Neuropeptide Bursicon and its receptor-mediated the transition from summer-form to winter-form of Cacopsylla chinensis
Figures
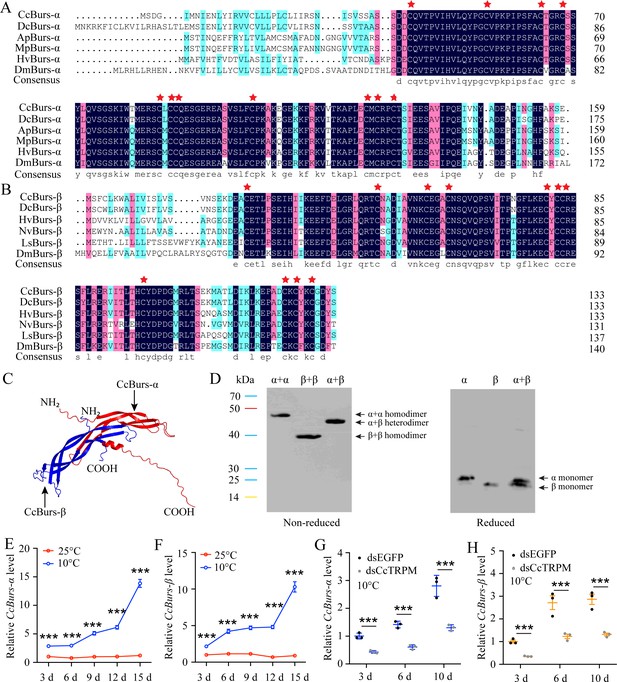
Molecular characteristic of CcBurs-α and CcBurs-β in C. chinensis.
(A) Multiple alignments of the amino acid sequences of CcBurs-α with homologs from five other insect species. Black represents 100% identity, red represents 75% identity, and blue represents <75% identity. CcBurs-α (C. chinensis, OR488624), DcBurs-α (Diaphorina citri, XP_008468249.2), ApBurs-α (Acyrthosiphon pisum, XP_001946341.1), MpBurs-α (Myzus persicae, XP_022171710.1), HvBurs-α (Homalodisca vitripennis, XP_046670477.1), DmBurs-α (Drosophila melanogaster, CAH74223.1). The corresponding GenBank accession numbers are as follows. (B) Multiple alignments of the amino acid sequences of CcBurs-β with homologs from five other insect species. Black represents 100% identity, red represents 75% identity, and blue represents <75% identity. CcBurs-β (C. chinensis, OR488625), DcBurs-β (D. citri, AWT50591.1), HvBurs-β (H. vitripennis, XP_046671521.1), NvBurs-β (Nezara viridula, AZC86173.1), LsBurs-β (Laodelphax striatellus, AXF48186.1), DmBurs-β (D. melanogaster, CAH74224.1). The corresponding GenBank accession numbers are as follows. (C) Predicted protein tertiary structures of CcBurs-α and CcBurs-β. (D) Western blot analysis of Bursicon proteins using anti-His-Tag antibody with non-reduced and reduced SDS-PAGE. The left numbers indicate the positions of pre-stained protein markers. Lanes of α, β, and α+β represent separate expression of CcBurs-α, CcBurs-β, or co-expressed of α+β. Monomers were not present under non-reduced conditions. (E-F) Relative mRNA expression of CcBurs-α and CcBurs-β after 25 °C or 10 °C treatments at 3, 6, 9, 12, and 15 days (n=3). (G-H) Effect of temperature receptor CcTRPM knockdown on the mRNA expression of CcBurs-α and CcBurs-β at 3, 6, and 10 days under 10 °C condition (n=3). Data in 1E-1H are shown as the mean ± SE with three independent biological replications, with at least 50 nymphs for each biological replication. Statistically significant differences were determined using pair-wise Student’s t-test in SPSS 26.0 software, and significance levels were denoted by ***p<0.001.
-
Figure 1—source data 1
Labelled file for the western blot analysis in Figure 1D (reduced gel).
- https://cdn.elifesciences.org/articles/97298/elife-97298-fig1-data1-v1.tif
-
Figure 1—source data 2
Original, uncropped file for the western blot analysis in Figure 1D (reduced gel).
