CCDC113 stabilizes sperm axoneme and head-tail coupling apparatus to ensure male fertility
Figures
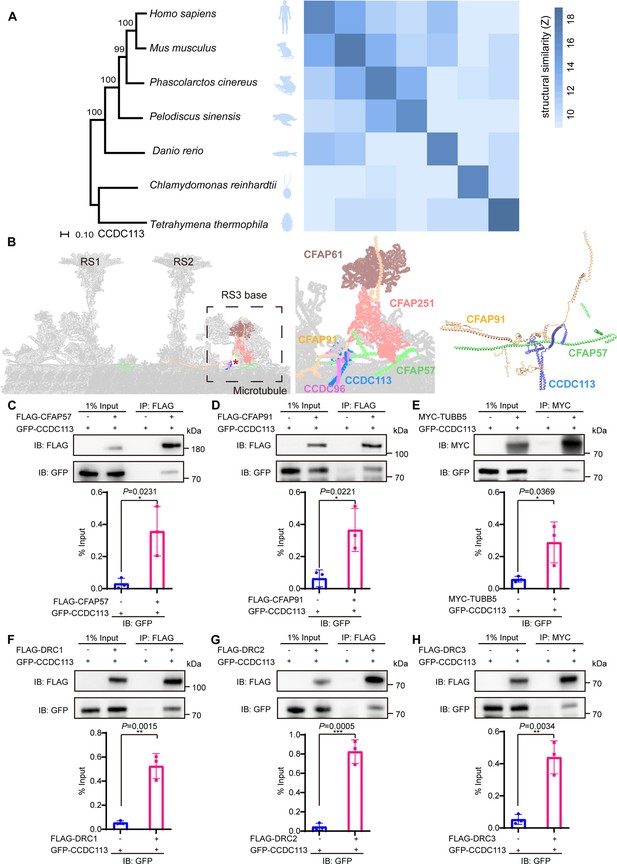
CCDC113 is an evolutionarily conserved axoneme-associated protein.
(A) Multiple species phylogenetic tree of CCDC113. Structural similarity scores (Z scores) of CCDC113 orthologs in H. sapiens, Mus musculus, Phascolarctos cinereus, Danio rerio, Chlamydomonas reinhardtii, and T. thermophila were derived through the DALI webserver for pairwise structure comparisons (Holm and Laakso, 2016). (B) Positioning of CCDC113 within the 96 nm repeat of human axoneme (Walton et al., 2023). CCDC113 forms a complex with CCDC96, is located at the base of RS3, and is adjacent to CFAP91 and CFAP57. CFAP91 originates at the base of RS2 and links the RS3 subunits (CFAP251 and CFAP61). (C–H) Neighboring axoneme-associated proteins were expressed alone or co-expressed with CCDC113 in HEK293T cells, and the interactions between CCDC113 and CFAP57, CFAP91, TUBB5, DRC1, DRC2, or DRC3 were examined by co-immunoprecipitation. IB: immunoblotting; IP: immunoprecipitation. The % Input is displayed below the corresponding figures for quantification. n=3 independent experiments. Data are presented as mean ± SD; *p<0.05, **p<0.01, ***p<0.001.
-
Figure 1—source data 1
Original files for western blot in Figure 1C-H.
- https://cdn.elifesciences.org/articles/98016/elife-98016-fig1-data1-v1.zip
-
Figure 1—source data 2
Labelled files for western blot in Figure 1C-H.
- https://cdn.elifesciences.org/articles/98016/elife-98016-fig1-data2-v1.zip
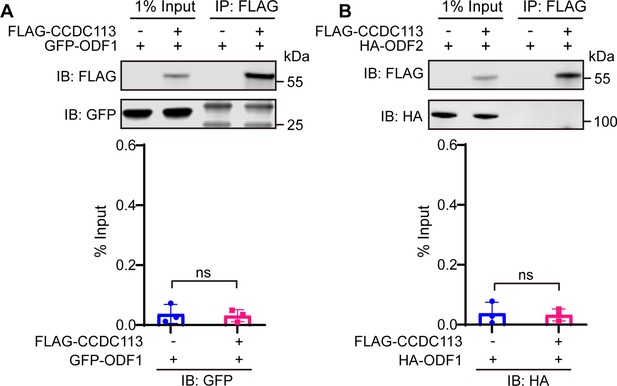
CCDC113 could not bind to ODF1 and ODF2.
