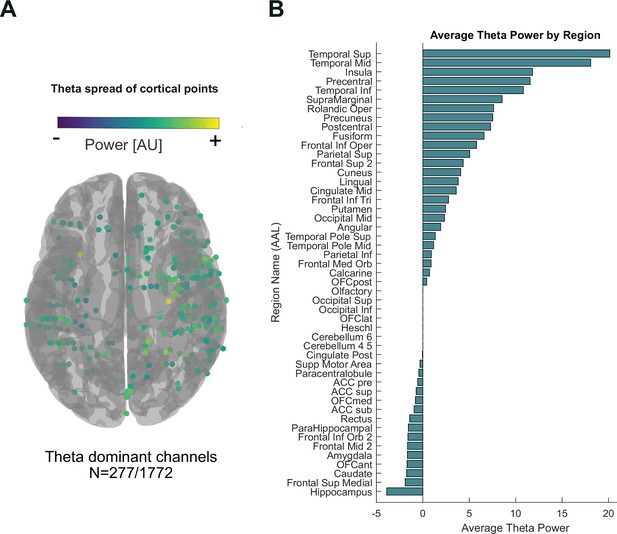
Cortical spread and amplitudes of theta peaks.
(A) 277/1772 channels showed higher maximum peak power in theta (4–8 Hz) than beta (13–30 Hz), alpha (8–12 Hz), and gamma (30–100 Hz). (B) Theta-dominant peaks were concentrated around the temporal regions; however, interestingly not in hippocampus. We suspect this is due to the sparse nature of electrodes placed in the region.

MRI connectomics reveal shared cortico-subcortical beta networks.
Individual network fingerprints (A) seeded from representative beta (top) and alpha (bottom)-dominant electrodes highlight the methodological approach and showcase the distinct connectivity patterns that arise in dependence of the connected brain regions (for raw signal traces from these locations, see Figure 1). Aggregated functional (B) and structural (C) connectivity maps across all beta (top) and alpha (bottom)-dominant electrodes were subjected to mass univariate t-tests comparing beta vs. alpha networks with Statistical Parametric Mapping software and visualised as T-maps with significant clusters identified using family-wise error correction (shown opaque). Channel locations from beta-dominant channels were associated with more robust functional and structural connectivity to frontal cortex and the basal ganglia compared to alpha channel locations, which were more connected to the occipital cortex.
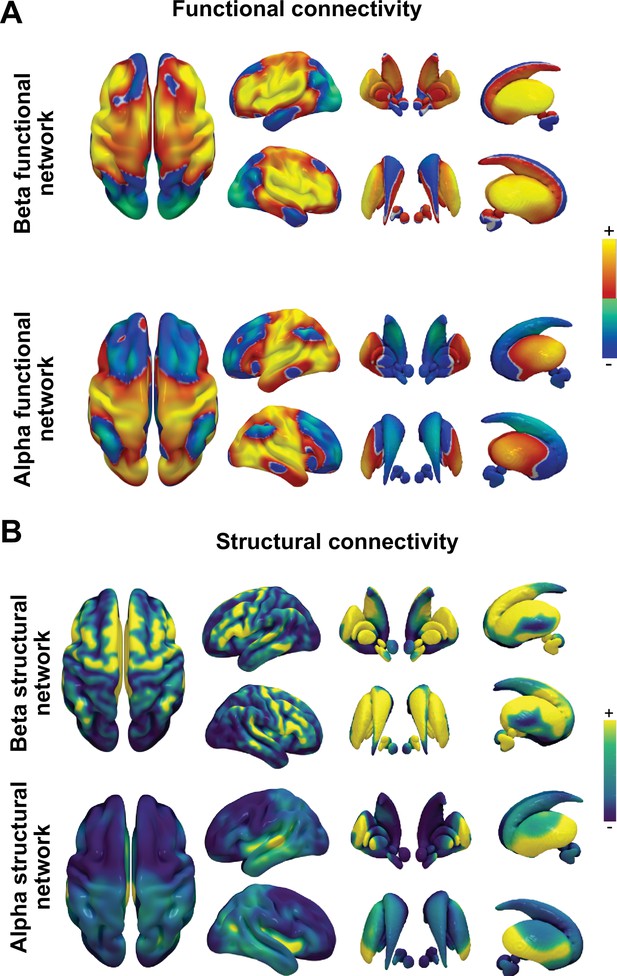
Unthresholded averages of functional and structural connectivity for alpha- and beta-dominant recording locations.