- https://cdn.elifesciences.org/articles/97298/elife-97298-fig1-data2-v1.tif
-
Figure 1—source data 3
Labelled file for the western blot analysis in Figure 1D (non-reduced gel).
- https://cdn.elifesciences.org/articles/97298/elife-97298-fig1-data3-v1.tif
-
Figure 1—source data 4
Original, uncropped file for the western blot analysis in Figure 1D (non-reduced gel).
- https://cdn.elifesciences.org/articles/97298/elife-97298-fig1-data4-v1.tif
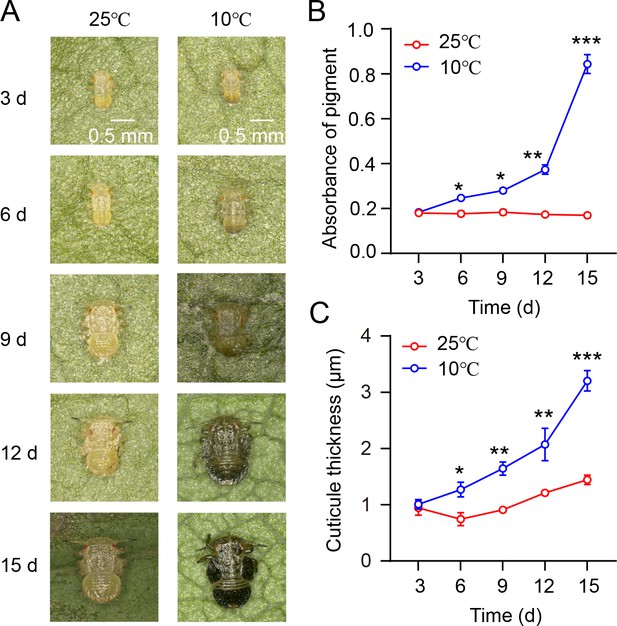
Investigation of the relationship between nymph phenotype, cuticle pigment absorbance, and cuticle thickness during the transition from summer-form to winter-form in C. chinensis.
(A) The nymph morphology during the transition from summer form to winter form of C. chinensis at varying time intervals (3, 6, 9, 12, 15 days). (B–C) The absorbance of total cuticle pigment and cuticle thickness of nymphs in C. chinensis under different temperature conditions.
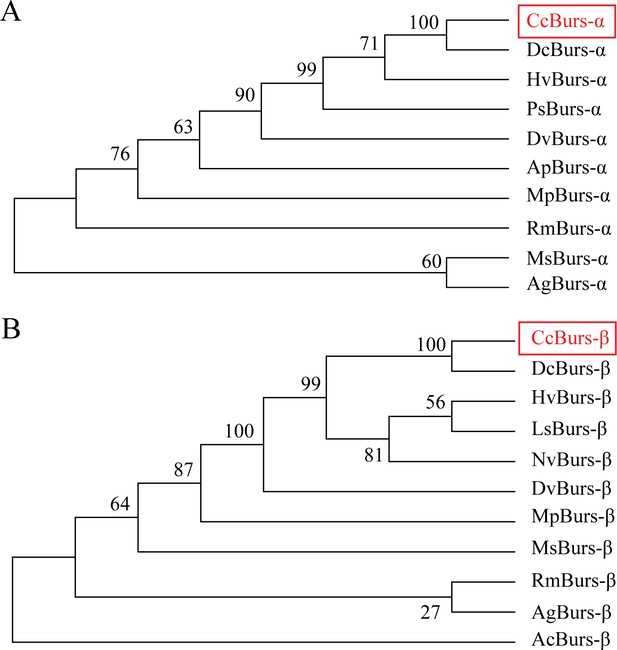
Phylogenetic tree analysis of CcBurs-α and CcBurs-β with its homologs in other insect species.