(A–B) CCDC113 was expressed alone or co-expressed with ODF1 (A) or ODF2 (B) in HEK293T cells, and the interactions between CCDC113 and ODF1 or ODF2 were examined by co-immunoprecipitation. IB: immunoblotting; IP: immunoprecipitation. The % Input is displayed below the corresponding figures for quantification. n=3 independent experiments. Data are presented as mean ± SD; ns indicates no significant difference.
-
Figure 1—figure supplement 1—source data 1
Original files for western blot in Figure 1—figure supplement 1A-B.
- https://cdn.elifesciences.org/articles/98016/elife-98016-fig1-figsupp1-data1-v1.zip
-
Figure 1—figure supplement 1—source data 2
Labelled files for western blot in Figure 1—figure supplement 1A-B.
- https://cdn.elifesciences.org/articles/98016/elife-98016-fig1-figsupp1-data2-v1.zip
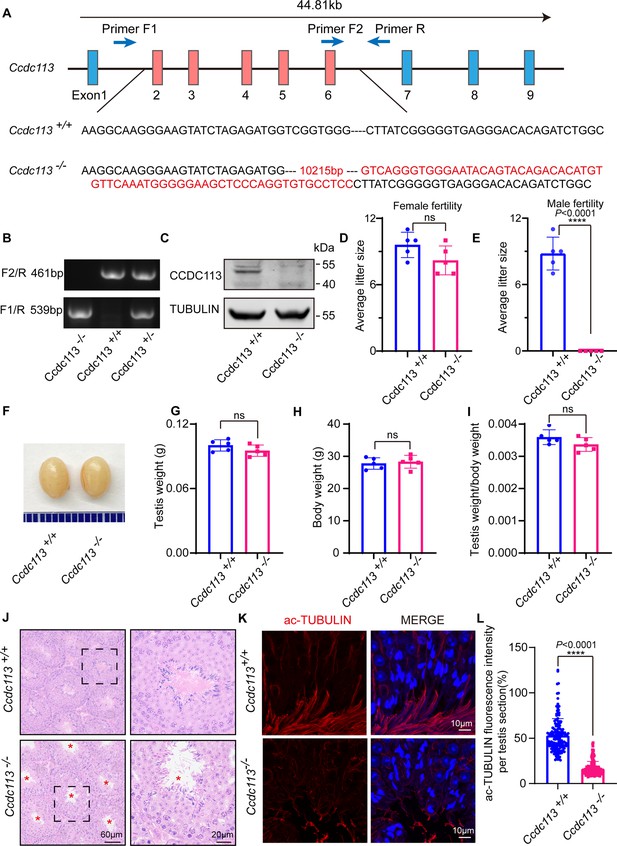
Ccdc113 knockout leads to male infertility.
(A) The CRISPR-Cas9 strategy for generating the Ccdc113 knockout mice. (B) Genotyping to identify Ccdc113 knockout mice. (C) Immunoblotting of CCDC113 in Ccdc113+/+ and Ccdc113–/– testes. TUBULIN served as the loading control. (D) The average litter size of Ccdc113+/+ and Ccdc113–/– female mice in 2 months (n=5 independent experiments). Data are presented as mean ± SD; ns indicates no significant difference. (E) The average litter size of Ccdc113+/+ and Ccdc113–/– male mice in 2 months (n=5 independent experiments). Data are presented as mean ± SD; ****p<0.0001. (F) The size of testes was similar in Ccdc113+/+ and Ccdc113–/– mice. (G) The testis weights of Ccdc113+/+ and Ccdc113–/– male mice (n=5 independent experiments). Data are presented as mean ± SD; ns indicates no significant difference. (H) The body weights of Ccdc113+/+ and Ccdc113–/– male mice (n=5 independent experiments). Data are presented as mean ± SD; ns indicates no significant difference. (I) The ratio of testis weight/body weight in Ccdc113+/+ and Ccdc113–/– male mice (n=5 independent experiments). Data are presented as mean ± SD; ns indicates no significant difference. (J) Hematoxylin-eosin (H&E) staining of testis sections from Ccdc113+/+ and Ccdc113–/– male mice. Red asterisks indicate the abnormal sperm flagellum in the Ccdc113–/– testis seminiferous tubule. (K) Immunofluorescence of acetylated-tubulin (red) in testis sections from Ccdc113–/– male mice showed flagellar defects. (L) Acetylated-tubulin fluorescence intensity was measured per testis section in 155 sections from 3 Ccdc113+/+ mice and 153 sections from 3 Ccdc113–/– male mice. Data are presented as mean ± SD; ****p<0.0001.
