Mechano-regulation of GLP-1 production by Piezo1 in intestinal L cells
eLife Assessment
This study focuses on the regulation of GLP-1 in enteroendocrine L cells and how this may be stimulated by the mechanogated ion channel Piezo1 and the CaMKKbeta-CaMKIV-mTORC1 signaling pathway. The work is innovative and is considered valuable, as the hypothesis that is being tested may have significant mechanistic and translational implications. Data to support the proposed mechanism were considered incomplete, yet data to support the overall physiological characterization were considered solid.
https://doi.org/10.7554/eLife.97854.3.sa0Valuable: Findings that have theoretical or practical implications for a subfield
- Landmark
- Fundamental
- Important
- Valuable
- Useful
Solid: Methods, data and analyses broadly support the claims with only minor weaknesses
- Exceptional
- Compelling
- Convincing
- Solid
- Incomplete
- Inadequate
During the peer-review process the editor and reviewers write an eLife Assessment that summarises the significance of the findings reported in the article (on a scale ranging from landmark to useful) and the strength of the evidence (on a scale ranging from exceptional to inadequate). Learn more about eLife Assessments
Abstract
Glucagon-like peptide 1 (GLP-1) is a gut-derived hormone secreted by intestinal L cells and vital for postprandial glycemic control. As open-type enteroendocrine cells, whether L cells can sense mechanical stimuli caused by chyme and thus regulate GLP-1 synthesis and secretion is unexplored. Molecular biology techniques revealed the expression of Piezo1 in intestinal L cells. Its level varied in different energy status and correlates with blood glucose and GLP-1 levels. Mice with L cell-specific loss of Piezo1 (Piezo1 IntL-CKO) exhibited impaired glucose tolerance, increased body weight, reduced GLP-1 production and decreased CaMKKβ/CaMKIV-mTORC1 signaling pathway under normal chow diet or high-fat diet. Activation of the intestinal Piezo1 by its agonist Yoda1 or intestinal bead implantation increased the synthesis and secretion of GLP-1, thus alleviated glucose intolerance in diet-induced-diabetic mice. Overexpression of Piezo1, Yoda1 treatment or stretching stimulated GLP-1 production and CaMKKβ/CaMKIV-mTORC1 signaling pathway, which could be abolished by knockdown or blockage of Piezo1 in primary cultured mouse L cells and STC-1 cells. These experimental results suggest a previously unknown regulatory mechanism for GLP-1 production in L cells, which could offer new insights into diabetes treatments.
Introduction
The gastrointestinal (GI) tract represents the largest endocrine organ in the human body. The enteroendocrine cells (EECs) located throughout the GI tract secrete a large number of gastrointestinal hormones to regulate a variety of physiological processes and are key regulators for energy homeostasis (Bany Bakar et al., 2023). GLP-1 is one of the gut-derived peptide hormones essential for postprandial glycemic control (Song et al., 2019). It is produced from Proglucagon (Gcg) by proprotein convertase in the intestinal L cells, a group EECs predominantly situated in the distal gut (Drucker, 2006; Rouillé et al., 1997). The circulating GLP-1 levels rapidly increase after meal and reduce postprandial blood glucose fluctuations by augmenting insulin secretion, suppressing glucagon secretion and slowing gastric emptying (Drucker, 2006; Willms et al., 1996). Nowadays, GLP-1-based therapy is well-recognized and commonly used in treatment of type 2 diabetes mellitus (T2DM; Saxena et al., 2021; Tan et al., 2022). Elucidation of the mechanism that regulates GLP-1 production is essential for the development of new drug targets for the treatment of diabetes.
EECs can be divided into two categories according to their morphology: open type and closed type. The open type EECs possess microvilli protruding into the gut lumen and have direct contact with the luminal contents. In contrast, the closed type EECs are located basolaterally without direct contact with the lumen (Gribble and Reimann, 2016). Both types of EECs synthesize and store peptides or hormones in secretory granules and release them by exocytosis at the basolateral membrane (Atanga et al., 2023). As open-type EECs, L cells received both chemical and mechanical signals from the luminal contents, and neural signals from the nerves (Furness et al., 2013). It has been well-documented that nutrients such as glucose, lipids, and amino acids in the intestinal lumen can stimulate the secretion of GLP-1 from L cells (Diakogiannaki et al., 2012). GLP-1 secretion can also be stimulated by intrinsic cholinergic nerves (Anini et al., 2002; Drucker, 2006). However, whether and how L cells coordinate mechanical stimuli from intestinal lumen to regulate GLP-1 production remain poorly understood.
Piezo channels, including Piezo1 and Piezo2 have recently been identified as mechanosensitive ion channels involved in the sensation of multiple mechanical stimuli, such as shear stress, pressure, and stretch (Gudipaty et al., 2017; Li et al., 2014; Romac et al., 2018). They allow the influx of cations such as Ca2+ and Na+ in response to mechanical tension and converts mechanical stimuli into various electrical and chemical signals. Piezo1 plays a crucial role in blood pressure regulation, red blood cell volume regulation, bone homeostasis, pulmonary and cardiac functions (Cahalan et al., 2015; Lai et al., 2022; Wang et al., 2023; Wang et al., 2016). Previous studies have reported that Piezo1 is expressed in the intestinal epithelium, regulating gut peristalsis, barrier function, mucus secretion, and inflammation (Jiang et al., 2021; Liu et al., 2022a; Sugisawa et al., 2020; Xu et al., 2021). Interestingly, accumulating evidence demonstrates the regulation of insulin and ghrelin secretion by Piezo1 (Deivasikamani et al., 2019; Ye et al., 2022; Zhao et al., 2024). Recent studies have also reported that Piezo2 is expressed in a population of EECs and convert force into serotonin release (Alcaino et al., 2018; Treichel et al., 2022). These findings suggest a critical role of Piezo channels in the mechano-regulation of hormone production. However, whether Piezo channels are expressed L cells and play a role in GLP-1 production remain unknown.
The current study has shown that Piezo1 channels on intestinal L cells mediate mechanosensing of intestinal contents and regulate glucose homeostasis by triggering GLP-1 synthesis and secretion via the CaMKKβ/CaMKIV-mTORC1 signaling pathway. This finding provides new insights into the treatment of T2DM and lays a theoretical foundation for the development of antidiabetic drugs targeting Piezo1.
Results
Assessment of Piezo1 in human and mouse intestine in different energy status
Piezo1 mRNA was found to be highly expressed in both mouse ileal mucosa and STC-1 cells (Figure 1—figure supplement 1A). Moreover, Piezo1 was co-localized with GLP-1 in immunofluorescent staining on NCD fed mouse ileal sections, indicating its expression in L cells (Figure 1—figure supplement 1B). Interestingly, increased body weight and impaired glucose tolerance were observed in high-fat diet-induced diabetic mice, while Piezo1 and Proglucagon expression levels in the ileal mucosa of diabetic mice were significantly lower than that in mice feed with normal chow diet (Figure 1—figure supplement 1C–F). Moreover, ileal mucosal Piezo1 mRNA levels were positively correlated with Gcg mRNA levels (Figure 1—figure supplement 1G), but negatively correlated with the AUC of glucose tolerance test (Figure 1—figure supplement 1H). Obese T2DM patients who underwent Roux-en-Y gastric bypass (RYGB) surgery showed decreased BMI (Figure 1—figure supplement 1I) and increased Piezo1 and GLP-1 in ileal mucosa (Figure 1—figure supplement 1J, K) compared to that before surgery. These findings indicated that Piezo1 is expressed in intestinal L cells and its level varies in different energy status.
Generation and characterization of Piezo1 IntL-CKO mice
To investigate the potential role of Piezo1 in GLP-1 production, we tried to knockout Piezo1 in L cells by Cre-loxP system driven by an L cell-specific promoter. Proglucagon (encoded by Gcg gene) is mainly expressed in both L cells and pancreatic α cells (Jin, 2008). Villin-1 (encoded by Vil1 gene) is expressed in gastrointestinal epithelium, including L cells, but not in pancreatic α cells (Maunoury et al., 1992; Rutlin et al., 2020). Since neither Gcg nor Villin are specific markers for L cells, we tried to generate a new line of mice enabling loss of Piezo1 expression specifically in the intestine L cell by combination of FLP-Frt and Cre-loxP system. We inserted a Flippase (FLP) expression cassette in the 3’UTR of Vil1 to generate a Vil1 promoter-driven FLP mice (Vil1FLP; Figure 1A). Then, we generated Flippase-dependent Gcg promoter driven-Cre (GcgCre) mice by inserting an Frt-flanked Cre expression cassette in reverse orientation within the 3’- UTR of Gcg gene (Figure 1A). We further crossed the Vil1FLP mice with GcgCre mice to obtain L-cell-specific Cre mice (Vil1FLP::GcgfrtCre), in which Vil1 promoter-driven Flippase flipped the reverse Cre cassette into a correct orientation in Villin-positive cells (including L cells, but not pancreatic α cells), and thus Cre can only be expressed under the Gcg promoter in L cells. The genotypes of the Vil1FLP, GcgCre and Vil1FLP::GcgfrtCre mice were identified by PCR with specific primers (Figure 1B). The flipping of the reverse Cre cassette was validated by PCR, which confirmed that the flipping only occurred the intestine, but not in the pancreas (Figure 1C). To confirm the cell type specificity of Cre activity, we crossed Vil1FLP::GcgfrtCre mice to Rosa26mT/mG reporter mice. All tissues and cells of Rosa26mT/mG mice express red fluorescence (membrane-targeted tdTomato; mT) at baseline, and switch to membrane-targeted EGFP in the presence of cell-specific Cre (Figure 1D). EGFP expression was only observed scatteredly in the intestine, but not in the pancreas, indicating the intestine-specific Cre activity in the Vil1FLP::GcgfrtCre mice (Figure 1E). Finally, we bred Vil1FLP::GcgfrtCre mice with Piezo1loxp/loxp mice to generate Piezo1 IntL-CKO mice (Figure 1F).
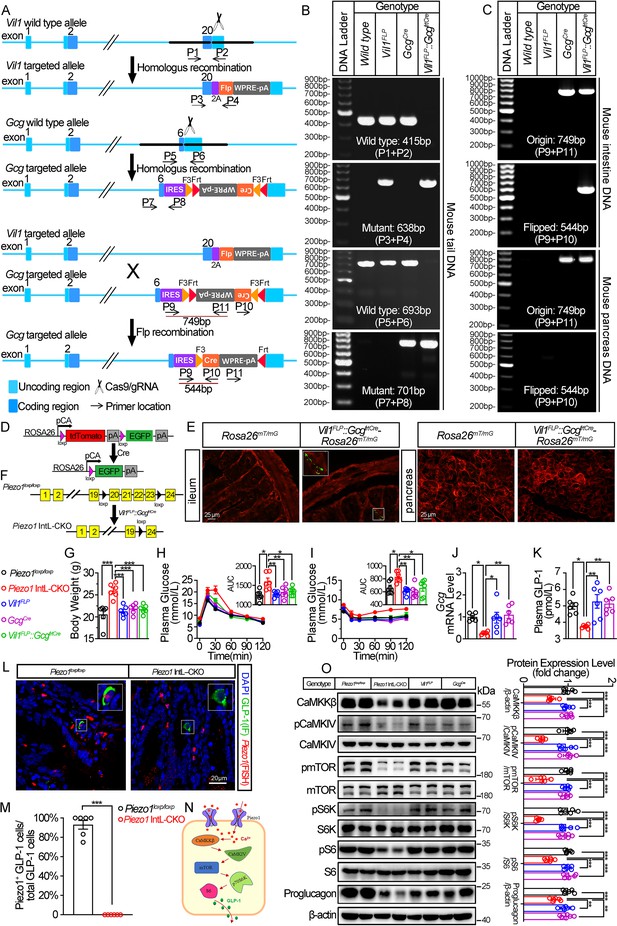
Generation, validation, and characterization of Piezo1 IntL-CKO mice.