HvBurs-α (Homalodisca vitripennis, XP_046670477.1), PsBurs-α (Phenacoccus solenopsis, QEY08365.1), DvBurs-α (Daktulosphaira vitifoliae, XP_050533746.1), RmBurs-α (Rhopalosiphum maidis, XP_026815117.1), MsBurs-α (Melanaphis sacchari, XP_025201519.1), AgBurs-α (Aphis gossypii, XP_027848520.1). DvBurs-β (D. vitifoliae, XP_050533747.1), MpBurs-β (Myzus persicae, XP_022171709.1), MsBurs-β (M. sacchari, XP_025201526.1), RmBurs-β (R. maidis, XP_026815130.1), AgBurs-β (A. gossypii, XP_027848521.1), AcBurs-β (Adelges cooleyi, XP_050428069.1).
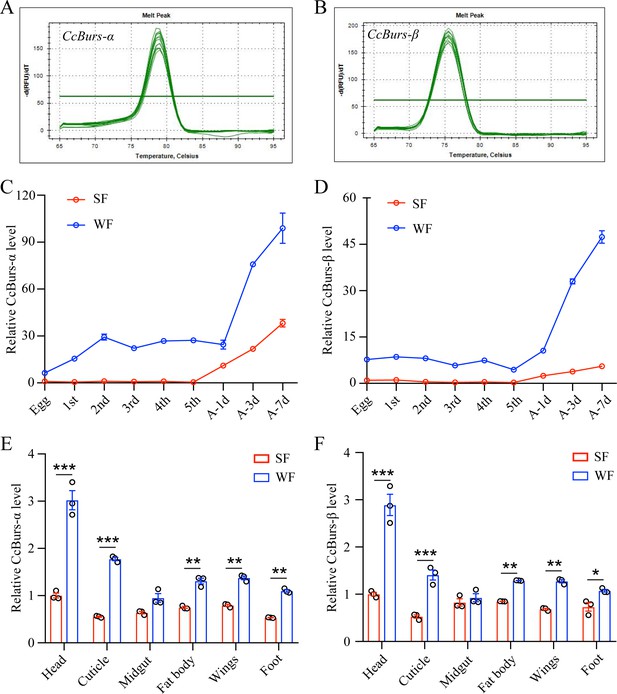
Spatio-temporal expression patterns of CcBurs-α and CcBurs-β.
(A–B) Melting curve for qRT-PCR primers of CcBurs-α and CcBurs-β. (C-D) The relative mRNA expression of CcBurs-α and CcBurs-β at different development ages of summer-form and winter-form by qRT-PCR (n=3). First, second, third, fourth, and fifth are the nymphs at the first, second, third, fourth, and fifth instar, separately. A-1d, A-3d, and A-7d are the adults on the first day, third day, and seventh day, respectively. (E-F) Tissue expression patterns of CcBurs-α and CcBurs-β in both summer-form and winter-form by qRT-PCR (n=3). The data in S1C-S1F are shown as the mean ± SEM with three independent biological replications of at least 50 nymphs for each biological replication. Statistically significant differences were determined with the pair-wise Student’s t-test in SPSS 20.0 software, and significance levels were denoted by *p<0.05, **p<0.01, and ***p<0.001.
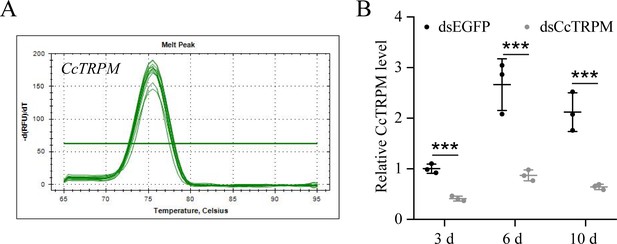
RNAi efficiency of CcTRPM after dsRNA treatment at 3, 6, and 10 days by qRT-PCR under 10 °C condition (n=3).