-
Figure 2—source data 1
Original file for gel in Figure 2B.
- https://cdn.elifesciences.org/articles/98016/elife-98016-fig2-data1-v1.zip
-
Figure 2—source data 2
Labelled file for gel in Figure 2B.
- https://cdn.elifesciences.org/articles/98016/elife-98016-fig2-data2-v1.zip
-
Figure 2—source data 3
Original files for western blot in Figure 2C.
- https://cdn.elifesciences.org/articles/98016/elife-98016-fig2-data3-v1.zip
-
Figure 2—source data 4
Labelled file for western blot in Figure 2C.
- https://cdn.elifesciences.org/articles/98016/elife-98016-fig2-data4-v1.zip
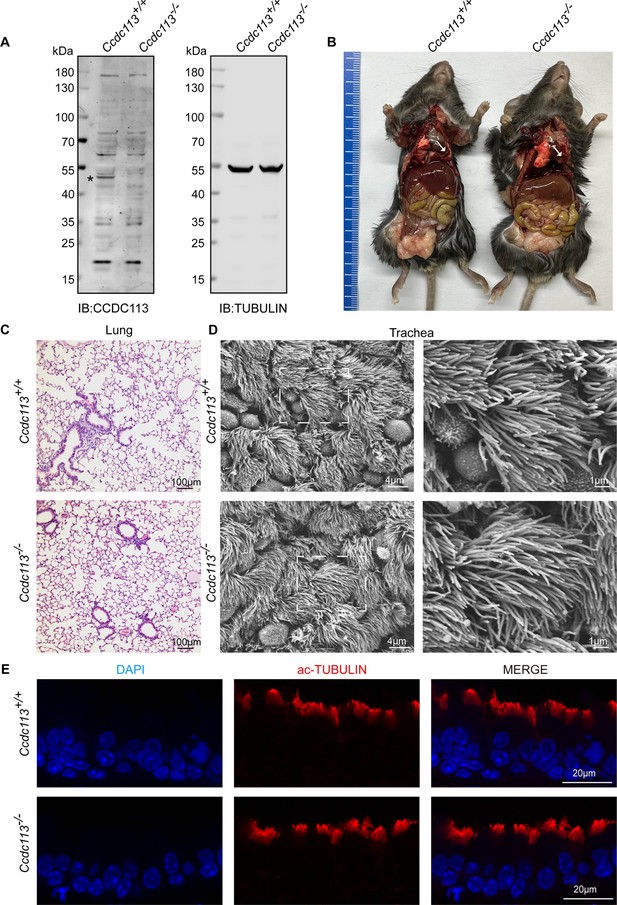
Ccdc113–/– mice did not exhibit the ciliopathies, such as hydrocephalus, situs inversus, and abnormal ciliogenesis of tracheal cilia.
(A) Immunoblotting of CCDC113 in Ccdc113+/+ and Ccdc113–/– testes, with TUBULIN serving as the loading control. An asterisk indicates the CCDC113 band. IB: immunoblotting. (B) The Ccdc113–/– mice did not exhibit hydrocephalus or left-right asymmetry defects. (C) Histology staining of the lung from Ccdc113+/+ and Ccdc113–/– mice. (D) Scanning electron micrography of Ccdc113+/+ and Ccdc113–/– tracheal epithelium at low and high magnifications of the boxed areas. (E) Immunofluorescence analysis of acetylated-tubulin (red) from Ccdc113+/+ and Ccdc113–/– trachea cilia.
-
Figure 2—figure supplement 1—source data 1
Original files for western blot in Figure 2—figure supplement 1A.
- https://cdn.elifesciences.org/articles/98016/elife-98016-fig2-figsupp1-data1-v1.zip
-
Figure 2—figure supplement 1—source data 2
Labelled file for western blot in Figure 2—figure supplement 1A.