(A) Schematic description for the generation of Vil1FLP and Flippase-dependent GcgCre mice. Vil1FLP flip the inverted Cre gene in the GcgCre cassette in Vil1FLP::GcgfrtCre mice to restrict Cre expression in intestinal L cells. As shown, locations of genotyping primers are also indicated. (B) Tail DNA genotyping PCR results using genotyping primer for Vil1FLP, GcgCre and Flippase-activated Cre (Vil1FLP::GcgfrtCre) mice. (C) Intestine and pancreas DNA genotyping results. The ‘Original’ band represents the original GcgCre cassette with inverted Cre, while the ‘Flipped’ band represents recombined GcgCre cassette with Cre flipped into the correct direction. (D) Schematic description for the validation of Vil1FLP::GcgfrtCre efficacy by crossing with Rosa26mT/mG reporter mice. (E) Fluorescence was detected in the ileal and pancreatic tissues from Rosa26mT/mG and Vil1FLP::GcgfrtCre-Rosa26mT/mG mice by frozen tissue confocal microscopy. Green fluorescence represents successful deletion of TdTomato and reactivation of EGFP in the Cre-expressing cells. (F) Schematic description for the generation of Intestinal L cell-Piezo1-/- mice (Piezo1 IntL-CKO) by crossing Piezo1loxp/loxp mice with Vil1FLP::GcgfrtCre mice. (G) Body weight of 14- to 16-week-old male mice of the indicated genotypes fed with NCD (n=6/group). (H, I) IPGTT (H) and ITT (I) and associated area under the curve (AUC) values of 14- to 16-week-old male mice of the indicated genotypes fed with NCD (n=6/group). (J) Gcg mRNA levels in ileum of 14- to 16-week-old male mice of the indicated genotypes fed with NCD (n=6/group). (K) The plasma GLP-1 levels in 14- to 16-week-old male mice of the indicated genotypes fed with NCD (n=6/group). (L) Representative images for Piezo1 RNA-FISH and GLP-1 immunofluorescent staining in the ileum of 14-week-old male mice of indicated genotypes fed with NCD (n=6/group). (M) Percentage of Piezo1-positive GLP-1 cells in total GLP-1 cells in the ileal mucosa of 14-week-old male mice of indicated genotypes fed with NCD (n=6/group). (N) A schematic diagram depicting the potential mechanisms linking the CaMKKβ/CaMKIV-mTOR signaling pathway and GLP-1 production. (O) Representative western blots are shown for indicated antibodies in the ileal mucosa (n=6/group). Data are represented as mean ± SEM. Significance was determined by Student’s t test for comparison between two groups, and by one-way ANOVA for comparison among three groups or more, *p<0.05, **p<0.01, ***p<0.001.
-
Figure 1—source data 1
PDF file containing original gels and blots for Figure 1B, C and O, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig1-data1-v1.zip
-
Figure 1—source data 2
Original files for gel and western blot analysis displayed in Figure 1B, C and O.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig1-data2-v1.zip
-
Figure 1—source data 3
Original data for Figure 1.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig1-data3-v1.zip
Under normal chow diet, Piezo1 IntL-CKO mice exhibited increased body weight (Figure 1G) and greater glycemic excursions compared to control groups (Piezo1loxp/loxp, Vil1FLP, GcgCre and Vil1FLP::GcgfrtCre; Figure 1H and I), while the food and water intake were not changed (Figure 1—figure supplement 2A, B). The morphology of islet (Figure 1—figure supplement 3A) and ileum (Figure 1—figure supplement 4A) were not affected. Ileal mucosal Proglucagon expression and plasma GLP-1 level were significantly lower in Piezo1 IntL-CKO mice than that in all littermate controls such as Piezo1loxp/loxp, Vil1FLP, GcgCre and Vil1FLP::GcgfrtCre mice (Figure 1J and K), while no significant alteration was observed in the expression of pancreatic Piezo1 and Proglucagon (Figure 1—figure supplement 3B–D). According to in situ hybridization of Piezo1 and immunofluorescence analysis of GLP-1, the expression of Piezo1 disappeared in GLP-1 positive cells, suggesting successful knockout of Piezo1 in L cells in Piezo1 IntL-CKO mice (Figure 1L and M). Also depicted in Figure 1—figure supplement 5, Piezo1 is expressed in GLP-1-positive cells of the duodenum, jejunum, ileum, and colon in control mice, but not in Piezo1 IntL-CKO mice. However, Piezo1 remains expressed in intestinal ghrelin positive cells and pancreatic glucagon-positive cells of Piezo1 IntL-CKO mice (Figure 1—figure supplement 6). Moreover, while GLP-1 levels were reduced in L cells of Piezo1 IntL-CKO mice, levels of PYY, another hormone secreted by L cells, were unaffected (Figure 1—figure supplement 7A–D). Additionally, ileal mucosal cholecystokinin (CCK), a hormone secreted by I cells with metabolic effects similar to GLP-1, was also unchanged in Piezo1 IntL-CKO mice (Figure 1—figure supplement 7E). Previous study showed that Piezo1 affected intestinal tight junctions and epithelial integrity (Jiang et al., 2021). To access whether loss of Piezo1 in L cells affect epithelial integrity of the intestine, we examined the expression of tight junction proteins, including ZO-1 and Occludin. As shown in Figure 1—figure supplement 8, the expression of ZO-1 and Occludin remained unchanged in Piezo1 IntL-CKO mice when compared to littermate controls.
Piezo1 is a non-selective cationic channel that allows passage of Ca2+ and Na+. CaMKKβ is the main calcium/calmodulin dependent protein kinase kinase involved in the regulation of metabolic homeostasis (Marcelo et al., 2016). It is activated by binding calcium-calmodulin (Ca2+/CaM), resulting in downstream activation of kinases CaMKIV. The activation of CaMKIV modulate the gene expression of nutrient- and hormone-related proteins (Ban et al., 2000; Chen et al., 2011; Takemoto-Kimura et al., 2017). Previous studies have reported that Ca2+ and mTOR signaling regulate the production of GLP-1 (Tolhurst et al., 2011; Xu et al., 2015; Yu and Jin, 2010). Drawing from these findings, this research study proposed a hypothesis that Piezo1 may regulate GLP-1 synthesis via the CaMKKβ/CaMKIV-mTOR signaling pathway (Figure 1N). As shown in Figure 1O, abrogated GLP-1 production was associated with decreased CaMKKβ/CaMKIV-mTOR signaling in the ileal mucosa of Piezo1 IntL-CKO mice (Figure 1O).
Derangements of glucose metabolism and GLP-1 production were induced by HFD in Piezo1 IntL-CKO mice, which was mitigated by Exendin-4
We next assessed the effect of L-cell-specific Piezo1 gene deletion on GLP-1 and glucose tolerance in diet-induced diabetic mice. Piezo1 IntL-CKO and control mice were exposed to HFD for 10 weeks. Compared to the controls, higher body weight (Figure 2A), greater glucose excursions (Figure 2B) were observed in Piezo1 IntL-CKO mice exposed to HFD. Ileal mucosal Proglucagon expression levels were lower in Piezo1 IntL-CKO than control mice (Figure 2C–F). Impaired CaMKKβ/CaMKIV-mTORC1 signaling pathway in ileal mucosa as evidenced by a decrease in CaMKKβ, reduced phosphorylation levels of CaMKIV, mTOR, S6K, and S6 was also observed in Piezo1 IntL-CKO mice (Figure 2F). No significant alteration in morphology, Piezo1 or Proglucagon levels were observed in the pancreas of Piezo1 IntL-CKO mice (Figure 2—figure supplement 1A–D). Together these data demonstrate that Piezo1 IntL-CKO mice with prolonged HFD feeding exhibit impaired glucose metabolism phenotype and reduced GLP-1.
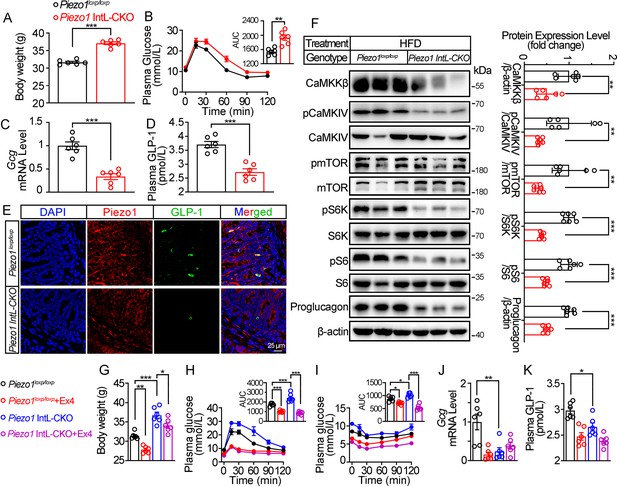
Validation and phenotype of Piezo1 IntL-CKO mice fed with high-fat diet.
(A) Body weight of 14- to 16-week-old male Piezo1loxp/loxp and Piezo1 IntL-CKO mice fed with HFD for 10 weeks (n=6/group). (B) IPGTT and associated area under the curve (AUC) values of 14- to 16-week-old male Piezo1loxp/loxp and Piezo1 IntL-CKO mice fed with HFD (n=6/group). (C) Gcg mRNA levels in the ileal mucosa of 14- to 16-week-old male Piezo1loxp/loxp and Piezo1 IntL-CKO mice fed with HFD (n=6/group). (D) The plasma GLP-1 level in 14- to 16-week-old male Piezo1loxp/loxp and Piezo1 IntL-CKO mice fed with HFD (n=6/group). (E) Double immunofluorescent staining of Piezo1, and GLP-1 in the ilea of 14- to 16-week-old male Piezo1loxp/loxp and Piezo1 IntL-CKO mice fed with HFD (n=6/group). (F) Representative western blots are shown for indicated antibodies in the ileal mucosa (n=6/group). (G) Body weight after 7 consecutive days infusion of saline or Ex-4 (100 µg/kg body weight) in 14- to 16-week-old male Piezo1loxp/loxp and Piezo1 IntL-CKO mice fed with HFD (n=6/group). (H, I) IPGTT (H) and ITT (I) and associated area under the curve (AUC) values after consecutive infusion of saline or Ex-4. (J) Gcg mRNA levels in the ileal mucosa (n=6/group) after consecutive infusion of saline or Ex-4. (K) The plasma GLP-1 level after consecutive infusion of saline or Ex-4 (n=6/group). Data are represented as mean ± SEM. Significance was determined by Student’s t test for comparison between two groups, and by one-way ANOVA for comparison among three groups or more, *p<0.05, **p<0.01, ***p<0.001.