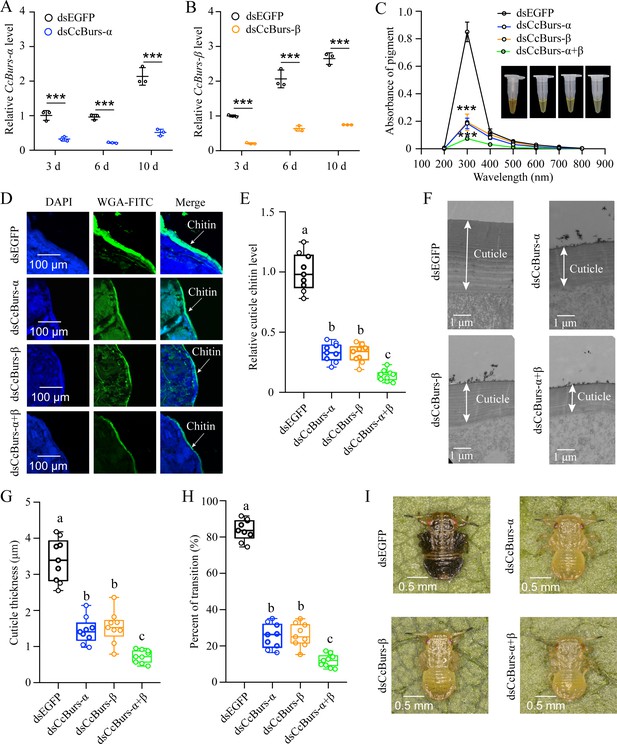
Neuropeptide Bursicon was essential for the transition from summer-form to winter-form in C. chinensis.
(A-B) RNAi efficiency of CcBurs-α and CcBurs-β after dsRNA treatment at 3, 6, and 10 days by qRT-PCR under 10 °C condition (n=3). (C-I) Effect of RNAi-mediated knockdown of CcBurs-α and CcBurs-β on absorbance of total cuticle pigment, relative cuticle chitin content, cuticle thickness of the thorax, transition percent, and phenotypic changes of first instar nymphs compared to dsEGFP treatments (n=9). Data in 2 A and 2B are shown as the mean ± SE with three independent biological replications, with at least 50 nymphs for each replication. Data in 2 C, 2E, and 2 G are presented as mean ± SE with three biological replications, with three technical replications for each biological replication. Data in 2 H are presented as mean ± SE with nine biological replications. Statistically significant differences were determined using pair-wise Student’s t-test, and significance levels were denoted by ***p<0.001. Different letters above the bars indicate statistically significant differences (p<0.05), as determined by ANOVA followed by a Turkey’s HSD multiple comparison test in SPSS 26.0 software.
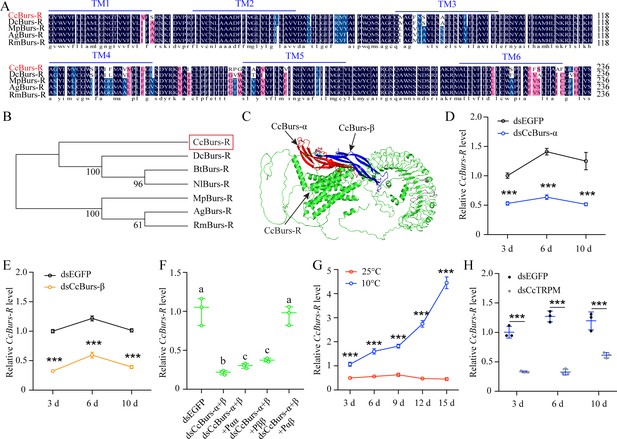
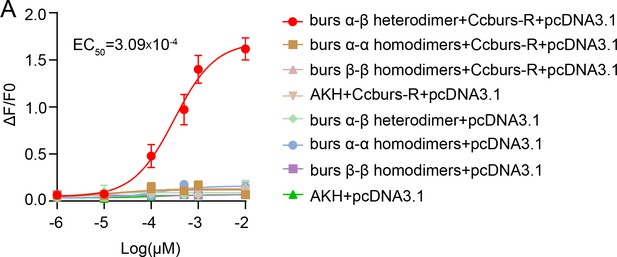
CcBurs-R was identified as the Bursicon receptor in C. chinensis.