- https://cdn.elifesciences.org/articles/98016/elife-98016-fig2-figsupp1-data2-v1.zip
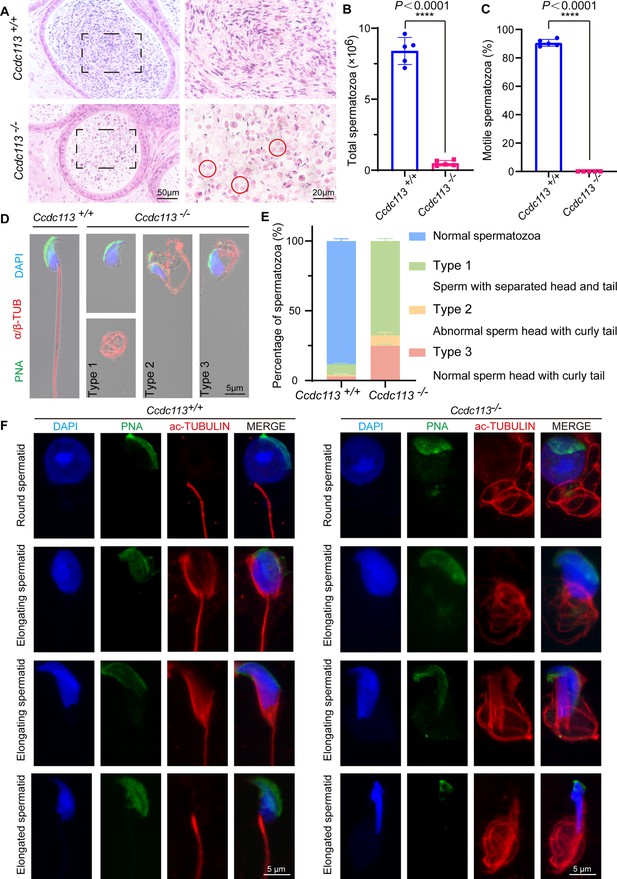
Ccdc113 knockout results in sperm flagellar defects and sperm head-tail detachment.
(A) Hematoxylin-eosin (H&E) staining of the cauda epididymis from 2-month-old Ccdc113+/+ and Ccdc113–/– male mice. Red circles indicate coiled eosin-stained structures without sperm heads in the epididymal lumen. (B) Analysis of sperm counts in Ccdc113+/+ and Ccdc113–/– male mice (n=5 independent experiments). Mature spermatozoa were extracted from the unilateral cauda epididymis and dispersed in phosphate-buffered saline (PBS). Sperm counts were measured using hemocytometers. Data are presented as mean ± SD; ****p<0.0001. (C) Motile sperm in Ccdc113+/+ and Ccdc113–/– mice (n=5 independent experiments). Data are presented as mean ± SD; ****p<0.0001. (D) Ccdc113+/+ and Ccdc113–/– spermatozoa were co-stained with a flagellar marker α/β-tubulin (red) and an acrosomal marker peanut agglutinin (PNA). Nuclei were stained with DAPI (blue). (E) Quantification of different categories of Ccdc113+/+, Ccdc113–/– spermatozoa (n=3 independent experiments). Data are presented as mean ± SD. (F) Immunofluorescence analysis of acetylated-tubulin (green) and PNA (red) from Ccdc113+/+ and Ccdc113–/– spermatids.
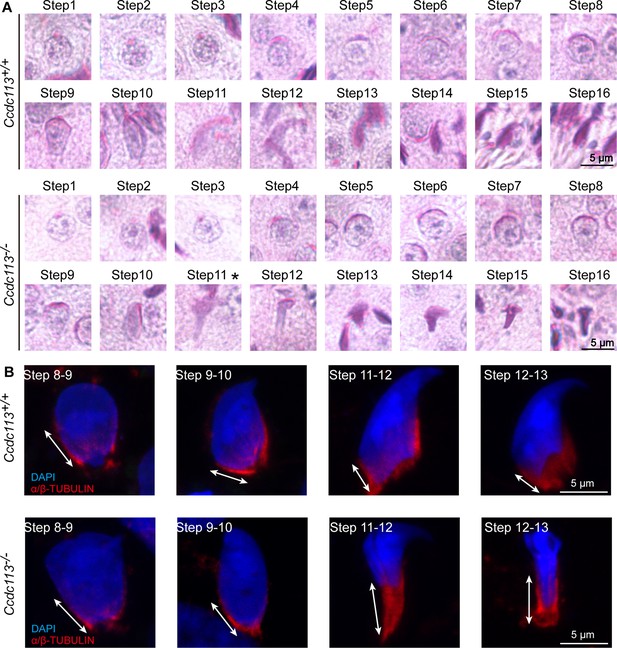
Ccdc113 knockout leads to abnormal sperm head shaping defect.
(A) Periodic acid Schiff (PAS) staining of spermatid at different steps from Ccdc113+/+ and Ccdc113–/– mice. Asterisk indicates abnormal spermatid shapes were found from step 11. (B) Spermatids from different manchette-containing steps were stained with α/β-tubulin antibody (red) to visualize the manchette. Step 11 of Ccdc113–/– spermatids displayed abnormal elongation of the manchette.