Functional (A) and structural (B) group averages without statistical comparison replicate the statistical differences, but show more relative overlap in some regions, for example, see higher alpha band activity in posterior basal ganglia nuclei.
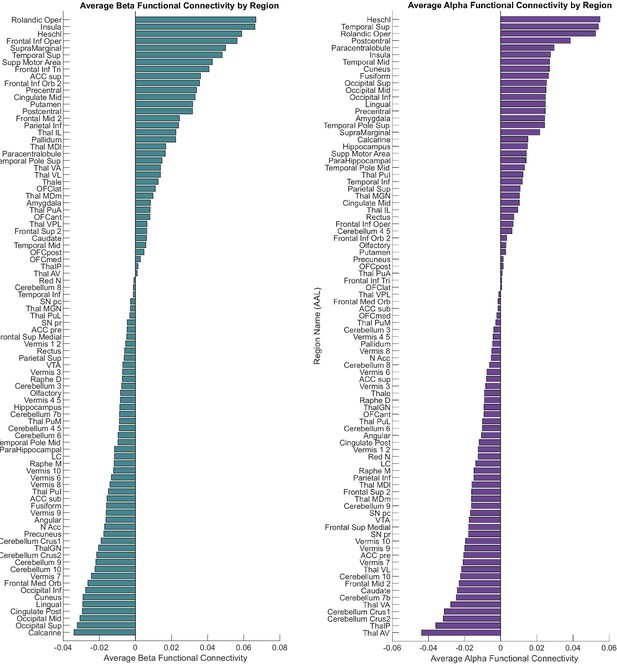
Region-wise bar plots of functional connectivity values for every parcel based on the automatic anatomical labelling (AAL) atlas.
For functional connectivity, the highest intensities for beta (teal) channels were in frontal, insula, cingulate, basal ganglia, and temporal regions, while for alpha (purple) the highest intensities were found in temporal and occipital regions.
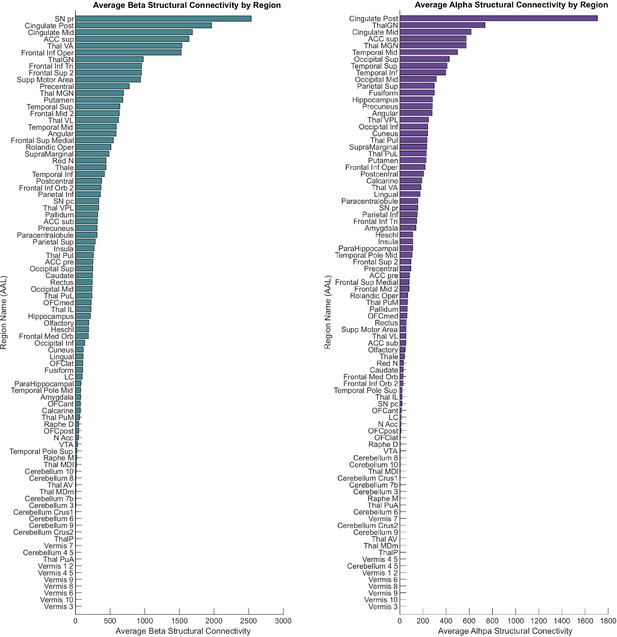
Region-wise bar plots of structural connectivity values for every parcel based on the automatic anatomical labelling (AAL) atlas.
For structural connectivity, the highest intensities for beta (teal) channels were in basal ganglia, cingulate, frontal, basal ganglia, and parietal regions, while for alpha (purple) the highest connectivity estimates were found in cingulate, temporal, and occipital regions.