(A) Multiple alignments of the amino acid sequences of the CcBurs-R transmembrane domain with homologs from four other insect species. The transmembrane domain from TM1 to TM6 is indicated by blue horizontal lines. CcBurs-R (C. chinensis, OR488626), DcBurs-R (D. citri, KAI5703609.1), MpBurs-R (M. persicae, XP_022172830.1), AgBurs-R (Aphis gossypii, XP_027844917.2), RmBurs-R (Rhopalosiphum maidis, XP_026817427.1). The corresponding GenBank accession number is as follows. (B) Phylogenetic tree analysis of CcBurs-R with its homologs in six other insect species.BtBurs-R (Bemisia tabaci, XP_018898471.1), NlBurs-R (N. lugens, XP_022198758.2). (C) Predicted protein tertiary structure of CcBurs-R and its binding with CcBurs-α and CcBurs-β. (D-E) Effect of CcBurs-α and CcBurs-β knockdown on the mRNA expression of CcBurs-R at 3, 6, and 10 d, respectively (n=3). (F) CcBurs-α+β heterodimer protein could rescue the CcBurs-R expression after knockdown of CcBurs-α and CcBurs-β together. (G) Relative mRNA expression of CcBurs-R after 25 °C or 10 °C treatment at 3, 6, 9, 12, and 15 days (n=3). (H) Effect of temperature receptor CcTRPM knockdown on the mRNA expression of CcBurs-R at 3, 6, and 10 days (n=3).Data in 3D-3H are shown as the mean ± SE with three independent biological replications, with at least 50 nymphs for each replication. Statistically significant differences were determined using pair-wise Student’s t-test in SPSS 26.0 software, and significance levels were denoted by ***p<0.001. Different letters above the bars indicated statistically significant differences (p<0.05), as determined by ANOVA followed by a Turkey’s HSD multiple comparison test in SPSS 26.0 software.
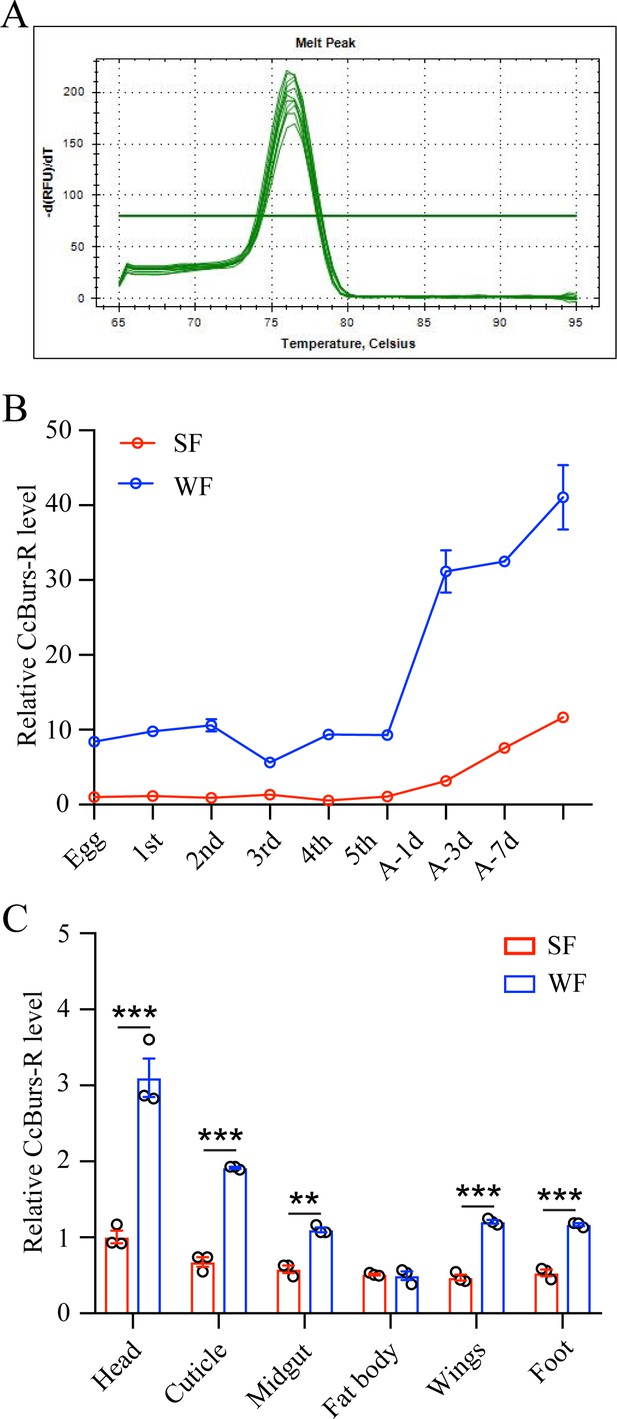
Spatio-temporal expression patterns of CcBurs-R in both summer-form and winter-form by qRT-PCR (n=3).