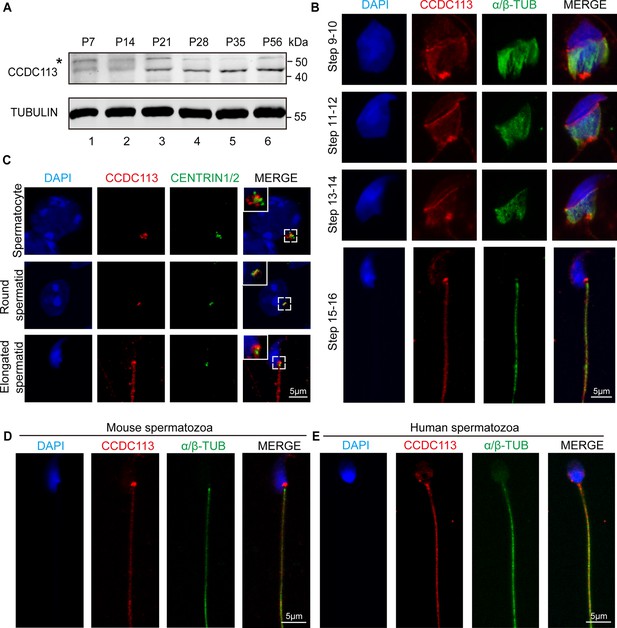
CCDC113 localizes to the head-tail coupling apparatus (HTCA), manchette, and sperm flagellum.
(A) CCDC113 was expressed starting in postnatal day 7 (P7) testes. TUBULIN served as the loading control. An asterisk indicates nonspecific bands. (B) Immunofluorescence of CCDC113 (red) and CENTRIN1/2 (green) in developing germ cells. CCDC113 partially colocalize with centriolar protein CENTRIN1/2. (C) Immunofluorescence of CCDC113 (red) and α/β-tubulin (green) in developing germ cells. The manchette was stained with the anti-α/β-tubulin antibody. (D–E) CCDC113 localizes to the HTCA and flagellum in mature mouse spermatozoa and human spermatozoa. Nuclei were stained with DAPI (blue).
-
Figure 4—source data 1
Original files for western blot in Figure 4A.
- https://cdn.elifesciences.org/articles/98016/elife-98016-fig4-data1-v1.zip
-
Figure 4—source data 2
Labelled file for western blot in Figure 4A.
- https://cdn.elifesciences.org/articles/98016/elife-98016-fig4-data2-v1.zip
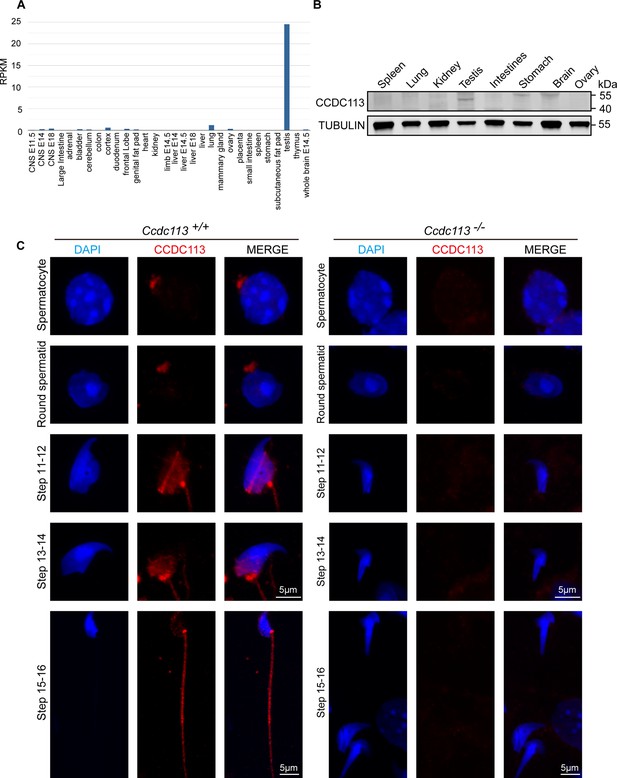
Subcellular localization of CCDC113 in testicular germ cells from Ccdc113+/+ and Ccdc113–/– mice.