-
Figure 2—source data 1
PDF file containing original western blots for Figure 2F, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig2-data1-v1.zip
-
Figure 2—source data 2
Original files for western blot analysis displayed in Figure 2F.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig2-data2-v1.zip
-
Figure 2—source data 3
Original data for Figure 2.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig2-data3-v1.zip
Injection of GLP-1 analog Exendin-4 (Ex-4) decreased the body weight (Figure 2G) and improved both glucose tolerance (Figure 2H) and insulin resistance (Figure 2I) in control and Piezo1 IntL-CKO mice, while endogenous synthesis of GLP-1 was not changed by Ex-4 injection in Piezo1 IntL-CKO mice (Figure 2J and K). These data suggested that decreased GLP-1 synthesis and secretion contribute to impaired glucose metabolism in Piezo1 IntL-CKO mice.
The pharmacological and mechanical activation of ileal Piezo1 stimulates GLP-1 synthesis
We next examined whether activation of Piezo1 could rescued the impaired glucose metabolism in diet-induced diabetic mice. Injection of Piezo1 activator Yoda1 after 10 weeks of high-fat diet, led to reduced body weight and improved the impaired glucose metabolism significantly in diabetic mice, while Piezo1 antagonist GsMTx4 reversed the weight loss and glucose-lowering effect of Yoda1 (Figure 3A and B). Yoda1 remarkably induced an increase in GLP-1 synthesis and secretion (Figure 3C and D), as well as an increment of CaMKKβ/CaMKIV-mTORC1 signaling in ileal mucosa (Figure 3E), while GsMTx4 abolished the effect of Yoda1 (Figure 3C–E). However, weight loss, improved plasma glucose and increased GLP-1 production induced by Yoda1 were not observed in Piezo1 IntL-CKO mice (Figure 3F–J).
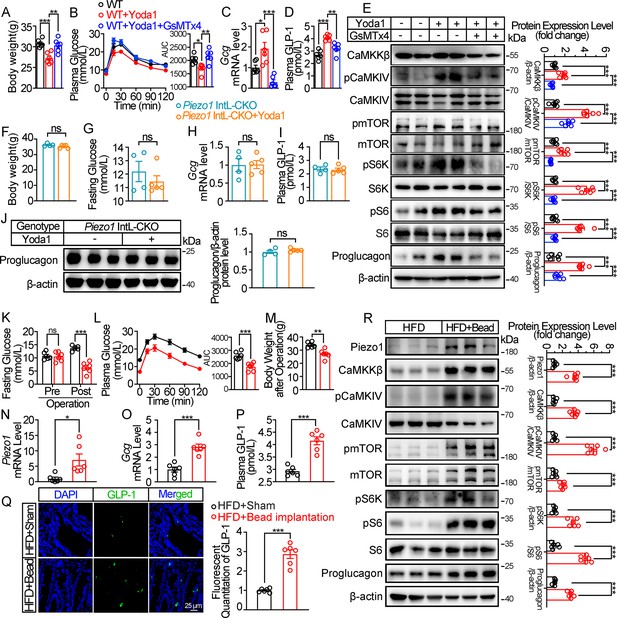
Chemical and mechanical interventions of Piezo1 regulate GLP-1 synthesis in mice.
(A–E) 14- to 16-week-old male C57BL/6 J mice fed with HFD for 10 weeks were infused with vehicle, Yoda1 (2 μg per mouse) or GsMTx4 (250 μg/kg) by i.p. for 7 consecutive days. (n=6/group). (A) Body weight after consecutive drug infusion. (B) IPGTT and associated area under the curve (AUC) values. (C) Gcg mRNA levels in the ileal mucosa. (D) Plasma GLP-1. (E) Representative western blots are shown for indicated antibodies in the ileal mucosa. (F–J) 14- to 16-week-old male Piezo1 IntL-CKO mice fed with HFD for 10 weeks were infused with vehicle, Yoda1 (2 μg per mouse) by i.p. for 7 consecutive days. (n=4 or 5/group). (F) Body weight after 7 consecutive days’ drug infusion. (G) Fasting blood glucose levels. (H) Ileal mucosal Gcg mRNA levels. (I) Plasma GLP-1 levels. (J) Ileal mucosal Proglucagon protein levels. (K–R) 14- to 16-week-old male C57BL/6 J mice fed with HFD were subjected to sham operation, or intestinal bead implantation (n=6/group). (K) Fasting blood glucose levels. (L) IPGTT and associated area under the curve (AUC) values. (M) Body weight. (N, O) Piezo1 (N) and Gcg (O) mRNA levels in the ileal mucosa. (P) The plasma GLP-1 levels. (Q) Immunofluorescence staining of GLP-1 in ileum and quantification of GLP-1-positive cells. (R) Representative western blots images and densitometry quantification for indicated antibodies in the ileal mucosa. Data are represented as mean ± SEM. Significance was determined by Student’s t test for comparison between two groups, and by one-way ANOVA for comparison among three groups or more, *p<0.05, **p<0.01, ***p<0.001.
-
Figure 3—source data 1
PDF file containing original western blots for Figure 3E, J and R, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig3-data1-v1.zip
-
Figure 3—source data 2
Original files for western blot analysis displayed in Figure 3E, J and R.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig3-data2-v1.zip
-
Figure 3—source data 3
Original data for Figure 3.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig3-data3-v1.zip
The intestine receives mechanical stimulation from the chyme, which may activate Piezo1 in the intestine epithelium, including L cells. To mimic the mechanical pressing and stretching induced by intestinal contents, a small silicon bead was implanted into the high-fat diet-induced diabetic mouse ileum. To exclude the possibility of bowel obstruction and abdominal pain caused by bead implantation, we measured the fecal mass and gastrointestinal transit time, and accessed abdominal mechanical sensitivity in both sham and bead-implanted mice. As shown in Figure 3—figure supplement 1A, B, there was no significant difference in fecal mass and gastrointestinal transit time between the sham-operated mice and those implanted with beads. The results of abdominal mechanical sensitivity indicated that no difference in abdominal pain threshold was observed between sham and bead implanted mice (Figure 3—figure supplement 1C). Intestinal bead implantation improved the impaired glucose metabolism in diabetic mice (Figure 3K and L). Body weight loss, activated ileal mucosal CaMKKβ/CaMKIV-mTOR signaling, increased mRNA and protein levels of ileal mucosal Piezo1 and Proglucagon, as well as the circulating levels of GLP-1 were observed in diabetic mice after operation (Figure 3M–R). The above data suggest that mechanical stimuli induced by intestinal bead implantation activates ileal Piezo1 in diabetic mice, stimulating GLP-1 production via CaMKKβ/CaMKIV-mTOR signaling axis, thus improving glucose homeostasis.
Piezo1 regulates GLP-1 synthesis and secretion in primary cultured mouse L cells and isolated mouse ileum
To obtain primary L cells, we isolated cell from the ileum of Vil1FLP::GcgfrtCre-Rosa26mT/mG mice, in which tdTomato expression switched to EGFP expression in L cells as shown in Figure 1E. EGFP-positive cells (mouse L cells) were then sorted from isolated single cells (Figure 4A). Immunofluorescence showed that the sorted EGFP+ cells were Piezo1 positive (Figure 4B).
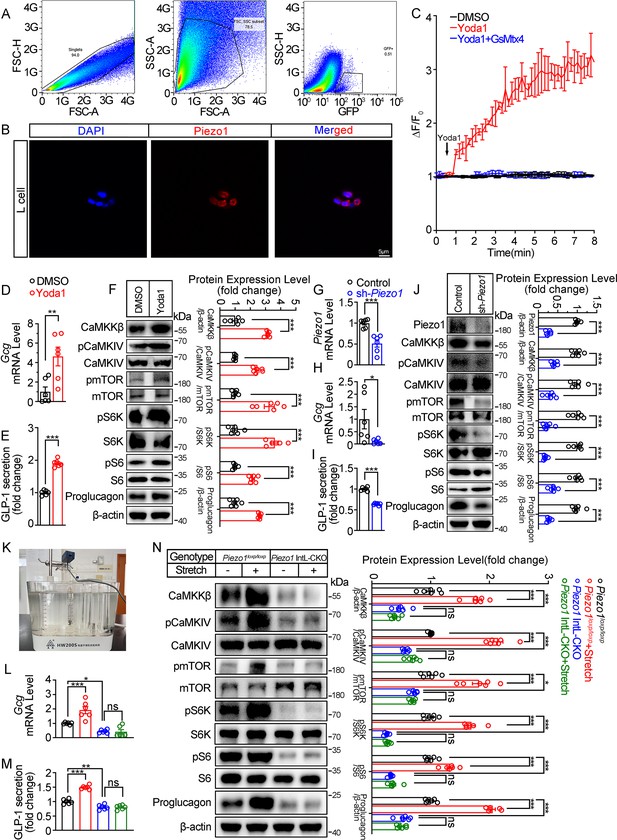
Piezo1 regulates GLP-1 synthesis and secretion in primary cultured mouse L cells and isolated mouse ileum.
(A) Isolation of mouse L cells (GFP positive) from ileal tissue by FACS. The gating in flowcytometry for sorting of GFP-positive cells. (B) Immunofluorescent staining of Piezo1 in sorted GFP-positive L cells. (C) Intracellular Ca2+ imaging by fluo-4-AM calcium probe. The change of fluorescent intensity (ΔF/F0) was plotted against time. (D–F) L cells were treated with vehicle or Yoda1 (5 μM) for 24 hr. (D) Gcg mRNA expression. (E) GLP-1 concentrations in the culture medium. (F) Western blot images and densitometry quantification for the indicated antibodies. (G–J) Knockdown of Piezo1 in L cells by shRNA for 48 hours. (G) Piezo1 mRNA expression. (H) Gcg mRNA expression. (I) GLP-1 levels in the culture medium. (J) Western blot images and densitometry quantification for the indicated antibodies. (K–N) Ileal tissues from Piezo1loxp/loxp and Piezo1 IntL-CKO mice were subjected to tension force (n=6/group). (K) A representative photograph showing the traction of isolated ileum. (L) Gcg mRNA levels. (M) GLP-1 concentrations in the medium. (N) Western blot images and densitometry quantification for the indicated antibodies. Data are represented as mean ± SEM and are representative of six biological replicates. Significance was determined by Student’s t test for comparison between two groups, and by one-way ANOVA for comparison among three groups or more, *p<0.05, **p<0.01, ***p<0.001.
-
Figure 4—source data 1
PDF file containing original western blots for Figure 4F, J and N, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig4-data1-v1.zip
-
Figure 4—source data 2
Original files for western blot analysis displayed in Figure 4F, J and N.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig4-data2-v1.zip
-
Figure 4—source data 3
Original data for Figure 4.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig4-data3-v1.zip
Yoda1 at the dose of 5 μM triggered an increase in intracellular Ca2+ level in primary cultured mouse L cells, which was blocked by pre-incubation of cells with GsMTx4 (0.1 μM) for 15 min (Figure 4C). Yoda1 also stimulated Proglucagon expression and GLP-1 secretion, as well as CaMKKβ/CaMKIV-mTOR signaling pathway in primary cultured mouse L cells (Figure 4D–F). In contrast, knockdown of Piezo1 by shRNA led to significant decrease in Proglucagon expression and GLP-1 secretion, as well as inhibition of CaMKKβ/CaMKIV/mTOR signaling pathway (Figure 4G–J).