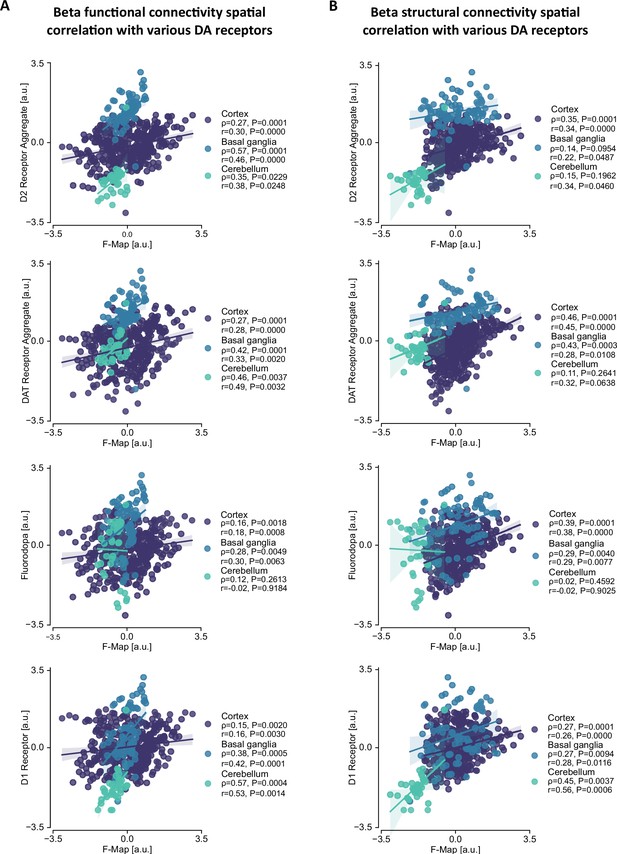
The connectomic beta network correlates with molecular markers of dopamine signalling.
Dopamine uptake significantly correlates with beta functional connectivity. (A) A method schematic explaining how correlations were calculated. Each point on the scatterplot represents a parcellation from the compound brain atlas. For every parcel Xi, a correlation is calculated for that parcel in the PET scan and in the beta network (B) in cortex (rho = 0.22, p=0.0001) and basal ganglia (rho = 0.5, p=0.0001) but not cerebellum (p>0.05) and beta structural connectivity (C) in cortex (rho = 0.4, p=0.0001) and basal ganglia (rho = 0.33, p=0.001) but not cerebellum (p>0.05). GABA was used as a control molecule which revealed no significant correlations with beta functional connectivity (D) in cortex, basal ganglia, or cerebellum (p>0.05). Beta structural connectivity (E) revealed weaker positive correlation with GABA in cortex (rho = 0.17, p=0.0003), a negative correlation in the basal ganglia (rho = −0.55, p=0.0001), and no correlation in cerebellum (p>0.05).
Tables
Spread of invasive electrophysiological channels.
Given the low number of channels exhibiting resting-state gamma activity, we excluded those from this table.
| Lobe | Channels/lobe | ECoG | sEEG | Sum theta channels | Sum alpha channels | Sum beta channels | |||
|---|---|---|---|---|---|---|---|---|---|
| Frontal | 616 | 122 | 494 | 73 | 11.80% | 38 | 6.20% | 458 | 73.40% |
| Temporal | 519 | 68 | 451 | 106 | 20.40% | 180 | 35.00% | 216 | 41.60% |
| Cingulate | 116 | 0 | 116 | 12 | 10.30% | 21 | 18.10% | 71 | 61.20% |
| Insula | 128 | 2 | 126 | 19 | 14.80% | 21 | 16.40% | 86 | 67.20% |
| Amygdala | 3 | 0 | 3 | 1 | 33.30% | 0 | 0% | 1 | 33.30% |
| Parietal | 272 | 52 | 220 | 50 | 18.40% | 73 | 26.80% | 139 | 51.10% |
| Occipital | 107 | 13 | 94 | 15 | 14.00% | 62 | 57.90% | 27 | 25.20% |
| Subcortex | 11 | 1 | 10 | 1 | 9.10% | 2 | 18.20% | 7 | 63.60% |
-
ECoG, electrocorticography; sEEG, stereoelectroencephalography.
-
Table 1—source data 1
sEEG channels (high and low beta calculated separately to calculate total beta peaks).
- https://cdn.elifesciences.org/articles/97184/elife-97184-table1-data1-v1.xlsx
-
Table 1—source data 2
ECoG channels.
Lobes with only zero values removed (high and low beta calculated separately to calculate total beta peaks).
- https://cdn.elifesciences.org/articles/97184/elife-97184-table1-data2-v1.xlsx