(A) RNA expression data from various tissues using RNA-Seq data from Mouse ENCODE (https://www.ncbi.nlm.nih.gov/gene/244608). CCDC113 is highly expressed in the testis, but not significantly in the ovary and brain. (B) CCDC113 was predominately expressed in testis. Immunoblotting of CCDC113 was performed in the spleen, lung, kidney, testis, intestine, stomach, brain, and ovary. TUBULIN served as the loading control. (C) The immunofluorescence of CCDC113 in Ccdc113+/+ and Ccdc113–/– mice. Testicular germ cells were stained with anti-CCDC113 antibody (red), and the nucleus was stained with DAPI.
-
Figure 4—figure supplement 1—source data 1
Original files for western blot in Figure 4—figure supplement 1B.
- https://cdn.elifesciences.org/articles/98016/elife-98016-fig4-figsupp1-data1-v1.zip
-
Figure 4—figure supplement 1—source data 2
Labelled file for western blot in Figure 4—figure supplement 1B.
- https://cdn.elifesciences.org/articles/98016/elife-98016-fig4-figsupp1-data2-v1.zip
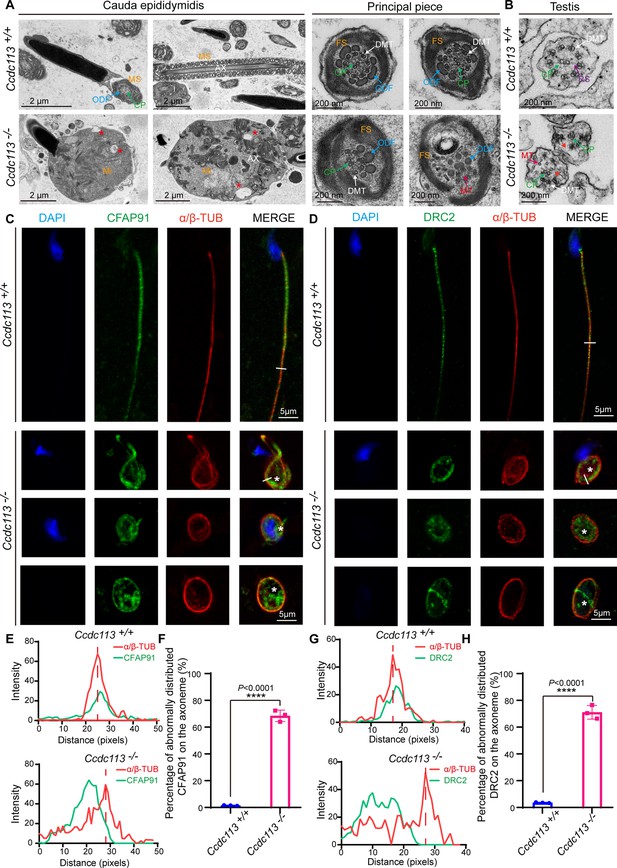
CCDC113 is indispensable for the docking of CFAP91 and DRC2 to the doublet microtubules (DMTs) to maintain the structural integrity of the axoneme.
(A) Transmission electron microscopy (TEM) analysis of spermatozoa from the cauda epididymidis of Ccdc113+/+ and Ccdc113–/– male mice. The flagellar longitudinal sections of Ccdc113–/– spermatozoa revealed unremoved cytoplasm, including disrupted mitochondria, damaged axonemes, and large vacuoles. Asterisks indicate large vacuoles. Cross sections of the principal piece of Ccdc113–/– spermatozoa further revealed partial loss or unidentifiable ‘9+2’ structures, along with the disruption of the fibrous sheath and outer dense fibers. (B) TEM analysis of the axoneme in testicular spermatids from Ccdc113+/+ and Ccdc113–/– male mice. The red arrowheads indicate the absence of significant radial spokes (RSs). MS: mitochondrial sheath; Mi: mitochondrial; AX: axoneme; FS: fibrous sheath; DMT: doublet microtubule; MT: microtubule; CP: central pair; ODF: outer dense fiber; RS: radial spokes. (C) The immunofluorescence analysis for CFAP91 (green) and α/β-tubulin (red) was performed in Ccdc113+/+ and Ccdc113–/– spermatozoa. Nuclei were stained with DAPI (blue). White asterisks indicate regions not colocalized with tubulin. (D) The immunofluorescence analysis for DRC2 (green) and α/β-tubulin (red) was performed in Ccdc113+/+ and Ccdc113–/– spermatozoa. Nuclei were stained with DAPI (blue). White asterisks indicate regions not colocalized with tubulin. (E, G) Line-scan analysis (white line) was performed using ImageJ software. (F, H) Percentage of abnormally distributed CFAP91 and DRC2 on the axoneme of Ccdc113+/+ and Ccdc113–/– spermatozoa (n=3 independent experiments). At least 200 spermatozoa were analyzed from each mouse. Data are presented as mean ± SD; ****p<0.0001.