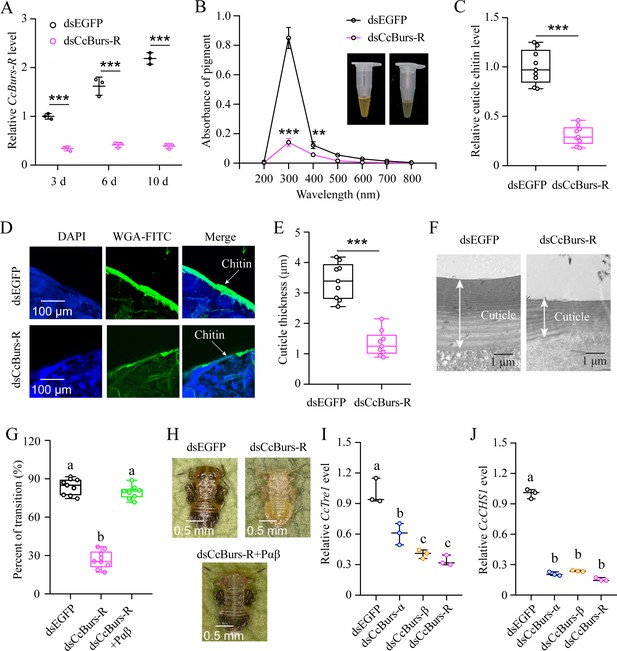
CcBurs-R directly mediated the transition from summer-form to winter-form in C. chinensis. (A) RNAi efficiency of CcBurs-R after dsRNA treatment at 3, 6, and 10 days by qRT-PCR under 10 °C condition (n=3). (B-H) Effect of RNAi-mediated knockdown of CcBurs-R on the absorbance of total cuticle pigment, relative cuticle chitin content, cuticle thickness of the thorax, transition percent, and phenotypic changes of first instar nymphs compared to dsEGFP treatments under 10 °C condition (n=9). (I-J) Relative mRNA expression of CcTre1 and CcCHS1 afterknockdown of CcBurs-α,CcBurs-β, and CcBurs-R at 10 d, separately (n=3). Data in 4 A, 4I, and 4 J are shown as the mean ± SE with three independent biological replications, with at least 50 nymphs for each replication. Data in 4B, 4 C, and 4E are presented as mean ± SE with three biological replications, with three technical replications for each biological replication. Data in 4 G are presented as mean ± SE with nine biological replications. Statistically significant differences were determined using pair-wise Student’s t-test, and significance levels were denoted by **p<0.01 and ***p<0.001. Different letters above the bars indicate statistically significant differences (p<0.05), as determined by ANOVA followed by a Turkey’s HSD multiple comparison test in SPSS 26.0 software.
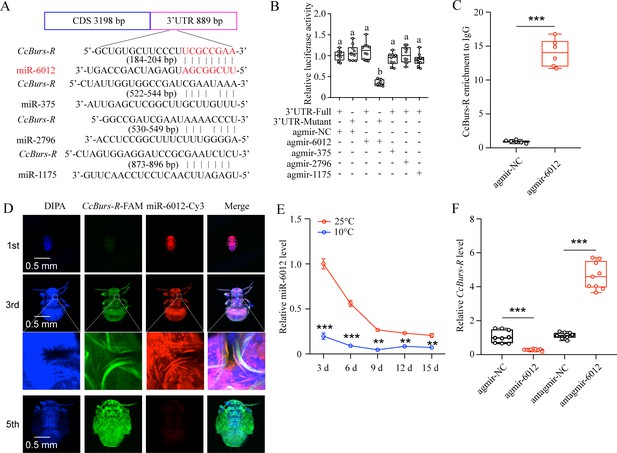
miR-6012 directly targeted CcBurs-R to inhibit its expression.