Given the ability of Piezo1 in sensing mechanical force, tension of 1.5 g was applied to the isolated mouse ileum bathed in Tyrode’s solution for four hours. Tension stimulated Proglucagon expression, GLP-1 secretion and activated CaMKKβ/CaMKIV-mTOR signaling pathway in the ileum of control mice, but not in Piezo1 IntL-CKO mice (Figure 4K–N), suggesting the involvement of Piezo1 of the L cells in mediating the force-induced GLP-1 production and CaMKKβ/CaMKIV-mTOR signaling.
Pharmacological, mechanical and genetic activation of Piezo1 stimulates GLP-1 synthesis and secretion in STC-1 cells
To further validate the role of Piezo1 in regulating GLP-1, we examined the effect of manipulating Piezo1 on GLP-1 production in an intestinal neuroendocrine cell line STC-1. Pharmacological activation of Piezo1 by Yoda1 triggered an inward current in STC-1 cell recorded by whole cell patch-clamp, which could be inhibited by pre-incubation of GsMTx4 (Figure 5A). Yoda1 also triggered an increase in intracellular Ca2+ level in STC-1 cells. Pre-incubation of cells with GsMTx4 (0.1 μM) for 15 min inhibited [Ca2+]i increase (Figure 5B and C). Yoda1 induced a concentration-dependent activation of CaMKKβ/CaMKIV-mTOR pathway and GLP-1 synthesis and secretion (Figure 5D–F). GsMTx4 blocked the effect of Yoda1 on STC-1 cells in both GLP-1 and CaMKKβ/CaMKIV-mTOR activation (Figure 5G–I).
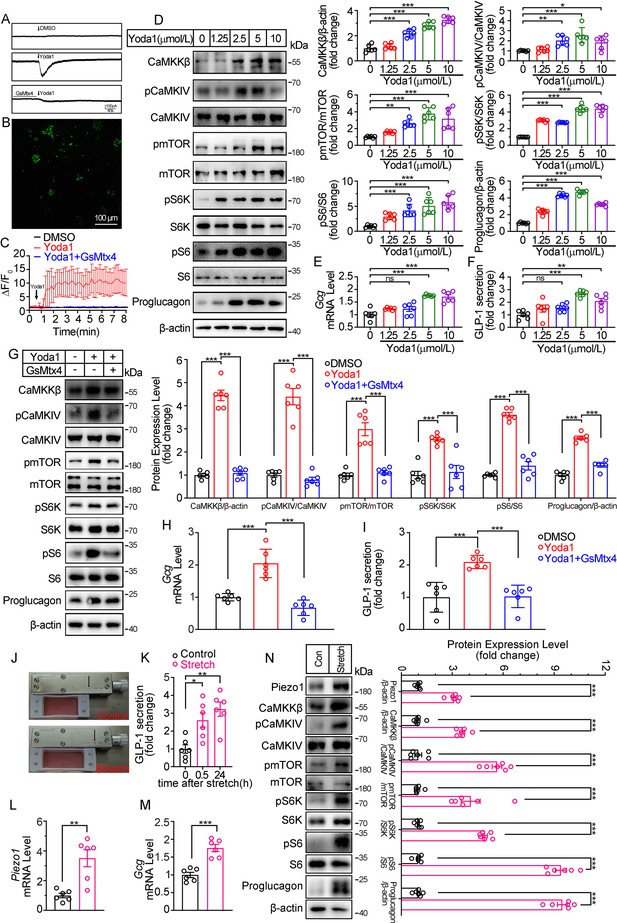
Modulation of GLP-1 synthesis and secretion by pharmacological and mechanical activation of Piezo1 in STC-1 cells.
(A) Whole-cell currents induced by Yoda1 (5 μM) were recorded from STC-1 cells or STC-1 cells pretreated with GsMTx4 for 30 min. (B, C) Intracellular calcium imaging in STC-1 cells. (B) STC-1 cells were loaded with fluo-4 AM for 1 hr. The representative time-lapse image showing the intracellular Ca2+ signals. (C) The change of fluorescent intensity (ΔF/F0) was plotted against time. (D–F) STC-1 cells were treated with various concentrations of Yoda1 for 24 hr. (D) Whole-cell extracts underwent western blot with indicated antibodies. (E) Gcg mRNA levels. (F) GLP-1 concentrations in the culture medium. (G–I) STC-1 cells were treated with Yoda1 (5 μM) in the presence or absence of GsMTx4 (0.1 μM) for 24 hr. (G) Whole-cell extracts underwent western blot with indicated antibodies. (H) Gcg mRNA levels. (I) GLP-1 concentrations in the culture medium. (J–N) STC-1 were subjected to mechanical stretch. (J) STC-1 cells were cultured in elastic chambers and the chambers were subjected to mechanical stretch by 120% extension of their original length. (K) The medium GLP-1 concentrations were detected at indicated time. (L) Piezo1 mRNA levels. (M) Gcg mRNA levels. (N) Whole-cell extracts underwent western blot with indicated antibodies. Data are represented as mean ± SEM and are representative of six biological replicates. Significance was determined by Student’s t test for comparison between two groups, and by one-way ANOVA for comparison among three groups or more, *p<0.05, **p<0.01, ***p<0.001.
-
Figure 5—source data 1
PDF file containing original western blots for Figure 5D, G and N, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig5-data1-v1.zip
-
Figure 5—source data 2
Original files for western blot analysis displayed in Figure 5D, G and N.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig5-data2-v1.zip
-
Figure 5—source data 3
Original data for Figure 5.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig5-data3-v1.zip
To mimic the activation of Piezo1 by mechanical stretching in vivo, STC-1 cells grown on elastic chambers were subjected to mechanical stretch to 120% of their original length. Mechanical stretch upregulated Piezo1 and Proglucagon expression, promoted GLP-1 secretion (Figure 5J–N), and activated CaMKKβ/CaMKIV- mTOR signaling pathways (Figure 5N).
Consistent to the pharmacological and mechanical activation of Piezo1, over-expression of Piezo1 in STC-1 cells resulted in a significant increase in GLP-1 production, as well as activation of the CaMKKβ/CaMKIV-mTOR signaling pathway (Figure 6A–D). Conversely, knockdown of Piezo1 by shRNA led to a significant decrease in GLP-1 production and inhibition of CaMKKβ/CaMKIV-mTOR signaling pathway (Figure 6E–H).
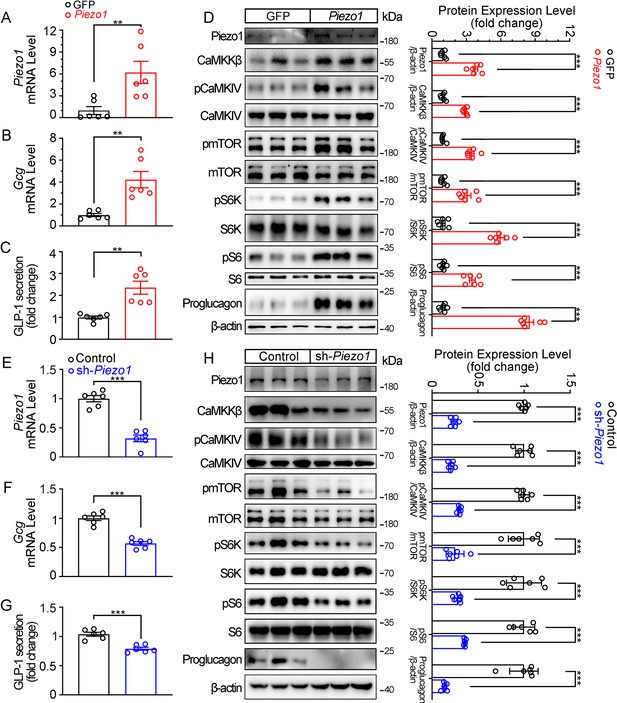
Genetic interference of Piezo1 regulates GLP-1 production in STC-1 cells.
(A–D) STC-1 cells were transfected with mouse control or Piezo1 expression plasmids for 48 hr. Piezo1 (A) and Gcg (B) mRNA levels in STC-1 cells. (C) GLP-1 concentrations in culture medium. (D) Whole-cell extracts underwent western blot with indicated antibodies. (E–H) Stable knockdown of Piezo1 in STC-1 cells. Piezo1 (E) and Gcg (F) mRNA levels in STC-1 cells. (G) GLP-1 concentrations in culture medium. (H) Whole-cell extracts underwent western blot with indicated antibodies. Data are represented as mean ± SEM Data are represented as mean ± SEM and are representative of six biological replicates. Significance was determined by Student’s t test, *p<0.05, **p<0.01, ***p<0.001.
-
Figure 6—source data 1
PDF file containing original western blots for Figure 6D and H, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig6-data1-v1.zip
-
Figure 6—source data 2
Original files for western blot analysis displayed in Figure 6D and H.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig6-data2-v1.zip
-
Figure 6—source data 3
Original data for Figure 6.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig6-data3-v1.zip
Piezo1 regulates GLP-1 production through CaMKKβ/CaMKIV and mTOR in STC-1 cells
Next, we examined whether CaMKKβ/CaMKIV and mTOR signaling mediates the effects of Piezo1 on GLP-1 production. Overexpression of CaMKKβ or CaMKIV increased CaMKKβ/CaMKIV and mTOR signaling activity, resulting in increased synthesis and secretion of GLP-1 (Figure 7A–C). In contrast, the CaMKKβ inhibitor STO-609, downregulated CaMKKβ/CaMKIV and mTOR signaling, as well as GLP-1 synthesis and secretion (Figure 7D–F). Inhibition of mTORC1 activity by rapamycin suppressed GLP-1 production induced by Yoda1, which was associated with inhibition of mTOR signaling (Figure 7G–I).
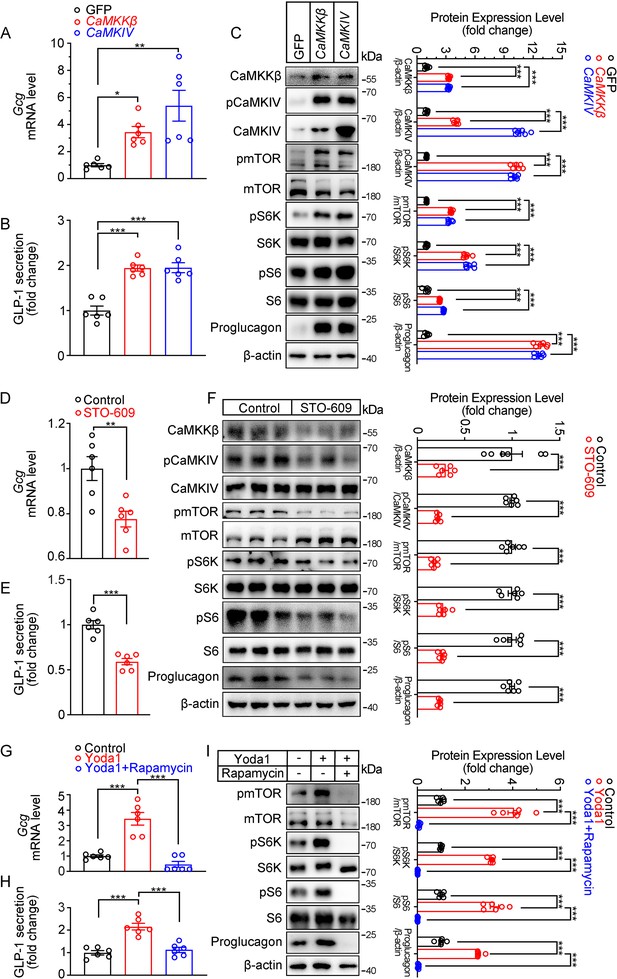
Modulation of GLP-1 production by CaMKKβ/CaMKIV and mTOR signaling activity in STC-1 cells.