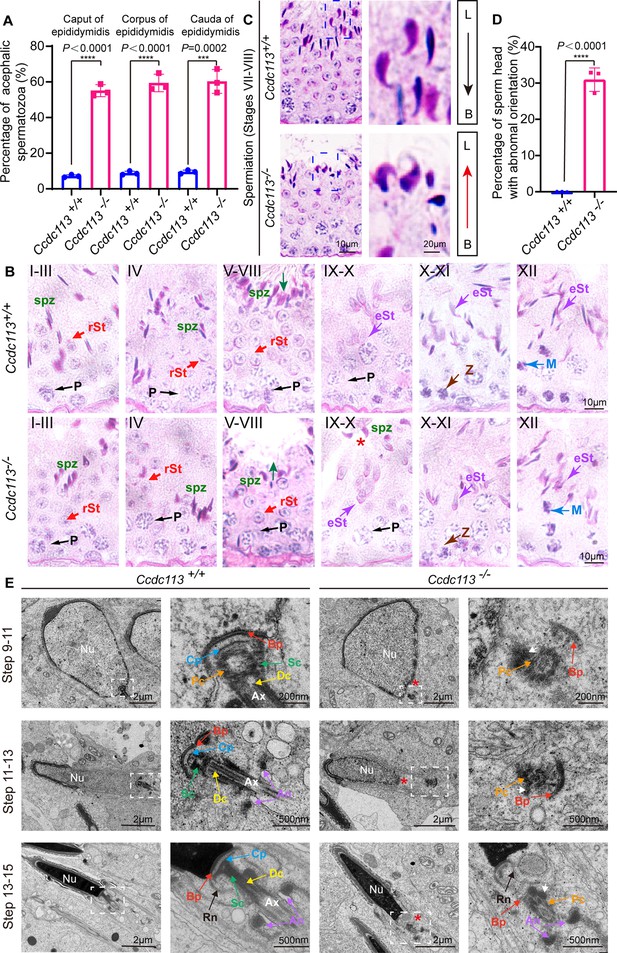
Ccdc113 knockout spermatids display impaired head-tail coupling apparatus (HTCA).
(A) The proportion of decapitated tails in Ccdc113+/+ and Ccdc113–/– corpus, caput, and cauda epididymis (n=3 independent experiments). At least 200 spermatozoa were analyzed from each mouse. Data are presented as mean ± SD; ****p<0.0001, ***p<0.001. (B) Periodic acid Schiff (PAS) staining of testis sections from Ccdc113+/+ and Ccdc113–/– mice. The green arrows indicate the orientation of the sperm heads in stages V–VIII seminiferous epithelia. Ccdc113–/– sperm head could still be detected in stages IX–X seminiferous epithelia. P: pachytene spermatocyte, spz: spermatozoa, rSt: round spermatid, eSt: elongating spermatid, Z: zygotene spermatocyte, M: meiotic spermatocyte. (C) Ccdc113–/– spermatids lost their head orientation toward the basement membrane during spermiation in stages VII–VIII of the seminiferous epithelium. L: lumen, B: basement membrane. (D) Percentage of sperm heads with abnormal orientation in stages VII–VIII of the seminiferous epithelium in Ccdc113+/+ and Ccdc113–/– mice (n=3 independent experiments). At least 200 spermatozoa were analyzed from each mouse. Data are presented as mean ± SD; ****p<0.0001. (E) Defective HTCA formation in Ccdc113–/– spermatids. Transmission electron microscopy (TEM) analysis of the stepwise development of the HTCA was performed in Ccdc113+/+ and Ccdc113–/– testes. In Ccdc113+/+ spermatids, the well-defined coupling apparatus was tightly attached to the sperm head. In Ccdc113–/– spermatids, segmented columns (Scs), the capitulum (Cp) were absent. The red asterisks indicate the distance between the sperm head and HTCA. The white arrows indicate the dense material surrounding the proximal centriole. Nu: nuclear; Bp: basal plate; Cp: capitulum; Sc: segmented column; Pc: proximal centriole; Dc: distal centriole; An: annulus; Ax: axoneme; Rn: redundant nuclear envelope.