(A) Predicted binding sites of four miRNAs in the 3’UTR of CcBurs-R. (B) In vitro confirmation of the target relationship between miR-6012 and CcBurs-R using dual luciferase reporter assays. (C) In vivo validation of miR-6012 directly targeting CcBurs-R using RNA-binding protein immunoprecipitation (RIP) assay. (D) Co-localization of miR-6012 and CcBurs-R in different development stages of C. chinensis using FISH. (E) Effect of different temperature treatments on the expression of miR-6012 by qRT-PCR. (F) Effect of miR-6012 agomir and antagomir treatments on the mRNA level of CcBurs-R at 6 days under 10 °C conditions. Data in 5B and 5 F are presented as the mean ± SE with nine biological replicates. Results of 5 C and 5E are indicated as the mean ± SE with six or three biological replicates. Statistically significant differences were determined using pair-wise Student’s t-test, and significance levels were denoted by **p<0.01 and ***p<0.001. Different letters above the bars represent statistically significant differences (p<0.05), as determined by ANOVA followed by a Turkey’s HSD multiple comparison test in SPSS 26.0 software.
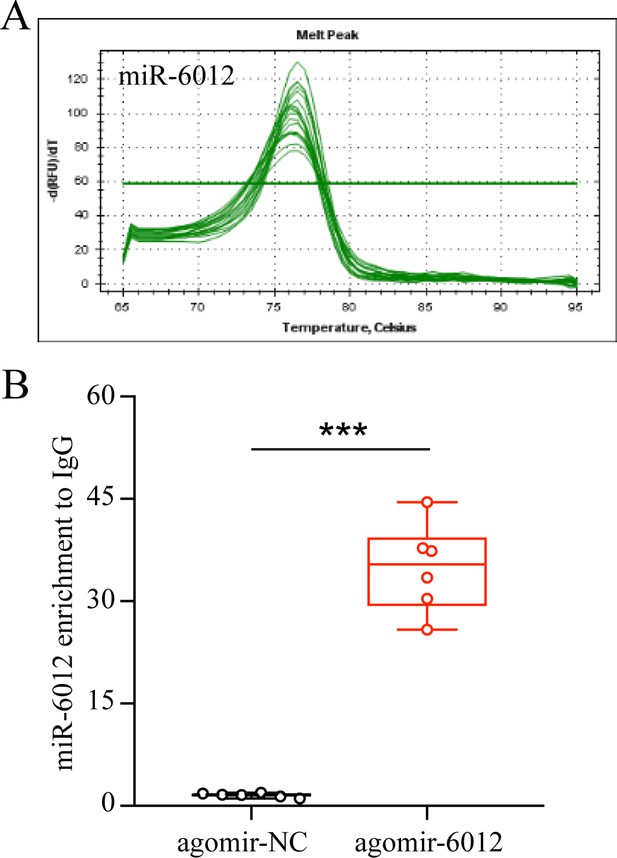
Enrichment of miR-6012 by antibody against Ago1 in agomir-6012 treated group compared with agomir-NC group.
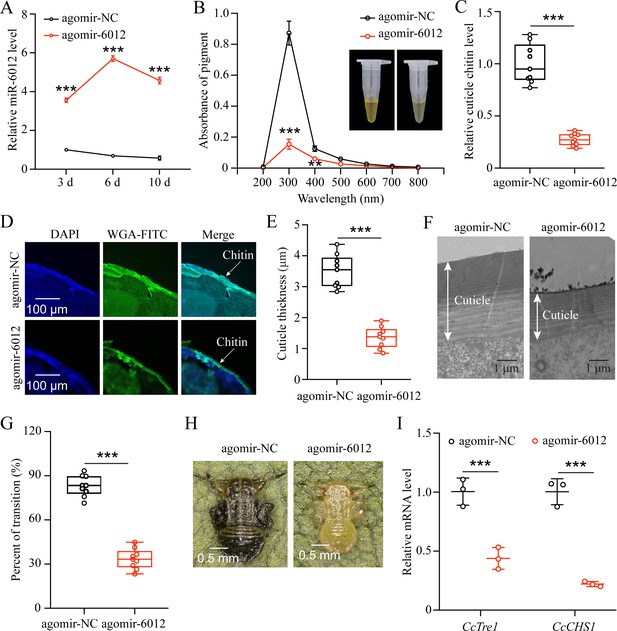
miR-6012 targeted CcBurs-R to mediate the seasonal polyphenism in C. chinensis.