(A–C) STC-1 cells were transfected with GFP, CaMKKβ or CaMKIV plasmids for 48 hr. (A) Gcg mRNA levels in STC-1 cells. (B) GLP-1 concentrations in culture medium. (C) Whole-cell extracts underwent western blot with indicated antibodies. (D–F) STC-1 cells were treated with CaMKKβ inhibitor STO-609 (10 μmol/L) for 24 hr. (D) Gcg mRNA levels in STC-1 cells. (E) GLP-1 concentrations in culture medium. (F) Whole-cell extracts underwent western blot with indicated antibodies. (G–I) STC-1 cells were pretreated with Rapamycin (50 nmol/L) for 1 hr, then treated with Yoda1 (5 μmol/L) for 24 hr. (G) Gcg mRNA levels in STC-1 cells. (H) GLP-1 concentrations in the culture medium. (I) Whole-cell extracts underwent western blot with indicated antibodies. Data are represented as mean ± SEM and are representative of six biological replicates. Significance was determined by Student’s t test for comparison between two groups, and by one-way ANOVA for comparison among three groups or more, *p<0.05, **p<0.01, ***p<0.001.
-
Figure 7—source data 1
PDF file containing original western blots for Figure 7C, F and I, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig7-data1-v1.zip
-
Figure 7—source data 2
Original files for western blot analysis displayed in Figure 7C, F and I.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig7-data2-v1.zip
-
Figure 7—source data 3
Original data for Figure 7.
- https://cdn.elifesciences.org/articles/97854/elife-97854-fig7-data3-v1.zip
Discussion
It has been known for decades that GLP-1 secretion from the intestinal L cells is stimulated by meal intake and is essential for postprandial glycemic control (Drucker, 2006; Song et al., 2019). However, the mechanism underlying the regulation of GLP-1 production is not completely understood. One of the problems that impeded the investigation of regulation mechanism of GLP-1 is the lack of an L-cell-specific genetically engineered animal model. Here, an L-cell-specific Cre mouse line was generated for the first time through the combination of the FLP-Frt and Cre-LoxP systems. This enables genetic manipulation specifically in the L cells and creates a valuable tool for investigating molecular mechanisms in L cells.
Previous studies have shown that L cells are able to sense nutrients in the intestinal lumen such as glucose and other carbohydrates, lipids and amino acids, which induce GLP-1 secretion through different mechanisms, including membrane depolarization-associated exocytosis, Ca2+/Calmodulin (Tolhurst et al., 2011), cAMP (Yu and Jin, 2010), mTORC1 (Xu et al., 2015), and AMPK (Jiang et al., 2016) signaling pathways. However, it is innegligible that as open type endocrine cells, L cells not only receive the chemical stimulations from the nutrients, but also mechanical stimulation when the chyme passing through the intestine, including stretching, pressure and shear force (Sensoy, 2021). While the food needs to be digested and nutrients absorbed before L-cells can detect the nutritive signals, mechanical stimulation may be more direct and faster. The expression of Piezo1, a mechanosensitive ion channel, was demonstrated in human and mouse intestinal sections, primary mouse L cell culture, and intestinal neuroendocrine cell line STC-1, indicating the mechanosensing ability of L cells and the potential regulatory effect of GLP-1 on mechanical stimulation. The results showed a significant increase in GLP-1 secretion by implantation of intestinal beads, stretching of intestinal tissue, or stretching of STC-1 cells, providing further evidence that mechanical regulation of GLP-1 secretion does exist. In addition, the selective deletion of Piezo1 (Piezo1 IntL-CKO) mice showed reduced circulating GLP-1 level, increased body weight, and impaired glucose homeostasis, while pharmacological activation of Piezo1 in mice, primary L cells, and STC-1 cells showed opposite effects. More importantly, Piezo1 IntL-CKO mice was unable to response to the tension-induced GLP-1 production. These further suggested a Piezo1-mediated mechanical sensing mechanism in L cells that regulates GLP-1 production and glucose metabolism by sensing the stimulation of intestinal luminal contents.
Interestingly, this intestinal Piezo1-mediated mechanical sensing mechanism may severely impaired in diabetic patients and rodents. Reduced expression of Piezo1 was demonstrated in the ileal mucosa of diet-induced diabetic mice, along with reduced GLP-1 production. When challenged with high-fat diet, Piezo1 IntL-CKO mice exhibited more severe symptoms of diabetes which was mitigated by Ex-4. These findings suggest that the impairment of Piezo1-mediated mechanical sensing function in the intestine is an important mechanism for the pathogenesis of T2DM. It is noteworthy that RYGB, a commonly performed weight-loss and hypoglycemic surgery (Cummings et al., 2004), significantly increased Piezo1 expression in L cells of obese diabetic patients. Yoda1 treatment or intestinal bead implantation enhanced GLP-1 production and improved glucose metabolism in the diet-induced diabetic mouse model, suggesting that restoring the mechano-sensing or enhancing the function of Piezo1 either pharmacologically or mechanically, may be a new strategy to improve the secretion of GLP-1 and alleviate T2DM. However, various data suggest that Piezo1-mediated regulation of GLP-1 production has only been demonstrated in transgenic mice, mouse primary L cells, and enteric neuroendocrine cell lines derived from mice. Whether Piezo1 plays the same role in human L cells awaits to be investigated. A number of studies have generated L cells culture from human intestinal organoid culture or human intestinal stem cell monolayer culture by manipulating the growth factors in the media (Goldspink et al., 2020; Petersen et al., 2014; Villegas-Novoa et al., 2022). It is worthy to validate our finding in human L cells in order to prove its translational potential in T2DM treatment.
The intragastric balloon is a current clinical weight loss measure that involves placing a space-occupying balloon in the stomach to reduce food intake and generate satiety signals, thus maintaining satiety. Investigations illustrated that intragastric balloon alter the secretion of hormones such as cholecystokinin and pancreatic polypeptide, delay the emptying of food in the stomach and reduce the appetite (Mathus-Vliegen and de Groot, 2013). Intragastric balloon provides a feasible weight loss intervention for obese people (Kim et al., 2016). In this study, a new intestinal implantation surgery of beads was adopted, which may offer a novel approach for weight loss and glucose control by activating the intestinal Piezo1-GLP-1 axis in the future.
Mechanistically, cellular and mouse models revealed that Piezo1 regulates GLP-1 production through the CaMKKβ/CaMKIV-mTOR signaling pathway. CaMKKβ/CaMKIV has been reported to mediate the Ca2+ signaling in many metabolic processes, including liver gluconeogenesis and de novo lipogenesis, adipogenesis, insulin sensitivity, and β cell proliferation (Anderson et al., 2012; Lin et al., 2011; Liu et al., 2012; Liu et al., 2022b). mTOR plays a central role in nutrient and energy sensing and regulates cellular metabolism and growth in response to different nutrient and energy status (Howell and Manning, 2011). Here, the data suggest that mTOR can also response to mechanical stimuli through a mechano-sensitive Ca2+ channel-mediated CaMKKβ/CaMKIV activation. Although it has not been demonstrated that CaMKIV directly phosphorylates mTOR or S6K in L cells, a previous study reported that CaMKKβ could serve as a scaffold to assemble CaMKIV with key components of the mTOR/S6K pathway and promote liver cancer cell growth (Lin et al., 2015), which lended support to the CaMKKβ/CaMKIV-mTOR signaling in our study. Recently, Knutson et. al. found that ryanodine and IP3-triggered calcium release from intracellular calcium store could amplified the initial Peizo2 - Ca2+ signal triggered by mechanical stimulation, and was required for the mechanotransduction in the serotonin release from enterochromaffin cells (Knutson et al., 2023). Primary L-cell and STC-1 cell results showed a persistent intracellular Ca2+ increase triggered by Yoda1, which also suggests that intracellular Ca2+ stores are involved in Ca2+ relay. Beside Ca2+, cyclic AMP (cAMP) is another signaling molecule that active Gcg gene expression and GLP-1 production (Drucker et al., 1994; Jin, 2008; Simpson et al., 2007). cAMP was found to play a critical role in nutrients-induced GLP-1 secretion, including glucose (Ong et al., 2009), lipids (Hodge et al., 2016), and amino acids (Tolhurst et al., 2011). Previous study reported that Ca2+ can activate soluble adenylyl cyclase (sAC) to increase intracellular cAMP (Jaiswal and Conti, 2003). Whether sAC-cAMP can be activated by Piezo1-mediated Ca2+ influx and whether it is an alternative signaling pathway that mediates the Piezo1-regulated GLP-1 production remain to be explored.
Furthermore, recent studies have highlighted the role of Piezo1 in enhancing insulin secretion (Deivasikamani et al., 2019; Ye et al., 2022), while inhibiting ghrelin (Zhao et al., 2024) and glucagon production (Guo et al., 2024), as well as reducing intestinal nutrient absorption (Tao et al., 2024). The diverse functions of Piezo1 across various cell types can be attributed to several factors, including cellular context, specific signaling pathways, and the microenvironment surrounding the cells. The current study reveals Piezo1-mediated mechanosensory properties of intestinal L cells that play an important role in regulating GLP-1 production and glucose metabolism. This finding suggests the existence of a new mechanoregulatory mechanism in enteroendocrine cells in addition to chemical and neural regulation, which may provide new ideas for the treatment of metabolic diseases such as diabetes and obesity.