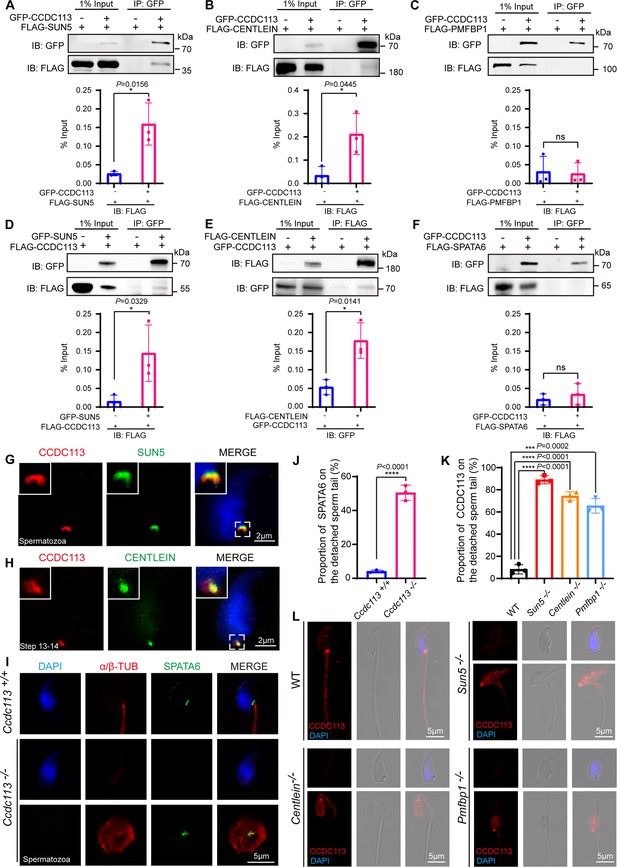
CCDC113 interacts with SUN5 and CENTLEIN, participating in sperm head-tail linkage.
(A–C, F) Head-tail coupling apparatus (HTCA)-associated proteins (SUN5, CENTLEIN, PMFBP1, SPATA6) were expressed alone or co-expressed with CCDC113 in HEK293T cells, and the interactions between CCDC113 and these HTCA-associated proteins were examined by co-immunoprecipitation. CCDC113 interacted with SUN5 and CENTLEIN, but did not interact with PMFBP1 and SPATA6. IB: immunoblotting; IP: immunoprecipitation. (D) SUN5 interacted with CCDC113. pECMV-FLAG-Ccdc113 and pEGFP-GFP-Sun5 were transfected into HEK293T cells. At 48 hr after transfection, the cells were collected for immunoprecipitation (IP) with anti-GFP antibody and analyzed with anti-FLAG and anti-GFP antibodies. (E) CENTLEIN interacted with CCDC113. pCDNA -FLAG-Centlein and pEGFP-GFP-Ccdc113 were transfected into HEK293T cells. At 48 hr after transfection, the cells were collected for IP with anti-FLAG antibody and analyzed with anti-FLAG and anti-GFP antibodies. The % Input is displayed below the corresponding figures for quantification. n=3 independent experiments. Data are presented as mean ± SD; *p<0.05, ns indicates no significant difference. (G) Immunofluorescence of CCDC113 (red) and SUN5 (green) in mature spermatozoa. Nuclei were stained with DAPI (blue). (H) Immunofluorescence of CCDC113 (red) and CENTLEIN (green) in testicular step 13–14 spermatid. Nuclei were stained with DAPI (blue). (I) Immunofluorescence analysis for SPATA6 (green) and α/β-tubulin (red) was performed in Ccdc113+/+ and Ccdc113–/– spermatozoa. Nuclei were stained with DAPI (blue). (J) Quantification ratio of SPATA6 on the detached sperm tail (n=3 independent experiments). At least 200 spermatozoa were analyzed for each mouse. (K) Quantification ratio of CCDC113 on the detached sperm tail (n=3 independent experiments). At least 200 spermatozoa were analyzed from each mouse. Data are presented as mean ± SD; ***p<0.001, ****p<0.0001. (L) Immunofluorescence analysis for CCDC113 (red) was performed in wild-type (WT), Sun5–/–, Centlein–/–, and Pmfbp1–/– spermatozoa. Nuclei were stained with DAPI (blue).
-
Figure 7—source data 1
Original files for western blot in Figure 7A-F.
- https://cdn.elifesciences.org/articles/98016/elife-98016-fig7-data1-v1.zip
-
Figure 7—source data 2
Labelled files for western blot in Figure 7A-F.
- https://cdn.elifesciences.org/articles/98016/elife-98016-fig7-data2-v1.zip