(A) Expression of miR-6012 after agomir-6012 treatment at 3, 6, and 10 days by qRT-PCR under 10 °C condition (n=3). (B-H) Effect of agomir-6012 treatment on absorbance of total cuticle pigment, relative cuticle chitin content, cuticle thickness of the thorax, transition percent, and phenotypic changes of first instar nymphs compared to agomir-NC treatments under 10 °C condition (n=9). (I) Relative mRNA expression of CcTre1 and CcCHS1 after agomir-6012 treatment at 6 days, separately (n=3). Data in 6 A and 6I are shown as the mean ± SE with three independent biological replications, with at least 50 nymphs for each replication. Data in 6 C and 6E are presented as mean ± SE with three biological replications of three technical replications for each biological replication. Data in 6B and 6 G are presented as mean ± SE with nine biological replications. Statistically significant differences were determined using pair-wise Student’s t-test, and significance levels were denoted by **p<0.01 and ***p<0.001.
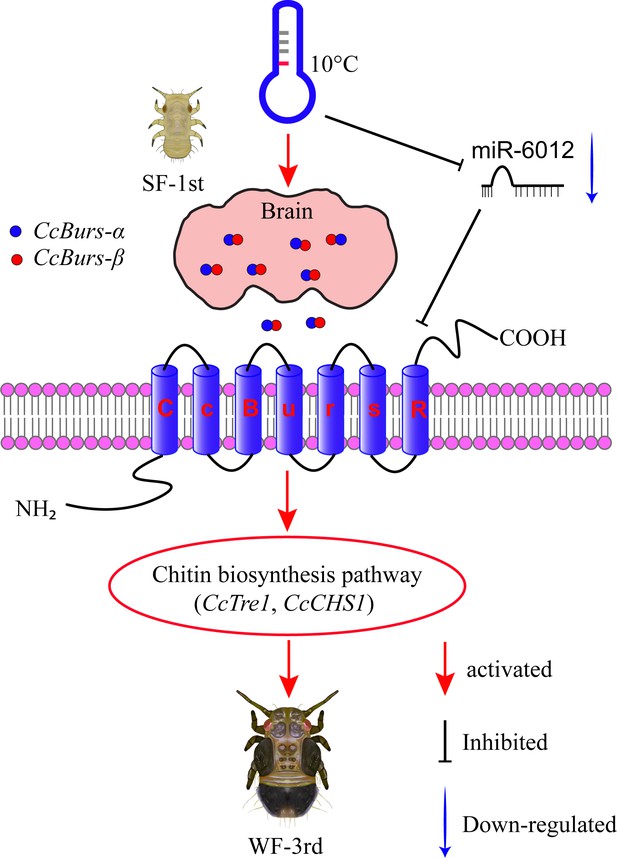
Schematic model of the novel functions of Bursicon signaling in the seasonal polyphenism of C. chinensis in response to low temperature.
Under 10 °C conditions, low temperature significantly upregulated the expression of the Bursicon signaling pathway. CcBurs-α and CcBurs-β then formed a heterodimeric neuropeptide to activate their receptor CcBurs-R, which mediated the transition from summer-form to winter-form in C. chinensis by acting on the chitin biosynthesis pathway. Furthermore, low temperature inhibited the expression of miR-6012, relieving its inhibitory effects on CcBurs-R. miR-6012 directly targeted CcBurs-R, contributing to the novel function of Bursicon signaling in seasonal polyphenism. Finally, the first instar nymphs of summer-form developed into third instar nymphs of winter-form in C. chinensis.
Additional files
-
Supplementary file 1
The primers used in current study and comparison of pigmentation and cuticle thickness after genes knockdown.
(a) List of primers used in this study. (b) Comparison of pigmentation and cuticle thickness after CcTRPM, CcBurs-a, CcBurs-β, and CcBurs-R knockdown.
- https://cdn.elifesciences.org/articles/97298/elife-97298-supp1-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/97298/elife-97298-mdarchecklist1-v1.pdf