Materials and methods
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus, C57BL/6 J) | Vil1FLP, GcgCre | Shanghai Model Organisms Center | N/A | |
| Strain, strain background (M. musculus, C57BL/6 J) | Vil1FLP::GcgfrtCre | This paper | N/A | Please refer to the "Genetic mouse generation" section. |
| Strain, strain background (M. musculus, C57BL/6 J) | Rosa26mTmG | Jackson Laboratory | Stock No. 007676 | |
| Strain, strain background (M. musculus, C57BL/6 J) | B6.Cg-Piezo1tm2.1Apat/J | Jackson laboratory | RRID:IMSR_JAX:029213 | |
| Cell line (M. musculus, mouse) | STC-1 | ATCC | CRL-3254 | |
| Biological sample (Mouse) | Primary mouse ileal L cells, Ileum, Pancreas, Liver, Skeletal muscle, Epididymal adipose, Hypothalamus | This paper | N/A | Freshly isolated from Mice. |
| Transfected construct (M. musculus) | pLKO.1-shPiezo1 | This paper | N/A | Lentiviral construct to transfect and express the shRNA. |
| Antibody | Anti-Piezo1 (Rabbit polyclonal) | Affinity Biosciences | Cat# DF12083, RRID:AB_2844888 | WB: 1:1000 IF: 1:400 |
| Antibody | Anti-CaMKKβ (mouse monoclonal) | Santa Cruz Biotechnology | Cat# sc-271674, RRID:AB_10708844 | WB: 1:1000 |
| Antibody | Anti-Phospho- CaMKIV (Thr200) (Rabbit polyclonal) | Affinity Biosciences | Cat# AF3460, RRID:AB_2834898 | WB: 1:1000 |
| Antibody | Anti-CaMKIV (Rabbit polyclonal) | Cell Signaling Technology | Cat# 4032, RRID:AB_2068389 | WB: 1:1000 |
| Antibody | Anti-Phospho- mTOR (Ser2448) (Rabbit Monoclonal) | Cell Signaling Technology | Cat# 5536, RRID:AB_10691552 | WB: 1:1000 |
| Antibody | Anti-mTOR (Rabbit monoclonal) | Cell Signaling Technology | Cat# 2983, RRID:AB_2105622 | WB: 1:1000 |
| Antibody | Anti-phospho-p70 S6 Kinase (Thr389) (Rabbit monoclonal) | Cell Signaling Technology | Cat# 9234, RRID:AB_2269803 | WB: 1:1000 |
| Antibody | Anti-p70 S6 Kinase (Rabbit Monoclonal) | Cell Signaling Technology | Cat# 2903, RRID:AB_1196657 | WB: 1:1000 |
| Antibody | Anti-phospho-S6 Ribosomal Protein (Ser235/236) (Rabbit Monoclonal) | Cell Signaling Technology | Cat# 4858, RRID:AB_916156 | WB: 1:1000 |
| Antibody | Anti-S6 Ribosomal Protein (Rabbit monoclonal) | Cell Signaling Technology | Cat# 2217, RRID:AB_331355 | WB: 1:1000 |
| Antibody | Anti-GLP-1 (Mouse monoclonal) | Abcam | Cat# ab23468, RRID:AB_470325 | WB: 1:1000 IF: 1:500 |
| Antibody | Anti-β-actin (Mouse monoclonal) | Cell Signaling Technology | Cat# 3700, RRID:AB_2242334 | WB: 1:1000 |
| Antibody | Horseradish peroxidase‐conjugated, Goat Anti-Rabbit IgG | Jackson ImmunoResearch Labs | Cat# 111-035-003, RRID:AB_2313567 | 1:10,000 |
| Antibody | Horseradish peroxidase‐conjugated, Goat Anti-Mouse IgG | Jackson ImmunoResearch Labs | Cat# 115-035-003, RRID:AB_10015289 | 1:10,000 |
| Antibody | Goat anti-mouse fluorescein isothiocyanate-conjugated IgG | EarthOx LLC | Cat# E031210-01 | 1:100 |
| Antibody | Dylight 594 affinipure donkey anti-rabbit IgG | EarthOx LLC | Cat# E032421-01 | 1:100 |
| Recombinant DNA reagent | pcDNA3.1-mPiezo1-IRES-GFP | Addgene | Cat# 80925 | |
| Recombinant DNA reagent | pcDNA3.1-IRES-GFP | Addgene | Cat# 51406 | |
| Recombinant DNA reagent | CaMKKβ (Plasmid) | This paper | N/A | Gifted by Professor Koji Murao from Kagawa University |
| Recombinant DNA reagent | CaMKIV (Plasmid) | This paper | N/A | Gifted by Professor Koji Murao from Kagawa University |
| Sequence-based reagent | P1 | This paper | PCR primers | GACCTTTGCCCTCTGGTCTC |
| Sequence-based reagent | P2 | This paper | PCR primers | GAGTGACGGTGCCAGAGAAA |
| Sequence-based reagent | P3 | This paper | PCR primers | GACTCCAGCTGCCTTCTCTG |
| Sequence-based reagent | P4 | This paper | PCR primers | CGGTGATCTCCCAGATGCTC |
| Sequence-based reagent | P5 | This paper | PCR primers | CCCTAACTCAGTCTCCAGCA |
| Sequence-based reagent | P6 | This paper | PCR primers | CGGTTACCAGGTGGTCATGT |
| Sequence-based reagent | P7 | This paper | PCR primers | CCCTAACTCAGTCTCCAGCA |
| Sequence-based reagent | P8 | This paper | PCR primers | CTGCAAAGGGTCGCTACAGA |
| Sequence-based reagent | P9 | This paper | PCR primers | AATGGCTCTCCTCAAGCGTAT |
| Sequence-based reagent | P10 | This paper | PCR primers | ACAGGAGGTAGTCCCTCACAT |
| Sequence-based reagent | P11 | This paper | PCR primers | TGTCGGGGAAATCATCGTCC |
| Sequence-based reagent | Piezo1_F (Human) | This paper | PCR primers | ATCGCCATCATCTGGTTCCC |
| Sequence-based reagent | Piezo1_R (Human) | This paper | PCR primers | TGGTGAACAGCGGCTCATAG |
| Sequence-based reagent | GCG_F (Human) | This paper | PCR primers | GCACATTCACCAGTGACTACAGCA |
| Sequence-based reagent | GCG_R (Human) | This paper | PCR primers | TGGCAGCTTGGCCTTCCAAATA |
| Sequence-based reagent | β-actin_F (Human) | This paper | PCR primers | TCATGAAGATCCTCACCGAG |
| Sequence-based reagent | β-actin_R (Human) | This paper | PCR primers | CATCTCTTGCTCGAAGTCCA |
| Sequence-based reagent | Piezo1_F (Mouse) | This paper | PCR primers | GCAGTGGCAGTGAGGAGATT |
| Sequence-based reagent | Piezo1_R (Mouse) | This paper | PCR primers | GATATGCAGGCGCCTATCCA |
| Sequence-based reagent | Gcg_F (Mouse) | This paper | PCR primers | ATTGCCAAACGTCATGATGA |
| Sequence-based reagent | Gcg_R (Mouse) | This paper | PCR primers | GGCGACTTCTTCTGGGAAGT |
| Sequence-based reagent | CCK_F (Mouse) | This paper | PCR primers | TAGCGCGATACATCCAGCAGGT |
| Sequence-based reagent | CCK_R (Mouse) | This paper | PCR primers | GGTATTCGTAGTCCTCGGCACT |
| Sequence-based reagent | Actb_F (Mouse) | This paper | PCR primers | CCACAGCTGAGAGGGAAATC |
| Sequence-based reagent | Actb_R (Mouse) | This paper | PCR primers | AAGGAAGGCTGGAAAAGAGC |
| Commercial assay or kit | Mouse Glucagon-Like Peptide 1 (GLP-1) ELISA Kit | Millipore | Cat# EGLP-35K | Mouse Glucagon-Like Peptide 1 (GLP-1) ELISA Kit |
| Commercial assay or kit | RT-PCR kit | Takara | Cat# RR014A | RT-PCR kit |
| Chemical compound, drug | 0.1% gelatine | Biological Industries | Cat# 01-944-1B | |
| Chemical compound, drug | DMEM high sugar medium | Gibco | Cat# 11965092 | |
| Chemical compound, drug | Fetal bovine serum | Gibco | Cat# 12484028 | |
| Chemical compound, drug | Equine serum | Gibco | Cat# 16050122 | |
| Chemical compound, drug | Immobilon western chemiluminescent HRP substrate | Millipore | Cat# WBKLS0500 | |
| Chemical compound, drug | Diprotin A | Sigma-Aldrich | Cat# 90614-48-5 | |
| Chemical compound, drug | Thermo Scientific TurboFect Transfection Reagent | Thermo Fisher Scientific | Cat# R0531 | |
| Chemical compound, drug | TRIzol | Thermo Fisher Scientific | Cat# 15596026 | |
| Chemical compound, drug | RIPA Lysis Buffer | Beyotime Biotechnology | Cat# P0013B | |
| Chemical compound, drug | GsMTx4 | Alomone Labs | Cat# STG-100 | |
| Chemical compound, drug | Rapamycin | Santa Cruz Biotechnology | Cat# sc-3504B | |
| Chemical compound, drug | STO-609 | Selleck | Cat# S8274 | |
| Chemical compound, drug | Yoda1 | Sigma-Aldrich | Cat# SML1558 | |
| Chemical compound, drug | Dimethyl sulfoxide | Sigma-Aldrich | Cat# D2650 | |
| Chemical compound, drug | Exendin-4 | Sigma-Aldrich | Cat# E7144 | |
| Chemical compound, drug | Fluo-4 AM | Thermo Fisher Scientific | Cat# F14201 | |
| Software, algorithm | GraphPad Prism | GraphPad Software, https://www.graphpad.com/ | RRID:SCR_002798 | |
| Software, algorithm | ImageJ | ImageJ, https://imagej.nih.gov/ij/ | RRID:SCR_003070 | |
| Software, algorithm | Adobe photoshop | Adobe, https://www.adobe.com/creativecloud/desktop-app.html | RRID:SCR_014199 | |
| Other | Normal chow diet | Research Diets | Cat# D12450B | Feed for feeding mice. |
| Other | High fat diet | Research Diets | Cat# D12492 | Feed for feeding mice. |
Collection of human intestine samples
Request a detailed protocolMale obese participants with type 2 diabetes (n=6, BMI = 45.87 ± 4.889 kg/m2) and one-year post-RYGB patients (n=6, BMI = 25.48 ± 1.085 kg/m2) were recruited in current study. Written informed consent was obtained from each donor. The study protocol was approved by the Institutional Review Board of Jinan University. Mucosal biopsies were obtained from human intestines by using a colonoscopy (CF-HQ290I; Olympus).
Genetic mouse generation
Vil1FLP mice
Request a detailed protocolVil1FLP knock-in mouse model was developed by Shanghai Model Organisms Center, Inc. The targeting construct was designed to insert a 2A-Flp-WPRE-pA coexpression cassette into the stop codon of mouse Vil1 gene via homologous recombination using CRISPR/Cas9 system. 5'-AGCCCCTACCCTGCCTTCAA-3' was chosen as Cas9 targeted guide RNA (sgRNA). The donor vector, sgRNA and Cas9 mRNA was microinjected into C57BL/6 J fertilized eggs. F0 generation mice positive for homologous recombination were identified by long PCR. The primers (I-IV) used for detection of the correct homology recombination were I: 5’-ACTTCAGGCCTAACGCTCAC-3’ and II: 5’-TGTCCTGCAGGCAGAGAAAG-3’ for the correct 5’ homology arm recombination, and III: 5’-GTGCCGTCTCTAAGCACAGT-3’and IV: 5’-TGTTGGTGCTTCGGAGTGTT-3’for the correct 3' homology arm recombination. The PCR products were further confirmed by sequencing. F0 mice were crossed with C57BL/6 J mice to obtain Vil1FLP heterozygous mice.
FLP-dependent glucagon-Cre (GcgCre) mice
Request a detailed protocolGcgCre mouse model was developed by Shanghai Model Organisms Center, Inc. The targeting construct was designed to insert an IRES-F3-Frt-Wpre-pA-Cre-Frt-F3 expression cassette into the 3’ UTR of mouse Gcg gene of via homologous recombination using CRISPR/Cas9 system. 5’-ATGCAAAGCAATATAGCTTC-3’ was chosen as Cas9 targeted guide RNA (sgRNA). The donor vector, sgRNA and Cas9 mRNA was microinjected into C57BL/6 J fertilized eggs. F0 generation mice positive for homologous recombination were identified by long PCR. The primers (I-IV) used for detection of the correct homology recombination were I: 5’-TGCTACACAGGAGGTCTGTC-3’ and II: 5’-AGGCATGCTCTGCTATCACG-3’ for the correct 5’ homology arm recombination, and III: 5'-CCCTCCTAGTCCCTTCTCAGT-3' and IV: 5'-GCCAAGGACATCTTCAGCGA-3’ for the correct 3’ homology arm recombination. The PCR products were further confirmed by sequencing. F0 mice were crossed with C57BL/6 J mice to obtain Gcgcre heterozygous mice.
Vil1FLP::GcgfrtCre mice
Request a detailed protocolVil1FLP mice were crossed with Gcgcre mice to obtain Intestinal L cell-specific Cre (Vil1FLP::GcgfrtCre) mice.
Piezo1 IntL-CKO mice
Request a detailed protocolPiezo1loxp/loxp mice (B6.Cg-Piezo1tm2.1Apat/J) purchased from Jackson laboratory were crossed with Vil1FLP::GcgfrtCre mice to generate Piezo1 IntL-CKO mice.
PCR is used to identify the genotype of mice during the subsequent mating and breeding process. The primers required for mouse genotyping are shown in the Key Resources Table.
Mouse validation
Request a detailed protocolRosa26mT/mG reporter mice were purchased from Jackson laboratory, Inc Vil1FLP::GcgfrtCre mice were bred with Rosa26mT/mG reporter mice to further validate Cre recombinase activity and specificity. Every single Vil1FLP::GcgfrtCre mouse was confirmed by Rosa26mT/mG reporter mice before breeding with Piezo1loxp/loxp mice to generate Piezo1 IntL-CKO mice.
Frozen tissue confocal imaging
Request a detailed protocolVil1FLP::GcgfrtCre-Rosa26mT/mG reporter mice were sacrificed. Fresh ileum and pancreas tissues were collected and embedded in O.C.T. compound for histological analysis immediately. Slices of the tissues were cut for confocal imaging, which was protected from light. Fluorescence was detected by laser scanning confocal microscopy (Li et al., 2022).
Animal housing and treatment
Request a detailed protocolMale mice were maintained on a 12 hr light/12 hr dark cycle environment. Normal chow diet (NCD) or a high-fat diet (HFD) and water were available ad libitum unless specified otherwise. The animal protocols were approved by the Animal Care and Use Committee of Jinan University (IACUC-20230517–01).
Male Piezo1 IntL-CKO mice and age-matched control littermates (Piezo1loxp/loxp, Vil1FLP, GcgCre, Vil1FLP::GcgfrtCre mice) fed with NCD or HFD were used in the experiments.
Male Piezo1loxp/loxp and Piezo1 IntL-CKO mice fed with 10 week-high fat diet were intraperitoneally injected with normal saline (NS) or the GLP-1R agonist Exendin-4 (100 µg/kg body weight) for 7 consecutive days.
High fat diet induced diabetic mice were randomly divided into 3 groups. When indicated, animals were injected intraperitoneally with Vehicle, Yoda1 (2 μg per mouse) or GsMTx4 (250 μg/kg) plus Yoda1 for 7 consecutive days.
High fat diet treated Piezo1 IntL-CKO mice were randomly divided into 2 groups. When indicated, animals were injected intraperitoneally with Vehicle, Yoda1 (2 μg per mouse) for 7 consecutive days.
Diet induce diabetic C57BL/6 J mice were divided into sham and intestinal bead implantation groups.
Food and water intake detection
Request a detailed protocolThe food and water intake were quantified using metabolic cages (Cat 41853, Ugo Basile, Comerio, Italy). The mice were individually housed in these specialized cages and given a period of 3 days to acclimate before data collection began. They had unrestricted access to food and water, which was continuously monitored throughout the study. The 41850–010 software/interface package, consisting of EXPEDATA (for data analysis) and METASCREEN (for data collection) software, along with the IM-2 interface module, was employed to record and analyze the data.
Intraperitoneal glucose tolerance test
Request a detailed protocolMice were fasted for 12 hr before measuring their fasting glucose levels. An intraperitoneal glucose tolerance test (IPGTT) was performed by administering 1.5 g/kg body weight of glucose. Blood glucose concentrations were measured at specified time points using a glucometer by collecting tail vein blood samples.
Insulin tolerance test
Request a detailed protocolMice were subjected to a 4 hr fast before measurement of fasting glucose were taken. Insulin tolerance tests (ITT) were conducted with a dose of 0.75 U/kg body weight of insulin. Blood glucose levels were measured at specified time points.
Intestinal bead implantation
Request a detailed protocolHigh-fat diet-induced type 2 diabetic C57BL/6 J mice were fasted 6–8 hr before the operation. Standard aseptic procedures were used throughout the operation. Intestinal bead implantation was similar to gastric bead implantation described in our previous study (Zhao et al., 2024). Briefly, a 1 cm incision was made on the abdominal wall to expose the intestine. A 1 cm incision was made approximately 1 cm above the ileocecal region. A 2.5 mm diameter bead was implanted into the ileum of the mouse through an incision. Then the wound was closed with suture. Finally, the abdominal wall was closed with suture. For sham operation, all the procedures were the same as the bead implantation except that the bead was not implanted.
Detection of abdominal mechanical sensitivity
Request a detailed protocolThe mice were familiarized with a metal mesh floor covered with plastic boxes for 2 hr each day for 2 days prior to testing. Their abdominal fur was shaved 1 day before the experiments. The abdominal area was then stimulated using calibrated von Frey filaments (VFFs) that applied varying forces: subthreshold mechanical stimuli (indicative of allodynia, 0.07 g force) and painful stimuli (indicative of hyperalgesia, 0.16 and 1 g force). Each filament was applied 10 times for 5–8 s with 10 s intervals between applications. To prevent learning or sensitization, the same area was not stimulated more than once consecutively. The data were presented as the number of withdrawal responses out of 10 applications, with 0 indicating no withdrawal and 10 indicating the maximum withdrawal. A withdrawal response was defined as (1) the mouse withdrawing its abdomen from the VFFs, (2) subsequent licking of the abdominal area, or (3) withdrawal of the entire body. All tests were conducted in a blinded manner.
Gastrointestinal transit time
Request a detailed protocolThe whole-gut transit time test was conducted as previously described (Qin et al., 2017). The duration between the oral administration of charcoal and the appearance of the first stained fecal pellet was recorded as the total gastrointestinal transit time.
Stretching of isolated ileum
Request a detailed protocolAbout 2 cm ileum was isolated from control and Piezo1 IntL-CKO mice and kept in the specimen chamber filled with Tyrode’s solution (KCl 0.2 g/L, NaCl 8 g/L, CaCl2 0.2 g/L, MgCl2 0.1 g/L, NaHCO3 1 g/L, NaH2PO4 0.05 g/L, Glucose 1 g/L) of 37℃ gassed with oxygen. The specimen was connected to the force transducer of organ bath system (HW200S, Techman, Chengdu, CN). Adjust the transducer to apply traction force of 1.5 g on the tissue and maintained for 4 hr.
Measurement of GLP-1 secretion
Request a detailed protocolThe measurement of GLP-1 secretion was carried out according to previously described methods (Zhai et al., 2018). Samples were collected in the presence of aprotinin (2 μg/mL), EDTA (1 mg/mL) and diprotin A (0.1 mmol/L), and stored at –80 °C before use. GLP-1 levels were assayed using enzyme immunoassay kits following the manufacturer’s instructions.
Histological analysis
Request a detailed protocolTissues were collected, fixed with 4% paraformaldehyde, embedded in paraffin, and cut into 4 μm sections. Standard protocols were followed for staining the sections with hematoxylin-eosin. Photomicrographs were captured under an inverted microscope (Leica, Germany).
Immunofluorescence
Request a detailed protocolParaffin-embedded tissue sections were dewaxed and rehydrated, followed by boiling in 0.01 mol/L citrate buffer (pH 6.0) for 10 min. Subsequently, the sections were blocked with 5% goat serum for 1 hr and then incubated overnight at 4 °C with the following primary antibodies: Piezo1 (1:400), Glucagon (1:200), Ghrelin (1:100), GLP-1(1:500), PYY (1:100), ZO-1 (1:200), or Occludin (1:200). The sections were then incubated with a mixture of secondary antibodies. Images were taken by laser scanning confocal microscopy (Leica SP8). Fluorescence intensity was quantified by ImageJ software.
In situ hybridization
Request a detailed protocolParaffin sections were dewaxed and rehydrated. After antigen retrieval in in citrate buffer (pH6.0), the sections were incubated with Proteinase K (5 µg/ml) at 37 °C for the 15 min. Then the sections were hybridized with the probes overnight in a temperature-controlled chamber at 40 °C. The Piezo1 probe sequences were as follows: 5′-CTGCAGGTGGTTCTGGATATAGCCC-3′,5′-AAGAAGCAGATCTCCAGCCCGAAT-3′, 5′-GCCATGGATAGTCAATGCACAGTGC-3′. After washing with SSC buffers, the sections were hybridized in pre-warmed branch probes at 40 °C for 45 min. After washing with SSC buffers, the sections were hybridized with signal probe at 42 °C for 3 hr. After washing with SSC buffers, the sections were blocked with normal serum and then incubated with mouse anti-GLP-1 (1:500) antibody at 4 °C overnight followed by secondary antibody. Images were taken laser scanning confocal microscopy and the fluorescence signals were quantified by ImageJ.
Western blot analysis
Request a detailed protocolTissues and cells were harvested. Ileal mucosa was scraped for protein extraction. Protein extraction was performed by using RIPA lysis buffer (50 mM Tris PH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1% Sodium deoxycholate, 1 mM PMSF and protease inhibitor cocktail.), then 40 µg of proteins were loaded onto an SDS-PAGE gel for separation. After the separation, the proteins were transferred onto a nitrocellulose membrane. The membrane was then incubated in blocking buffer at room temperature for 1 hur. For overnight incubation, the membrane and primary antibody (at the recommended dilution as stated in the product datasheet) were immersed in primary antibody dilution buffer, with gentle agitation, at 4 °C. Subsequently, the membrane was incubated with a secondary antibody that specifically recognizes and binds to the primary antibody. Finally, western blotting luminol reagent was used to visualize bands. The grey scale values of the bands were measured using ImageJ software.
RNA extraction, quantitative real-time PCR
Request a detailed protocolRNA was extracted and reverse-transcribed into cDNAs using RT-PCR kit. Real-time PCR was performed as previously described (Zhai et al., 2018). Sequences for the primer pairs used in this study were shown in Key Resources Table.
Isolation of mouse intestinal L cells
Request a detailed protocolA 5~6 cm long ileum segment was collected from the Vil1FLP::GcgfrtCre-Rosa26mT/mG mouse. The tissue was washed with ice-cold PBS twice to remove the chyme in the lumen. The tissue was minced into 0.5 mm3 pieces in ice-cold PBS and then digested in 100mIU collagenase I and 0.01 g/mL trypsin at 37 °C for 30 min with rotation. After digested tissue was passed through 40 μm and 30 μm cell strainers sequentially, then centrifuged for 7 min at 4 °C. The cell pellet was resuspended in red cell lysis buffer and incubated for 10 min at room temperature. The unlysed cells were collected by centrifugation and resuspended with 1 mL cold PBS. The GFP positive cells was sorted by fluorescence-activated cell sorting (FASC) on Beckman Coulter MoFlo XDP cell sorter system.
Cell culture and treatments
Request a detailed protocolSTC-1 cells were maintained in DMEM medium supplemented with 2.5% fetal bovine serum and 10% equine serum at 37 °C with 5% CO2 air. L cells were maintained in DMEM medium supplemented with 10% fetal bovine serum.
For cell transfection, cells were plated at optimal densities and grown for 48 hr. Cells were then transfected with GFP, Piezo1 (Addgene, MA, USA), CaMKKβ and CaMKIV constructs (Murao et al., 2009) by using lipofectamine reagent according to the manufacturer’s instructions.
For stable knockdown of Piezo1 in STC-1 cells, short hairpin RNA (shRNA) sequences for mouse Piezo1 interference were cloned in to pLKO.1 vector. To produce lentivirus, psPAX2, pMD2G and pLKO.1 or pLKO.1-shPiezo1 plasmids (siPiezo1: CCAACCTTATCAGTGACTT) were co-transfected into 293T cells with lipofectamine 2000 reagent. Supernatant containing lentivirus was collected 48 hr after transfection and filtered through 0.45 μm filter. The virus-containing supernatant was used to infect STC-1 cells. Forty-eight hours after infection, the STC-1 cells were subjected to 1 µg/mL puromycin selection for 2–3 days.
For cell stretching, cells were grown in silicone elastic chambers coated by 0.1% gelatin solution. After incubated at 37 °C for 24–48 hr, The chambers were subjected to mechanical stretch to 120% of their original length.
Calcium imaging
Request a detailed protocolCells were plated onto confocal dishes at optimal densities and grown for 24 hr. Cells were loaded with the calcium fluorescent probe fluo-4 AM (1 μM) for 1 hr at 37 °C, then the cells were treated with Yoda1 (5 μM) or GsMTx4. The intracellular calcium ions were measured at room temperature using a laser confocal microscope with an excitation wave length of 494 nm and an emission wave length of 516 nm. The change of fluorescent signal was presented as ΔF/F0 and plotted against time.
Whole-cell patch-clamp recording
Request a detailed protocolBorosilicate glass-made patch pipettes (BF150-86-7.5, Sutter Instrument Co, USA) were pulled with micropipette puller (P-1000, Sutter Instrument Co, USA) to a resistance of 3–5 MΩ after being filled with pipette solution: 138 mM KCl, 10 mM NaCl, 1 mM MgCl2, 10 mM Glucose and 10 mM HEPES (pH 7.4). Cells were bathed in Margo-Ringer solution: 130 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2, 10 mM Glucose, 20 mM HEPES (pH7.4). Whole-cell calcium currents of STC-1 cells were recorded with the EPC10 USB patch-clamp amplifier (HEKA, Germany) controlled by PatchMaster software.
Statistical analysis
Request a detailed protocolAll data were expressed as mean ± S.E.M. Statistical differences were evaluated by one-way ANOVA or Student’s t-test. The correlation was determined by Pearson analysis. p<0.05 was considered significant. (*p<0.05, **p<0.01, *** p<0.001, ns = not significance). In this study, the data collection and analysis processes were not conducted in a blinded manner with respect to the experimental conditions. For the administration of drugs to animals, we allocated mice of the same genetic background to various experimental cohorts using a randomization protocol. No data were excluded during the data analysis.
Data availability
All of the data supporting the findings of this study are included in the article and source data files.
References
-
Intestinal enteroendocrine cells: Present and future druggable targetsInternational Journal of Molecular Sciences 24:8836.https://doi.org/10.3390/ijms24108836
-
The intestine as an endocrine organ and the role of gut hormones in metabolic regulationNature Reviews. Gastroenterology & Hepatology 20:784–796.https://doi.org/10.1038/s41575-023-00830-y
-
Gastric bypass for obesity: mechanisms of weight loss and diabetes resolutionThe Journal of Clinical Endocrinology and Metabolism 89:2608–2615.https://doi.org/10.1210/jc.2004-0433
-
Nutrient detection by incretin hormone secreting cellsPhysiology & Behavior 106:387–393.https://doi.org/10.1016/j.physbeh.2011.12.001
-
The biology of incretin hormonesCell Metabolism 3:153–165.https://doi.org/10.1016/j.cmet.2006.01.004
-
The gut as a sensory organNature Reviews. Gastroenterology & Hepatology 10:729–740.https://doi.org/10.1038/nrgastro.2013.180
-
Enteroendocrine cells: Chemosensors in the intestinal epitheliumAnnual Review of Physiology 78:277–299.https://doi.org/10.1146/annurev-physiol-021115-105439
-
Mechano-sensor Piezo1 inhibits glucagon production in pancreatic α-cellsBiochimica et Biophysica Acta. Molecular Basis of Disease 1870:167185.https://doi.org/10.1016/j.bbadis.2024.167185
-
mTOR couples cellular nutrient sensing to organismal metabolic homeostasisTrends in Endocrinology and Metabolism 22:94–102.https://doi.org/10.1016/j.tem.2010.12.003
-
AMPK-dependent regulation of GLP1 expression in L-like cellsJournal of Molecular Endocrinology 57:151–160.https://doi.org/10.1530/JME-16-0099
-
Mechanisms underlying proglucagon gene expressionThe Journal of Endocrinology 198:17–28.https://doi.org/10.1677/JOE-08-0085
-
Current status of intragastric balloon for obesity treatmentWorld Journal of Gastroenterology 22:5495–5504.https://doi.org/10.3748/wjg.v22.i24.5495
-
Intestinal enteroendocrine cells rely on ryanodine and IP3 calcium store receptors for mechanotransductionThe Journal of Physiology 601:287–305.https://doi.org/10.1113/JP283383
-
Mechanosensing by Piezo1 and its implications for physiology and various pathologiesBiological Reviews of the Cambridge Philosophical Society 97:604–614.https://doi.org/10.1111/brv.12814
-
Piezo1 Channels as Force Sensors in Mechanical Force-Related Chronic InflammationFrontiers in Immunology 13:816149.https://doi.org/10.3389/fimmu.2022.816149
-
Calcium/calmodulin-dependent protein kinase IV regulates vascular autophagy and insulin signaling through Akt/mTOR/CREB pathway in ob/ob miceJournal of Physiology and Biochemistry 78:199–211.https://doi.org/10.1007/s13105-021-00853-6
-
The Ca(2+)/Calmodulin/CaMKK2 Axis: Nature’s Metabolic CaMshaftTrends in Endocrinology and Metabolism 27:706–718.https://doi.org/10.1016/j.tem.2016.06.001
-
Exendin-4 regulates glucokinase expression by CaMKK/CaMKIV pathway in pancreatic beta-cell lineDiabetes, Obesity & Metabolism 11:939–946.https://doi.org/10.1111/j.1463-1326.2009.01067.x
-
The role of the PDE4D cAMP phosphodiesterase in the regulation of glucagon-like peptide-1 releaseBritish Journal of Pharmacology 157:633–644.https://doi.org/10.1111/j.1476-5381.2009.00194.x
-
Role of the prohormone convertase PC3 in the processing of proglucagon to glucagon-like peptide 1The Journal of Biological Chemistry 272:32810–32816.https://doi.org/10.1074/jbc.272.52.32810
-
The villin1 gene promoter drives cre recombinase expression in extraintestinal tissuesCellular and Molecular Gastroenterology and Hepatology 10:864–867.https://doi.org/10.1016/j.jcmgh.2020.05.009
-
A review on the food digestion in the digestive tract and the used in vitro modelsCurrent Research in Food Science 4:308–319.https://doi.org/10.1016/j.crfs.2021.04.004
-
Calmodulin kinases: essential regulators in health and diseaseJournal of Neurochemistry 141:808–818.https://doi.org/10.1111/jnc.14020
-
Recent advances in incretin-based pharmacotherapies for the treatment of obesity and diabetesFrontiers in Endocrinology 13:838410.https://doi.org/10.3389/fendo.2022.838410
-
Mechanical regulation of lipid and sugar absorption by Piezo1 in enterocytesActa Pharmaceutica Sinica. B 14:3576–3590.https://doi.org/10.1016/j.apsb.2024.04.016
-
Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP releaseThe Journal of Clinical Investigation 126:4527–4536.https://doi.org/10.1172/JCI87343
-
The role of mechanosensor Piezo1 in bone homeostasis and mechanobiologyDevelopmental Biology 493:80–88.https://doi.org/10.1016/j.ydbio.2022.11.002
-
Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patientsThe Journal of Clinical Endocrinology and Metabolism 81:327–332.https://doi.org/10.1210/jcem.81.1.8550773
-
Intestinal mTOR regulates GLP-1 production in mouse L cellsDiabetologia 58:1887–1897.https://doi.org/10.1007/s00125-015-3632-6
-
Mechanical stimulation activates Piezo1 to promote mucin2 expression in goblet cellsJournal of Gastroenterology and Hepatology 36:3127–3139.https://doi.org/10.1111/jgh.15596
Article and author information
Author details
Funding
National Natural Science Foundation of China (82170818)
- Geyang Xu
National Natural Science Foundation of China (81770794)
- Geyang Xu
Guangdong Basic and Applied Basic Research Foundation (2024A1515010686)
- Geyang Xu
Fundamental Research Funds for the Central Universities (21620423)
- Geyang Xu
Guangzhou Science and Technology Plan Project Funding (202201011353)
- Jie Yang
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (82170818, 81770794), Guangdong Basic and Applied Basic Research Foundation (2024A1515010686), the Fundamental Research Funds for the Central Universities (21620423), Guangzhou Science and Technology Plan Project Funding (202201011353).
Ethics
Written informed consent was obtained from each donor. The study protocol was approved by the Institutional Review Board of Jinan University. Mucosal biopsies were obtained from human intestines by using a colonoscopy (CF HQ290I; Olympus).
The animal protocols were approved by the Animal Care and Use Committee of Jinan University (IACUC-20230517-01).
Version history
- Sent for peer review:
- Preprint posted:
- Reviewed Preprint version 1:
- Reviewed Preprint version 2:
- Version of Record published:
Cite all versions
You can cite all versions using the DOI https://doi.org/10.7554/eLife.97854. This DOI represents all versions, and will always resolve to the latest one.
Copyright
© 2024, Huang, Mo, Yang et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,771
- views
-
- 228
- downloads
-
- 11
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Citations by DOI
-
- 8
- citations for umbrella DOI https://doi.org/10.7554/eLife.97854
-
- 1
- citation for Reviewed Preprint v1 https://doi.org/10.7554/eLife.97854.1
-
- 2
- citations for Version of Record https://doi.org/10.7554/eLife.97854.3






